Abstract
Hesperidin, a flavanone glycoside comprised of the flavanone hesperetin and the disaccharide rutinose, is a plentiful and inexpensive by-product of citrus cultivation. It has been reported to exert a wide range of pharmacological effects that include antioxidant, anti-inflammatory, and anticarcinogenic properties. In this study, we attempt to determine whether hesperidin inhibits inflammatory mediators in the mouse allergic asthma model. Mice were sensitized and challenged by ovalbumin (OVA) to induce chronic airway inflammation and airway remodeling. The administration of hesperidin significantly decreased the number of infiltrating inflammatory cells and Th2 cytokines in bronchoalveolar lavage (BAL) fluid compared with the OVA-induced group of mice. In addition, hesperidin reduced OVA-specific IgE levels in serum. Hesperidin markedly alleviated the OVA-induced airway hyperresponsiveness (AHR) to inhaled methacholine. Based on lung histopathological studies using hematoxylin and eosin and alcian blue-periodic acid-Schiff staining, hesperidin inhibited inflammatory cell infiltration and mucus hypersecretion compared with the OVA-induced group of mice. These findings provide new insight into the immunopharmacological role of hesperidin in terms of its effects in a murine model of asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic asthma is a chronic inflammatory disease of the lung that is characterized by airway inflammation and airway hyperresponsiveness (AHR). Inflammation occurs due to the infiltration of eosinophils, neutrophils, and macrophages into the bronchial lumen and lung tissues [1, 2]. The recruitment of these inflammatory cells is a critical event in the development of airway inflammation. The recruitment of leukocytes to the site of inflammation is mediated by the Th2 cytokines, such as IL-4, IL-5, and IL-13, which are crucial for IgE synthesis, airway eosinophilia, mucus secretion, and ultimately AHR [3–5]. In vivo experimental studies have revealed that the overexpression or administration of either IL-4 or IL-13 in the airways can induce airway eosinophilia and AHR [6, 7]. The growth, differentiation, recruitment, and survival of eosinophils are associated with IL-5 [8], and IL-4 is the most influential factor regulating IgE production by B cells. Immunoglobulin E (IgE) has been associated with allergic diseases and asthma. Recent studies manifest that anti-IgE therapy plays an important role in these diseases [9–11].
The morbidity and mortality of asthma appear to be increasing, and it has been suggested that medications used to treat asthma contribute to this trend. Recently, citrus flavonoids have been reported to possess various pharmacological effects, such as anti-cancer and anti-inflammatory activities [12–14]. Hesperidin (Fig. 1) is the most prevalent flavanone glycoside found in sweet orange and lemon and is obtained as an abundant by-product of citrus cultivation [15, 16]. It has been implicated in the inhibition of enzymes involved in several diseases, including phospholipase A2, lipoxygenase, HMG-CoA reductase, and cyclooxygenase [17, 18]. As a result, hesperidin has antioxidant, anti-inflammatory, hypolipidemic, vasoprotective, and cholesterol-lowering properties [19]. In addition, hesperidin has anti-allergic qualities. Park et al. reported that an orally administrated hesperidin, flavanone glycoside, inhibited the passive cutaneous anaphylaxis (PCA) reaction of mice but exhibited no PCA-inhibitory activity when administered intraperitoneally [20]. Another group also demonstrated that hesperidin did not inhibit histamine release in vitro [21]. The anti-allergic activities of hesperidin are controversial because the mechanisms by which this citrus flavonoid exerts its activities have not yet been fully clarified. It has been reported that hesperidin possesses potent anti-inflammatory effects in both cellular and animal models of inflammation, including endotoxin-induced acute lung injury and infection-induced lethal shock [22–24]. Results from both infectious and non-infectious mouse airway inflammatory models show a distinct anti-inflammatory role for hesperidin. In the study described here, we have employed a non-infectious mouse airway inflammatory and bronchial asthma model to determine if there is an anti-allergic inflammatory effect of orally administrated hesperidin.
