Abstract
Background
Allergic rhinitis (AR) is a ubiquitous chronic disease with a growing incidence. We aimed to investigate the protective effect of naringenin against AR induced in rats.
Methods
Thirty-two Sprague Dawley rats were divided into four groups of eight animals each. Group 1 represented the control group. The other 24 rats were sensitized with intraperitoneal 0.3 mg ovalbumin (OVA) and 30 mg aluminum hydroxide every other day for 14 days to induce AR. Ten microliters OVA was administered to both nostrils by inhalation for the following seven days to provoke AR. Group 2 represented the AR group and received no treatment. Group 3 was treated as the reference group and received 5 mg/kg desloratadine every day between days 15 and 21. Group 4 received 100 mg/kg naringenin orally between days 15 and 21. All animal’s sneezing and nasal itching scores were recorded on day 22. The rats were then sacrificed. Serum total IgE, IL4 and IL5 values were studied, and nasal structures were extracted ‘en bloc’ for histopathological examination.
Results
Significant clinical recovery was achieved in the group treated with naringenin. Serum total IgE, IL4 and IL5 values in the naringenin group were significantly lower than in the AR group, and significant histopathological improvement was observed compared to the AR group.
Conclusions
Naringenin produced significant clinical, biochemical and histopathological benefits in rats with induced AR. These effects suggest that naringenin is a promising agent for the treatment of AR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent sneezing, watery nasal discharge and nasal obstruction are characteristically seen in allergic rhinitis (AR), an allergic type-1 disease of the nasal mucosa [1]. AR is a commonly seen form of non-infected rhinitis frequently mediated by immunoglobulin E (IgE) in association with aeroallergens resulting in chronic sinusitis due to recurrent nasal inflammation [2]. Although AR is not life-threatening, it compromises quality of life and entails an economic burden [3]. AR is one of the most common diseases worldwide. It is seen in all age groups, but generally commences at early ages and persists throughout life [4].
Various therapeutic approaches are available for the treatment of AR, including antihistaminics, steroids, immunotherapy, and leukotriene receptor antagonists. However, these have various side-effects, and also involve high costs due to the need for long-term use. New safe and natural therapeutic methods are needed to reduce side-effect rates and costs. Animal studies involving various substances with known antiallergic effects in alternative medicine are also being performed [5].
Naringenin is a flavanone group flavonoid found in citrus fruits such as orange and mandarin, and particularly grapefruit. It is also present in tomato and tomato-based products such as ketchup, and more widely in tomato peel [6]. Naringenin is an aglycone of naringin, and an active metabolite emerging through the conversion of naringin to naringenin by enterobacteria before absorption in the digestive system following oral intake. Naringenin (4′,5,7 thrihydroxyflavanone) can be obtained from hydrolysis of naringin (4′,5,7 thrihydroxyflavanone-7-rhamnoglucoside) with the enzyme naringinase. Naringenin exhibits free radical scavenging, lipid peroxidation reducing and anti-inflammatory effects by inhibiting the cyclo-oxygenase and 5-lipo-oxygenase pathways in arachidonic acid metabolism [7, 8].
The purpose of this study was to investigate the protective effect of naringenin against AR induced with ovalbumin (OVA) in an experimental animal model in comparison with desloratadine at the clinical, biochemical and pathological levels.
Materials and methods
Animals
This study commenced following receipt of approval from the Atatürk University Experimental Animals Local Ethical Committee. It was performed with 32 healthy female Sprague Dawley rats weighing 250–300 g obtained from the Atatürk University Medical Experimental Application and Research Center, Turkey.
Rats were housed in transparent plastic cages in a 12 h dark: 12 h light cycle, at a mean temperature of 23 ºC and 42% humidity. They were fed with standard chow, and no weight loss or mortality was observed during the study. The principles of the Helsinki Declaration concerning the care and use of experimental animals were scrupulously applied throughout the study.
Rats were randomly assigned into four groups of eight animals each. No allergic or infectious findings were determined at observational examination.