MATERIALS AND METHODS
Animals
Female BALB/c mice, weighing approximately 18 to 20 g, were purchased from Shanghai Jingke Industrial Co., LTD (Certificate SCXK2003-0003) (Shanghai, China).
The mice were housed in micro-isolator cages and received food and water ad libitum. The laboratory temperature was 24 ± 1°C, and relative humidity was 40–80%.
Animals were housed in a 12 h light/12 h dark cycle (6 am–6 pm) and provided with rodent diet and water ad libitum. All animal experiments were performed in accordance with the guide for the Care and Use of Laboratory Animals published by US National Institutes of Health.
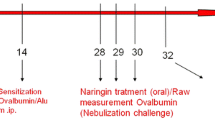
Sensitization and Challenge with Ovalbumin
These mice were divided into five groups (n = 10). Control group (Con), mice were sensitized with ovalbumin and nasal administration of phosphate-buffered saline (PBS); ovalbumin-challenged group (OVA), mice were sensitized and nasal administration of ovalbumin (local challenge); hesperidin group, ovalbumin-challenged mice were treated with hesperidin (5, 10, and 30 mg/kg). Hereafter, we used these group abbreviations to clarify the text. Mice were sensitized with 20 μg ovalbumin (Grade V; Sigma-Aldrich, St. Louis, MO) adsorbed in 100 μg/ml of Imject Alum (Pierce, Rockford, USA) by i.p. injection on days 0, 7, 14 (general sensitization) in all mice. On day 14, mice were anesthetized, and 100 μg of OVA in 50 μl of PBS administered intranasally in all mice except negative control sensitized with PBS. Mice were again anesthetized before being challenged with 100 μg of OVA in 50 μl of PBS on each of days 25–27. Control mice were given PBS in place of OVA in challenge stages of the protocol. On days 25–27, hesperidin at 5, 10, and 30 mg/kg were given with an oral administration 1 h prior to ovalbumin administration.
Collection of Blood and Bronchoalveolar Lavage
Twenty-four hours after the last administration, mice were anesthetized and bled via the brachial plexus for collection of blood samples used to estimate the IgE production. Bronchoalveolar lavage (BAL) fluids were performed by an intratracheal injection of 0.5 ml of PBS solution followed by gentle aspiration. The lavage was repeated twice to recover a total volume of 0.6–0.8 ml. The lavage was centrifuged, and the supernatant was processed for IL-4, IL-5, and IL-13 measurement. The cell pellets were resuspended in a 1 ml of PBS and then applied to a slide by cytospinning for differential cell counts by staining with a modified Giemsa method. At least 200 cells were counted per slide.
Mouse Anti-OVA IgE ELISA
To define serum levels of OVA-specific IgE, an ELISA analysis was carried out using a mouse-specific anti-IgE-antibody. Briefly, microplate wells were coated with 1% OVA in coating buffer (0.05 M sodium carbonate–bicarbonate, pH 9.6) overnight at 4°C. The wells were then incubated with blocking buffer (1% BSA in PBS, pH 7.2) at room temperature for 1 h and washed. Then, diluted (1/10) serum samples were introduced to the microplate, which was then incubated at room temperature for 2 h, washed, and incubated with Biotin anti-mouse IgE, followed by extravidin-peroxidase at room temperature for 30 min and with TMB substrate for 15 min. The enzymatic reaction was stopped with 2 M H2SO4, and absorbance was read at 450 nm. Units are reported as optical density at 450 nm.
Measurement of Cytokines
Levels of IL-4, IL-5, and IL-13 were quantified in BALF by enzyme immunoassays performed according to the manufacturer's protocol (BioLegend, Inc. Camino Santa Fe, Suite E San Diego, CA, USA).
Lung Histology
The lungs were removed, immersed in 4% phosphate-buffered formalin, and embedded in paraffin. Tissues were cut into 3-μm sections, which were then stained with hematoxylin and eosin (H&E) for general morphology, with alcian blue-periodic acid-Schiff (AB-PAS) for the identification of goblet cells in the epithelium.
Measurements of AHR
AHR was measured as previously described [24]. Mice were anesthetized by an intraperitoneal injection of 0.2 ml pentobarbital sodium (70–90 mg/kg), and tracheotomy was performed. The internal jugular vein was cannulated and connected to a microsyringe for intravenous methacholine administration. The mice were placed in a whole-body plethysmograph chamber (Buxco, Sharon, CT) and ventilated mechanically by a ventilator (Hugo Sachs Elektronik, Harvard Apparatus, March-Hugstetten, Germany) at a tidal volume of 200 ml/breath and a breath rate of 150/min. Airway resistance (RI) and lung compliance (Cdyn) in response to increasing concentrations of methacholine were recorded and expressed as a percentage of the respective basal values in response to baseline.
RESULTS
Ovalbumin-specific Serum IgE Levels
Antigen-specific Th2 responses are known to induce antigen-specific IgE antibody production. Therefore, we examined whether hesperidin influenced ovalbumin-specific IgE production in a murine allergic asthma model using ELISA. OVA-sensitized mice had high levels of serum anti-ovalbumin IgE antibodies compared to control mice (Fig. 2). Treatment with hesperidin (10 and 30 mg/kg) significantly reduced ovalbumin-specific IgE in mice (Fig. 2) (*p < 0.05, **p < 0.01 vs. ovalbumin-challenged mice).
Effect of Hesperidin on the Release of Il-4, Il-5, and Il-13 in BALF
As shown in Fig. 3. OVA inhalation in sensitized mice caused a notable increase in IL-4 (245.561 ± 33.74 pg/mL), IL-5 (289.815 ± 6.28 pg/mL), and IL-13 (147.1 ± 25.4 pg/mL) concentrations in BALF compared to PBS control mice. Hesperidin (10 and 30 mg/kg) treatment significantly decreased the concentrations of IL-4 in a dose-dependent manner (*p < 0.05, **p < 0.01).
Effects of Hesperidin on Ova-induced Total and Differential Leukocytes in BAL Fluid
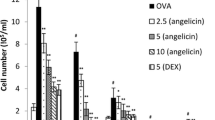
We examined the change of total and differential leukocytes in the BAL fluid following OVA immunization and challenge. In the OVA group, the number of total cells was increased by 3.3-fold, the number of eosinophils was increased significantly by 2.7-fold, the number of neutrophil was increased by 5.1-fold, and the number of macrophages was increased by 4-fold, compared to those in the control group (Fig. 4). Hesperidin significantly decreased the numbers of OVA-induced total cell, eosinophils, neutrophil, and macrophages in a dose-dependent manner. These results suggest that hesperidin inhibited the OVA-induced inflammatory response in a murine model of asthma.
Effect of hesperidin (milligram per kilogram) on the recruitment of inflammatory cells in BALF. The lavage fluid was centrifuged, and the cell pellets were resuspended in 1 ml of PBS and were applied to a slide by cytospinning to facilitate differential cell counts. At least 200 cells were counted per slide. The values represent the mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01. vs. OVA.
Effects of Hesperidin on Ova-induced Histopathological Changes in Lung
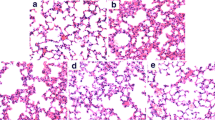
To assess the anti-inflammatory and/or anti-remodeling effect of hesperidin, histopathological studies were performed. Using H&E staining, inflammatory cell infiltration in peribronchial and perivascular areas was observed in OVA-challenged mice (Fig. 5a). Treatment with hesperidin (10 and 30 mg/kg) markedly reduced the degree of inflammatory cell infiltration in the peribronchial and perivascular areas. OVA also induced goblet cell hyperplasia and mucus hypersecretion into the lung tissue compared to those in the control group (using AB-PAS). Hesperidin inhibited the OVA-induced goblet cell hyperplasia and mucus hypersecretion compared to those in the OVA-inhaled group (Fig. 5b). These results suggest that hesperidin inhibited the OVA-induced inflammatory infiltration, goblet cell hyperplasia, and mucus hypersecretion in a murine model of asthma.