Experimental protocol and drug administration
Sensitization with antigens was performed on 24 rats, excluding the eight-member control group, to establish a model of AR. For that purpose, 0.3 mg OVA (Sigma-Aldrich Chemical Co. St. Louis) and 30 mg aluminum hydroxide were dissolved in 0.9% saline solution. A 1 ml mixture was prepared and administered to the rats every other day for 14 days in the same manner via the intraperitoneal route. From the 15th day onward, to trigger allergy, 20 mg/ml OVA was prepared with 0.9% saline solution. Next, 10 µl was administered via the intranasal route to each nostril of each rat with the help of a micropipette every day for seven days. Several similar studies have employed the same method to establish an AR model with OVA [9,10,11,12,13]. Our study design was as follows:
Group 1
The control group (control). The eight rats in this group received 8 mg/kg intraperitoneal saline solution throughout the study period.
Group 2
The AR group (AR). The eight rats in this group were sensitized and provoked with OVA, and received 8 mg/kg intraperitoneal saline solution throughout the treatment period.
Group 3
The AR and antihistaminic treatment group (AR-H). The eight rats with induced AR in this group received 10 mg/kg desloratadine (AERIUS 5 mg tb Schering Plough) dissolved in 2 ml 0.9% saline solution via intragastric gavage every day for seven days, 1 h before OVA administration on days 15–21 of the study.
Group 4
The induced AR and naringenin treatment group (AR-NAR). The eight rats in this group received 100 mg/kg naringenin dissolved in 2 ml saline solution with the help of 1% (1 mg/kg) carboxy methyl cellulose (CMC) via intragastric gavage every day for seven days 1 h before OVA administration on days 15–21 of the study.
Nasal symptom scoring
Nasal symptom scoring was performed on all rats 24 h after final drug administration for the purpose of clinical evaluation. All rats were placed into separate cages. After a 15 min adaptation period they were observed by eight observers blinded to the study groups. Nose scratching movements and numbers of sneezes were separately counted over a 15 min period. The total numbers of nasal scratching movement and sneezes were calculated.
Anesthesia application protocol: following clinical evaluation, general anesthesia was administered with ketamine hydrochloride (Ketalar ampoule, Pfizer, Istanbul) 40 mg/kg + xylazine hydrochloride (Rompun ampoule, Bayer, Istanbul) 10 mg/kg by the intraperitoneal route.
In-vivo blood collection process and excision of nasal structures
The diaphragm was carefully passed through an abdominal incision with animals under general anesthesia. Intracardiac blood specimens were collected from the functioning heart. Rats were then sacrificed, and the nasal region was extracted ‘en bloc’.
Biochemical analysis
The intracardiac blood specimens were immediately centrifuged for 10 min at 3000 rpm for plasma separation. The plasma was then stored at −80 °C until the day of study.
Plasma total Ig-E levels were determined using the ELISA method. A kit specially produced for rats, No. 201-11-0453 was employed for this purpose (Sunredbio, China). Ig-E levels were expressed as KU/L. Plasma IL-4 levels were determined using ELISA and a kit with a code number of 201-11-0134 (Sunredbio, China). IL-4 levels were expressed as pg/mL. Plasma IL-5 levels were determined using ELISA and a kit with a code number of 201-11-0135 (Sunredbio, China). IL-5 levels were expressed as pg/mL.
Histopathological analysis
Nasal septal tissues extracted at necropsy for histopathological examination were fixed in 10% formalin solution for 48 h. They were then softened for 96–120 h in Osteosoft (Merc, HC313331, Germany) solution for decalcification and washed in running water for 24 h. Tissues were then embedded in paraffin blocks being processed through 80% alcohol (12 h × 2 times), 90% alcohol (12 h × 2 times), 96% alcohol (12 h × 2 times), 100% alcohol (12 h × 2 times), chloroform (5 h × 3 times), and liquid paraffin (12 h). Sections 4 mm in thickness were taken from each block, and preparates were made ready on glass slides. The preparates were stained with hematoxylin–eosin (H and E) for histopathological examination and examined under a light microscope. Sections examined under light microscope were evaluated in terms of lesions; none (−), mild (+), moderate (++) or severe (+++) and photographed. The scale of histopathological examination was summarized in Table 1.