Effect of hesperidin on airway inflammation, airway goblet cell hyperplasia and mucus production. Representative hematoxylin–eosin and alcian blue-periodic acid-Schiff stained sections of lung from: a PBS-challenged mice; b OVA-challenged mice; c OVA-challenged mice treated with hesperidin (10 mg/kg); d OVA-challenged mice treated with hesperidin (30 mg/kg). The left panel is magnified 100×; the right panel is magnified 400×. Br bronchi, V vessel. Arrows indicate areas of alcian blue + cells.
Hesperidin Decreases Airway Hyperresponsiveness in Ova-induced Murine Asthma
Airway responsiveness was assessed as the percent alteration of RI and Cdyn in response to increasing doses of methacholine. RI is defined as the pressure driving respiration divided by flow. Cdyn refers to the distensibility of the lung and is defined as the change in volume of the lung produced by a change in pressure across the lung. OVA-challenged mice developed AHR, typically reflected by high RI and low Cdyn (Fig. 6). Hesperidin treatment (10 and 30 mg/kg) dramatically reduced RI and restored Cdyn in OVA-challenged mice in response to methacholine.
Effect of hesperidin on airway hyperresponsiveness in mice. Airway hyperresponsiveness was assessed by percentage change from the baseline level of (a) lung resistance (RI, n = 6 mice per treatment group) and (b) dynamic compliance (Cdyn, n = 6 mice per treatment group). Mch methacholine. *p < 0.05, **p < 0.01. vs. OVA.
DISCUSSION
Natural products are increasingly becoming important sources of pharmacotherapeutics, either as herbal drugs for treatment of chronic diseases or as raw materials form which compounds with particular biological activities are isolated. Hesperidin, which is the most prevalent flavanone glycoside found in sweet orange and lemon, obtained as an abundant by-product of citrus cultivation, has anti-inflammatory effects [25, 26]. Nevertheless, the effect of hesperidin on airway inflammation of asthma remains largely unknown. This study is the first to provide experimental evidence demonstrating that hesperidin inhibits OVA-induced airway inflammation in a murine model of asthma. The administration of hesperidin significantly inhibited asthmatic reactions such as leukocyte recruitment to the lungs, AHR, OVA-specific IgE levels in serum, and the production of Th2 cytokines. In addition, hesperidin alleviated inflammatory cell infiltration in lung tissue and goblet cell hyperplasia in the airway.
Based on previous studies, the immunological processes involved in airway inflammation of asthma are characterized by the proliferation and activation of Th2 cytokines, such as IL-4, IL-5, and IL-13. IL-4 causes B cells to synthesize IgE, which is involved in mast cell degranulation by cross-linking IgE receptors [27]. IL-5 enables the terminal differentiation and proliferation of eosinophil precursors and the eosinophil activation effect [28]. IL-13 also contributes to symptoms of asthma such as AHR, airway inflammation, goblet cell hyperplasia, airway luminal narrowing, and subepithelial fibrosis [29]. In this study, we demonstrated that hesperidin significantly suppressed the levels of IL-4, IL-5, IL-13, and OVA-specific IgE levels in serum in a dose-dependent manner. Hesperidin also significantly reduced the numbers of eosinophils in BALF (Fig. 4). Many studies have reported that the decrease of eosinophil numbers in BALF correlates with the attenuation of asthma symptoms in animal models [30]. We speculate that hesperidin suppresses the numbers of eosinophils, neutrophils, and macrophages in BALF through the suppression of these cytokines.
Moreover, it is believed that inflammatory mediators released during allergic inflammation play a vital role in causing asthma symptoms, such as goblet cell hyperplasia, airway luminal narrowing, AHR, and subepithelial fibrosis [31–34]. In our study, we found that hesperidin markedly inhibited OVA-induced AHR in response to increasing concentrations of methacholine (Fig. 6), the degree of inflammatory cell infiltration in the peribronchial and perivascular areas, and goblet cell hyperplasia (Fig. 5). Consequently, it is likely that anti-inflammatory therapy using hesperidin improves both airway inflammation and AHR. Therefore, we conclude that the effect of hesperidin on AHR, airway inflammation, and goblet cell hyperplasia may be attributed to the inhibition of Th2 cytokine production, eosinophilia, and serum IgE levels.