Statistical analysis
SPSS 17.0 software was used for statistical analysis. Distribution of nasal symptom scores and biochemical data was assessed using the Shapiro–Wilk test. Since distribution was normal, One-Way ANOVA was used for analysis. Two-way analyses were performed using the Post-hoc Tukey test. p values <0.05 were regarded as statistically significant.
Results
Nasal symptom scores
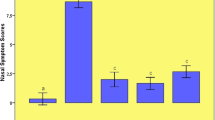
As both numbers of nasal scratching movements and number of sneezing were higher, the highest nasal symptom score was determined in the AR group (p < 0.05). Nasal scores in the two treatment groups were significantly lower compared to the AR group, but no significant difference was observed between nasal symptom scores in the AR-H and AR-NAR groups (Figs. 1, 2).
Biochemical results
Total Ig-E levels were significantly elevated in the AR group compared to the other groups (p < 0.001). Ig-E levels were significantly lower in both the AR-H and AR-NAR groups compared to the AR group. (p < 0.001) (Fig. 3).
Biochemical results. Data are expressed as mean values, and standard deviation values are shown above each bar. The letters above each bar indicate the statistical comparison. Different letters indicate a significant difference at the p < 0.05 level, while the same letters indicate no significant difference. a Comparison of Ig-E results. b Comparison of IL-4 results. c Comparison of IL-5 results
Plasma IL-4 levels were significantly higher in the AR group than in the other groups. Plasma IL-4 levels in rats treated with naringenin were significantly lower than in the AR group (p < 0.05) (Fig. 3).
Plasma IL-5 levels were significantly higher in the AR group than in the other groups (p < 0.001). IL-5 levels were significantly lower in both treatment groups compared to the AR group, but no significant difference was determined in IL-5 levels between the desloratadine and naringenin groups (p = 0.09) (Fig. 3). The biochemical test results are shown in Table 2.
Histopathological findings
A comparison of the histopathological findings is shown in Table 3. Histopathological examination of rat’s nasal cavities revealed the following findings:
Control group
A normal histological architecture was observed in the mucosa and submucosa (Fig. 4a).
Histopathological findings. H and E, Bar: 20 µm. a Control group: a normal histological architecture was observed in the mucosa and submucosa. c septal cartilage. b AR Group: severe desquamation and erosion in lamina epithelialis (arrows), degeneration and necrosis in the epithelium (arrow heads), eosinophilic cell infiltration (stars), dilatation of vessels and severe congestion (empty star). Severe edema was observed in the submucosa with dilatation of serous glands (thick arrows). c AR-H Group: desquamation and erosion in the lamina epithelialis (arrow heads), dilatation and congestion in vessels (empty star), dilatation of serous glands (thick arrows). d AR-NAR Group: mild desquamation and erosion in the lamina epithelialis (arrow heads), eosinophilic cell infiltration (stars), dilatation and congestion in vessels (empty star), dilatation of serous glands (thick arrows)
AR group
Desquamation, erosion and eosinophilic cell infiltration were observed in the mucosal epithelium in the nasal cavity. Severe edema was observed in the submucosa, together with dilatation and severe congestion in vessels (Fig. 4b).
AR-H group
Desquamation and erosion were observed in the lamina epithelialis, mild edema in the lamina propria, and hyperemia and eosinophilic cell infiltration in vessels (Fig. 4c)
AR-NAR group
Mild erosion was observed in the lamina epithelialis, edema in the lamina propria, and hyperemia and moderate eosinophilic cell infiltration in vessels (Fig. 4d).