In conclusion, we have demonstrated that hesperidin alleviated AHR, reduced the number of inflammatory cells, and enhanced Th2 cytokines levels in BALF and OVA-specific IgE levels in serum. Furthermore, goblet cell hyperplasia and airway luminal narrowing were also attenuated. These results suggest that hesperidin exhibits inhibitory activities not only for allergen-induced AHR or airway eosinophilic inflammation but also for airway remodeling, probably due to the downregulation of allergen sensitization and/or Th2 polarizing pathways. Our results suggest that hesperidin might offer a new therapeutic approach to allergic airway diseases.
References
Bochner, B.S., and W.W. Busse. 2004. Advances in mechanisms of allergy. The Journal of Allergy and Clinical Immunology 113: 868–875.
Bochner, B.S., and W.W. Busse. 2005. Allergy and asthma. The Journal of Allergy and Clinical Immunology 115: 953–959.
Busse, W.W., and R.F. Lemanske Jr. 2001. Asthma. The New England Journal of Medicine 344: 350–362.
Busse, W.W. 1993. Calhoun William WF, Sedgwick JD. Mechanism of airway inflammation in asthma. The American Review of Respiratory Disease 147: S20–S24.
Ngoc, P.L., D.R. Gold, A.O. Tzianabos, S.T. Weiss, and J.C. Celedon. 2005. Cytokines, allergy, and asthma. Current Opinion in Allergy and Clinical Immunology 5: 161–166.
Zhu, Z., R.J. Homer, Z. Wang, Q. Chen, G.P. Geba, J. Wang, et al. 1999. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. Journal of Clinical Investigation 103: 779–788.
Zavorotinskaya, T., A. Tomkinson, and J.E. Murphy. 2003. Treatment of experimental asthma by long-term gene therapy directed against IL-4 and IL-13. Molecular Therapy 7: 155–162.
Takatsu, K., and H. Nakajima. 2008. IL-5 and eosinophilia. Current Opinion in Immunology 20: 288–294.
Busse, W., J. Corren, B.Q. Lanier, M. McAlary, A. Fowler-Taylor, G.D. Cioppa, et al. 2001. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. The Journal of Allergy and Clinical Immunology 108: 184–190.
Djukanovic, R., S.J. Wilson, M. Kraft, N.N. Jarjour, M. Steel, K.F. Chung, et al. 2004. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. American Journal of Respiratory and Critical Care Medicine 170: 583–593.
Bousquet, J., P. Cabrera, N. Berkman, R. Buhl, S. Holgate, S. Wenzel, et al. 2005. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 60: 302–308.
Murakami, A., Y. Nakamura, K. Torikai, T. Tanaka, T. Koshiba, K. Koshimizu, et al. 2000. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Research 60: 5059–5066.
Sato, T., L. Koike, Y. Miyata, M. Hirata, Y. Mimaki, Y. Sashida, et al. 2002. Inhibition of activated protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinase-1 production and suppression of production of matrix metalloproteinases 1 and 9 in human fibrosarcoma HT-1080 cells. Cancer Research 62: 1025–1029.
Miyata, Y., T. Sato, M. Yano, and A. Ito. 2004. Activation of protein kinase CβII/ε-c-Jun NH2-terminal kinase pathway and inhibition of mitogen-activated protein/extracellular signal-regulated kinase1/2 phosphorylation in antitumor invasive activity induced by the polymethoxy flavonoid, nobiletin. Molecular Cancer Therapeutics 3: 839–847.
Barthe, G.A., P.S. Jourdan, C.A. McIntosh, and R.L. Mansell. 1988. Radioimmunoassay for the quantitative determination of hesperidin and analysis of its distribution in Citrus sinensis. Phytochemistry 27: 249–254.
Garg, A., S. Garg, L.J.D. Zaneveld, and A.K. Singla. 2001. Review article: chemistry and pharmacology of the citrus bioflavonoid hesperedin. Phytotherapy Research 15: 655–669.