Discussion
AR is an inflammatory disease of the nasal mucosa characterized by sneezing, and nasal itching, discharge and obstruction. CD4+ lymphocytes play a key role in this IgE-mediated inflammation, together with cytokines such as IL-4, -5, -10, and -13 released from Th2 cells. IL-4 is the principal cytokine that triggers sensitivity to allergens through the release of IgE from B lymphocytes. IL-5 plays a key role in the triggering of eosinopoiesis and in eosinophilic inflammation in AR. Scientific and epidemiological studies have shown that AR is a component of the inflammatory process and is associated with other inflammatory disorders of the mucosal membrane, such as rhinosinusitis and allergic conjunctivitis, and particularly asthma. Environmental allergens mediate early and late phase responses in AR, similarly to asthma [14].
Allergens in the nasal mucosa of sensitized individuals trigger the rapid release of stored mediators such as histamine by binding to the mast cell surface, resulting in early phase responses such as nasal itching, watery nasal discharge, and sneezing. IgE-mediated type-1 hypersensitivity reaction leads to the early phase of AR. In addition to histamine and tumor necrosis factor alpha (TNFα) stored with the production of endothelial molecules, lipid structures such as leukotriene C4 (LTC4) and prostaglandin D2 (PGD2) secreted from cells such as eosinophils, basophils, and CD+ T lymphocytes are also involved in the late phase of the allergic response. The principal symptom of the late phase of allergy is nasal obstruction. Edema of the nasal mucosa derives from mucosal vessel obstruction and plasma leakage and interstitial edema in the mucosa under the effect of platelet activating factor (PAF), PGD2, kinin and leukotrienes. Leukotrienes released from eosinophils in particular play the principal role in edema of the nasal mucosa observed in the late phase [15].
In addition to the lymph nodes and bone marrow, the principal production sites, IgE is also manufactured in B lymphocytes in the nasal mucosa. Increased allergen-specific IgE and eosinophilic inflammation, characteristic features of AR, differentiate the allergy from other disorders [14,15,16].
H1 antihistaminics, intranasal corticosteroids and immunotherapy used in combination are modalities commonly employed in the treatment of AR. However, these may give rise to side-effects such as mild numbness, vertigo, concentration disorder and arrhythmia. Many patients employ alternative and complementary therapies such as Chinese medicine and acupuncture that result in fewer side-effects. Alternative and complementary therapies, including herbal medications, and dietary supplements are also regarded as general therapeutic modalities for AR [17, 18]. Several studies have investigated herbal medications and alternative therapies for the treatment of AR. Such studies have attracted growing interest in recent years. Quercetin, curcumin, resveratrol, berberine and hesperidin have been used for therapeutic purposes in induced experimental AR models [10, 11, 19,20,21].
OVA is widely employed to induce an animal model of AR. OVA triggers such principal symptoms of AR as sneezing and nasal itching, and can also cause histopathological changes in the nasal mucosa. In their study involving an OVA-induced model of AR, Sakat et al. showed that OVA successfully induced AR and caused a significant increase in nasal symptom scores and in plasma immunoglobulin-E, IL-4, IL-13, MDA and NO levels [10]. Similarly, Kılıç et al. also reported that OVA induced AR by causing increases in nasal symptom scores and plasma immunoglobulin-E, IL-5, IL-13, and TAS levels [11]. We also determined allergic inflammation findings at histopathological examination in this study, together with a significant increase in numbers of nasal scratching and sneezing and an increase in plasma Ig-E, IL-4 and IL-5 levels. Our purpose was to investigate the protective effect of naringenin in the AR model we established in clinical, histopathological and serological terms.
Naringenin (4,5,7-trihidroxyflavone) is an aglycone of naringin and a natural flavanone generally found in citrus fruits, and also in cherry and tomato. Naringenin is regarded as harmless under the relative toxicity classification, and can be well-tolerated with no toxic effects up to 100 mM. Its health benefits, and disease prevention, pharmacological and nutritional properties have recently attracted considerable interest [22, 23]. Several previous studies have investigated the effects of naringenin.
Koçak et al. investigated the protective role of naringenin in a gentamycin-induced ototoxicity model. They administered 120 mg/kg gentamycin intraperitoneally and 50 mg/kg naringenin orally for 14 days. Audiological, biochemical and histopathological examinations revealed that simultaneous naringenin administration restricted the ototoxic effects of gentamycin [24].