Bok, S.H., S.H. Lee, Y.B. Park, K.H. Bae, K.H. Son, T.S. Jeong, et al. 1999. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferases are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. The Journal of Nutrition 129: 1182–1185.
Hirata, A., Y. Murakami, M. Shoji, Y. Kadoma, and S. Fujisawa. 2005. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Research 25: 3367–3374.
Yeh, M.H., S.T. Kao, C.M. Hung, et al. 2009. Hesperidin inhibited acetaldehyde-induced matrix metalloproteinase-9 gene expression in human hepatocellular carcinoma cells. Toxicology Letters 184: 204–210.
Park, S.H., E.K. Park, and D.H. Kim. 2005. Passive cutaneous anaphylaxis-inhibitory activity of flavanones from Citrus unshiu and Poncirus trifoliata. Planta Medica 71: 24–27.
Middleton, R., and G. Drzewiecki. 1984. Flavonoid inhibition of human basophil histamine release stimulated by various agents. Biochemical Pharmacology 33: 3333–3338.
Kim, H.P., K.H. Son, H.W. Chang, and S.S. Kang. 2004. Anti-inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Sciences 96: 229–245.
Yeh, C.C., S.J. Kao, C.C. Lin, et al. 2007. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Science 80: 1821–1831.
Kawaguchi, K., S. Kikuchi, R. Hasunuma, et al. 2004. A citrus flavonoid hesperidin suppresses infection-induced endotoxin shock in mice. Biological & Pharmaceutical Bulletin 27(5): 679–683.
Kim, J.Y., Y.P. Hwang, D.H. Kim, et al. 2006. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Bioscience, Biotechnology, and Biochemistry 70: 858–864.
Ahn, K.S., E.J. Noh, H.L. Zhao, et al. 2005. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappa B activation in RAW 264.7 cells. Life Science 76: 2315–2328.
Fish, S.C., D.D. Donaldson, S.J. Goldman, et al. 2005. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. Journal of Immunology 174: 7716–7724.
Kouro, T., and K. Takatsu. 2009. International Immunology 21: 1303–1309.
Patricia, C., and C. Fulkerson. 2006. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. The American Journal of Pathology 169(6): 2117–2126.
Wakahara, K., H. Tanaka, G. Takahashi, et al. 2008. Repeated instillations of Dermatophagoides farinae into the airways can induce Th2-dependent airway hyperresponsiveness, eosinophilia and remodeling in mice: effect of intratracheal treatment of fluticasone propionate. European Journal of Pharmacology 578: 87–96.
Cockcroft, D.W., and B.E. Davis. 2006. Mechanisms of airway hyperresponsiveness. The Journal of Allergy and Clinical Immunology 118: 551–559.
Hart, L.A., V.L. Krishnan, I.M. Adcock, P.J. Barnes, and K.F. Chung. 1998. Activation and localization of transcription factor, nuclear factor-κB, in asthma. American Journal of Respiratory and Critical Care Medicine 158: 1585–1592.
Vargaftig, B.B., and M. Singer. 2003. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. American Journal of Respiratory Cell and Molecular Biology 28: 410–419.
Leigh, R., R. Ellis, J.N. Wattie, J.A. Hirota, K.I. Matthaei, P.S. Foster, P.M. O’Byrne, and M.D. Inman. 2004. Type 2 cytokines in the pathogenesis of sustained airway dysfunction and airway remodeling in mice. American Journal of Respiratory and Critical Care Medicine 169: 860–867.
Acknowledgments
This work was supported by the National Science and Technology Supporting Plan of China (No. 2006BAD31B03-4).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dajun Wei and Xinxin Ci contribute equally to this work.
Rights and permissions
About this article
Cite this article
Wei, D., Ci, X., Chu, X. et al. Hesperidin Suppresses Ovalbumin-Induced Airway Inflammation in a Mouse Allergic Asthma Model. Inflammation 35, 114–121 (2012). https://doi.org/10.1007/s10753-011-9295-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9295-7