Zhang et al. investigated the effects of naringenin in 95 patients with osteosarcoma. They showed that naringenin reduced the progression and recurrence of the disease in osteosarcoma patients by increasing antioxidant capacity and glutathione and superoxide dismutase levels and reducing those of IL1 and IL6 [25].
In a study from 2017, Manchope et al. reported that naringenin exhibited analgesic, anti-inflammatory and oxidant properties [26]. Another study showed that in addition to maintaining its anti-inflammatory potency, heated naringenin significantly increased the anti-inflammatory effectiveness of spleen cells and the cytotoxic activity of natural killer (NK) cells and reduced T cell cytotoxicity [27]. A separate study reported that naringenin exhibited NF-κB inhibition with anti-inflammatory and anti-arthritic effects in an experimentally induced AR model in rats [28].
While numerous studies have investigated all these effects of naringenin for many years, there has also recently been a limited number of studies of its antiallergic properties.
Shi et al. investigated the effects of naringenin in an OVA-induced asthma model in mice. They reported that naringenin administered at 100 mg/kg via the intraperitoneal route exhibited a protective role, by significantly reducing airway hypersensitivity, lowering serum total IgE and IL4 and IL13 levels during bronchoalveolar lavage, and enhancing the increased effect of NF-κB that triggers allergic inflammation [29]. In support of these studies, other research by the same author and team investigated the effect of naringenin on potential structural changes in an OVA-induced model of asthma. They concluded that naringenin provides protection at the cellular level and reduces fibrosis formation [30]. Iwamura et al. showed that naringenin reduced airway hypersensitivity, produced a significant fall in leukocyte and eosinophil numbers in bronchoalveolar lavage (BAL), reduced pulmonary mononuclear inflammatory cell numbers and mucus hypersecretion at histological examination, and lowered concentrations of TH2 type cytokines released from CD4 T cells, such as IL4, IL5 and IL13 in mice with OVA-induced allergic airway inflammation [31]. We also determined significantly lower IL-4 and IL-5 levels in the naringenin group in the present study.
In their cell culture study, Moon et al. showed that naringenin significantly reduced thymic stromal lymphopoietin (TSLP) that plays a significant role in allergic diseases by inhibiting NF-κB, caspase 1 and receptor-interacting protein 2 [32]. Another study investigated the protective property of naringenin against the effects of TSLP in human mast cell line (HMC-1) cell culture and reported a protective effect against mast cell-mediated allergy formation through the reduction of mRNA expressions of IL13, TNF-α, TSLP receptor and IL7 receptor α [33].
In the present study, we observed a marked recovery in nasal symptom scores in the group treated with naringenin. At biochemical analysis, serum total IgE and the TH2 type cytokines IL4 and IL5 in the group treated with naringenin were lower than in the group receiving OVA. Histopathological analysis revealed that findings in the group with OVA-induced AR such as desquamation in the nasal epithelium in the nasal mucosa, erosion, eosinophilic cell infiltration, severe edema in the submucosa, dilatation in vessels, and severe congestion all recovered significantly in the group treated with naringenin. Naringenin appears to be as effective against AR as desloratadine. We therefore think that naringenin by itself may be effective in the treatment of AR. Further human studies are now needed for naringenin to be used in the clinical setting.
There are a number of limitations to this study. First, only a single dose of naringenin was employed. The use of different doses would make it possible to investigate its efficacy in a dose-dependent manner. Second, the addition of steroids and leukotriene antagonists, other medications with proven effectiveness against allergic diseases, would be useful in terms of comparing the effects of naringenin. Finally, immunohistochemical analysis might have enhanced the significance of this study.
Conclusion
This study investigated, for the first time in the literature, the protective effect of naringenin in an OVA-induced AR model using clinical, biochemical and histopathological methods. We determined that use of naringenin resulted in significant benefits against the clinical symptoms of AR, as well as significant biochemical and histopathological recovery. In the light of the current findings, we think that naringenin is a promising, potentially useful substance in the treatment of AR.
References
Okubo K, Kurono Y, Fujieda S, Ogino S, Uchio E, Odajima H, Takenaka H (2014) Japanese guideline for allergic rhinitis 2014. AllergolInt 63(3):357–75
Nuhoglu C, Nuhoglu Y, Bankaoglu M, Ceran O (2010) A retrospective analysis of adenoidal size in children with allergic rhinitis and nonallergic idiopathic rhinitis. Asian Pac J Allergy Immunol 28(2–3):136
Kashiwabara M, Asano K, Mizuyoshi T, Kobayashi H (2016) Suppression of neuropeptide production by quercetin in allergic rhinitis model rats. BMC Complement Altern Med 16(1):132
Bousquet J, Schunemann H, Fonseca J, Samolinski B, Bachert C, Canonica GW, Casale T, Cruz A, Demoly P, Hellings P (2015) MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 70(11):1372–92
Yasar M, Savranlar Y, Karaman H, Sagit M, Silici S, Ozcan I (2016) Effects of propolis in an experimental rat model of allergic rhinitis. Am J Otolaryngol 37(4):287–93
Erlund I (2004) Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res 24(10):851–74
Hsiu S-L, Huang T-Y, Hou Y-C, Chin D-H, Chao P-DL (2002) Comparison of metabolic pharmacokinetics of naringin and naringenin in rabbits. Life Sci 70(13):1481–9
Ribeiro IA, Rocha J, Sepodes B, Mota-Filipe H, Ribeiro MH (2008) Effect of naringin enzymatic hydrolysis towards naringenin on the anti-inflammatory activity of both compounds. J MolCatal B Enzym 52:13–8
Tatar A, Parlak SN, Yayla M, Ugan RA, Polat E, Halici Z Eds (2015) Effects of allergic rhinitis and desloratadine on the submandibular gland in a rat allergy model. International Forum of Allergy and Rhinology, Wiley Online Library.
Sakat MS, Kilic K, Kandemir FM, Yildirim S, Sahin A, Kucukler S, Saglam YS (2018) The ameliorative effect of berberine and coenzyme Q10 in an ovalbumin-induced allergic rhinitis model. Eur Arch Otorhinolaryngol 275(10):2495–505
Kilic K, Sakat MS, Yildirim S, Kandemir FM, Gozeler MS, Dortbudak MB, Kucukler S (2019) The amendatory effect of hesperidin and thymol in allergic rhinitis: an ovalbumin-induced rat model. Eur Arch Otorhinolaryngol 276(2):407–15
Zhang Z, Kang H (2020) Protective effect of Asarumsieboldii essential oil on ovalbumin induced allergic rhinitis in rat. Biosci Rep 40(6):BSR20191370
Li J, Wang B, Luo Y, Zhang Q, Bian Y, Wang R (2020) Resveratrol-mediated SIRT1 activation attenuates ovalbumin-induced allergic rhinitis in mice. MolImmunol 122:156–62
Greiner AN, Hellings PW, Rotiroti G, Scadding GK (2011) Allergic rhinitis. Lancet 378:2112–22
Ridolo E, Martignago I, Masieri S (2018) Mechanisms of allergic diseases in Otorhinolaryngology. J BiolRegulHomeost Agents 32(1 Suppl. 1):9
Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, Kawauchi H, Suzaki H, Fujieda S, Masuyama K (2020) Japanese guidelines for allergic rhinitis 2020. AllergolInt 69(3):331–45
Ren M, Tang Q, Chen F, Xing X, Huang Y, Tan X (2017) MahuangFuziXixin decoction attenuates Th1 and Th2 responses in the treatment of ovalbumin-induced allergic inflammation in a rat model of allergic rhinitis. J Immunol Res 2017:8254324
Jung HW, Jung J-K, Park Y-K (2011) Antiallergic effect of Ostericum koreanum root extract on ovalbumin-induced allergic rhinitis mouse model and mast cells. Asian Pac J Allergy Immunol 29(4):338–48
Sagit M, Polat H, Gurgen SG, Berk E, Guler S, Yasar M (2017) Effectiveness of quercetin in an experimental rat model of allergic rhinitis. Eur Arch Otorhinolaryngol 274(8):3087–95
Thakare VN, Osama M, Naik SR (2013) Therapeutic potential of curcumin in experimentally induced allergic rhinitis in guinea pigs. IntImmunopharmacol 17(1):18–25
Bozdemir K, Şahin E, Altintoprak N, Muluk NB, Cengiz BP, Acar M, Cingi C (2016) Is resveratrol therapeutic when used to treat allergic rhinitis in rats? Clin Invest Med 39(2):E63–E72
Rehman MU, Rahman Mir MU, Farooq A, Rashid SM, Ahmad B, Bilal Ahmad S, Ali R, Hussain I, Masoodi M, Muzamil S (2018) Naringenin (4, 5, 7-trihydroxyflavanone) suppresses the development of precancerous lesions via controlling hyperproliferation and inflammation in the colon of Wistar rats. Environ Toxicol 33(4):422–35
Da Pozzo E, Costa B, Cavallini C, Testai L, Martelli A, Calderone V, Martini C (2017) The citrus flavanonenaringenin protects myocardial cells against age-associated damage. Oxid Med Cell Longev 2017:9536148
Koçak İ, Sarac S, Aydogan E, Şentürk E, Akakın D, Koroglu K, Özer Ö (2017) Evaluation of the possible protective role of naringenin on gentamicin-induced ototoxicity: a preliminary study. Int J PediatrOtorhinolaryngol 100:247–53
Zhang L, Xu X, Jiang T, Wu K, Ding C, Liu Z, Zhang X, Yu T, Song C (2018) Citrus aurantiumnaringenin prevents osteosarcoma progression and recurrence in the patients who underwent osteosarcoma surgery by improving antioxidant capability. Oxid Med Cell Longev 2018:8713263
Manchope MF, Casagrande R, Verri WA Jr (2017) Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget 8(3):3766
Maatouk M, Elgueder D, Mustapha N, Chaaban H, Bzéouich IM, Loannou I, Kilani S, Ghoul M, Ghedira K, Chekir-Ghedira L (2016) Effect of heated naringenin on immunomodulatory properties and cellular antioxidant activity. Cell Stress Chaperones 21(6):1101–9
Fan R, Pan T, Zhu A-L, Zhang M-H (2017) Anti-inflammatory and anti-arthritic properties of naringenin via attenuation of NF-κB and activation of the heme oxygenase (HO)-1/related factor 2 pathway. Pharmacol Rep 69(5):1021–9
Shi Y, Dai J, Liu H, Li R-R, Sun P-L, Du Q, Pang L-l, Chen Z, Yin K-S (2009) Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-κB activity in a murine model of asthma. Can J PhysiolPharmacol 87(9):729–35
Shi Y, Tan Y, Mao S, Gu W (2014) Naringenin inhibits allergen-induced airway remodeling in a murine model of asthma. Mol Med Rep 9(4):1204–8
Iwamura C, Shinoda K, Yoshimura M, Watanabe Y, Obata A, Nakayama T (2010) Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. AllergolInt 59(1):67–73
Moon P-D, Choi I-H, Kim H-M (2011) Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur J Pharmacol 671(1–3):128–32
Han NR, Moon PD, Ryu KJ, Kim NR, Kim HM, Jeong HJ (2018) Inhibitory effect of naringenin via IL-13 level regulation on thymic stromal lymphopoietin-induced inflammatory reactions. ClinExpPharmacolPhysiol 45(4):362–9
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This study was performed in accordance with the PHS Policy on Human Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Animal Experiments ethical committee of Ataturk University. (No. E.1700320360-13:158;160).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Şahin, A., Sakat, M.S., Kılıç, K. et al. The protective effect of Naringenin against ovalbumin-induced allergic rhinitis in rats. Eur Arch Otorhinolaryngol 278, 4839–4846 (2021). https://doi.org/10.1007/s00405-021-06769-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06769-7