Abstract
Purpose
Obesity is the most important risk factor for obstructive sleep apnea (OSA); however, the exact underlying mechanisms are still not fully understood. The aim of this study was to examine the morphology of upper airways in overweight habitual snorers and in mild OSA patients. Furthermore, the associations between weight loss, parapharyngeal fat pad area and OSA were assessed in a 1-year randomised, controlled follow-up study originally conducted to determine the effects of lifestyle changes with weight reduction as a treatment of OSA.
Methods
Thirty-six overweight adult patients with mild OSA [apnea–hypopnea index (AHI) 5–15 events/h] and 24 weight-matched habitual snorers (AHI < 5 events/h) were included in the study. All patients underwent nocturnal cardiorespiratory recordings and multislice computed tomography (CT) of parapharyngeal fat pad area, the smallest diameter and area in naso-, oro- and hypopharynx, the smallest diameter and area of the whole pharyngeal airway, the distance from the hyoid bone to the mandibular plane and to cervical tangent as well as the distance between mandibular symphysis and cervical spine. In addition, OSA patients were further randomised to receive either an active 1-year lifestyle intervention with an early weight loss programme or routine lifestyle counselling. After 1 year, the cardiorespiratory recordings and CT scans were repeated.
Results
The pharyngeal fat pad area was significantly larger, and the distance from the hyoid bone to cervical spine was longer in patients with OSA than in habitual snorers (p = 0.002 and p = 0.018, respectively). The multiple regression analysis showed that besides a large pharyngeal fat pad area and a long distance from the cervical spine to hyoid bone, also a short distance from the mandibular symphysis to cervical tangent increased a risk to OSA. During the 1-year follow-up in OSA patients, the pharyngeal fat pad area and AHI decreased significantly in the intervention group (p = 0.003 and p < 0.001, respectively).
Conclusions
In the early stages of OSA, the pharyngeal fat pad seems to play an important role in the development of disease in overweight patients. Furthermore, weight reduction by lifestyle intervention-based programme reduces both central obesity and pharyngeal fat pads, resulting in an improvement of OSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterised by snoring and obstruction of upper airways during sleep, leading to deterioration of an individual's quality of life and working capacity. The prevalence of OSA has been estimated to be as high as 24 % in men and 9 % in women aged 30–60 years [1]. Obesity is also the most important risk factor for OSA, and in fact, most OSA patients are obese [2]. Furthermore, there are several reports that OSA deteriorates with weight gain and improves with weight reduction [3–6]. The pathogenesis leading to upper airway collapse is believed to result from a combination of predisposing anatomical factors and impaired neuromuscular compensatory responses. However, the exact underlying mechanisms are not yet fully understood. It has been postulated that the collapsibility of the upper airways may depend on the localisation of excess adipose tissue; e.g. increased fat tissue in the neck may have additional effects on pharyngeal neural and mechanical control mechanisms that mediate collapsibility, and thus, this tissue can increase OSA susceptibility [7, 8]. However, the size of parapharyngeal fat pad has not shown any association with the severity of OSA in previous clinical studies [9], whereas particularly visceral fat has been linked to adverse health consequences, including the risk of developing or worsening of OSA [10, 11].
Sleep-disordered breathing (SDB) represents a continuum of symptoms ranging from habitual snoring to obstructive sleep apnea syndrome. The disease has a tendency to worsen so that an originally mild OSA may progress to moderate or severe OSA, particularly if associated with weight gain [12]. Although, some of the risk factors for OSA have been well documented, the underlying mechanisms are still, at least partly, poorly known. There is a clear need for future studies identifying anatomic and soft tissue parameters that influence mechanical loading of the upper airway. In order to improve the understanding of the pathophysiology and development of OSA, it is important to evaluate the contributing risk factors to OSA in a homogenous population. The aim of the present study was to evaluate the morphology of upper airways by computed tomography (CT) in overweight habitual snorers and mild OSA patients. Moreover, the associations between weight loss, parapharyngeal fat pad area and OSA were assessed in a 1-year randomised, controlled follow-up study originally conducted to determine the effects of lifestyle intervention with a weight reduction programme in the most prevalent subgroup of OSA, i.e. overweight patients with mild OSA [6].
Subjects and methods
Study design
The present study comprises of two analyses: (1) cross-sectional comparison of CT scans of the upper airways between patients with mild OSA (AHI 5–15 events/h) and body mass index (BMI)-matched habitual snorers (AHI < 5) and (2) 1-year randomised, controlled trial on the effect of weight loss on the morphology of the upper airways in patients with mild OSA. In this follow-up study, patients were allocated into either an intensive lifestyle intervention group or a control group. The patients in the intervention group received a 1-year lifestyle intervention including an initial weight reduction programme with 12 weeks on a very low-calorie diet. In addition to the dietary counselling, the subjects in the intervention group were recommended to increase their overall level of daily physical activity. The control group consisted of subjects receiving a single general dietary and exercise counselling. The detailed design of the study has been previously reported [6]. The study was conducted in a single centre, Kuopio University Hospital, Finland. All patients were given verbal and written information about the trial, and they provided consent. The study protocol was approved by the Research Ethics Committee of the Hospital District of Northern Savo (Kuopio, Finland).
Participants
At the study site, a trained nurse measured height, weight and the BMI which was calculated as weight (in kilogram) divided by height squared (in square meter). The OSA group consisted of 36 patients (24 males and 12 females), and the habitual snorers were of 24 individuals (10 males and 14 females). The characteristics of the study population are presented in Table 1. Table 2 shows the characteristics of the OSA patients randomised to either intervention study group (22 patients; 17 males and 5 females) or control group (14 patients; 7 males and 7 females). The upper airways were clinically evaluated by an otorhinolaryngologist, and none of the participants had any significant adenotonsillar hypertrophy. The cardiorespiratory recordings and CT scans were performed at baseline and 1-year visit.
Procedures and measurements
In nocturnal cardiorespiratory monitoring, apnea was defined as a cessation (more than 90 %) of airflow of more than 10 s. Hypopnea was defined as a reduction (more than 30 %) of airflow of more than 10 s with oxygen desaturation of more than 4 %. The apnea–hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour, and mild OSA was defined with AHI of 5–15 events/h [13].
Computed tomography
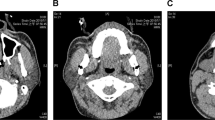
Multislice CT imaging was performed with Siemens Somatom 16 CT (Erlangen, Germany). The imaging was done in a standardised method, while patient was positioned supine on the scanning table. A three-dimensional (3-D) volume was obtained from a sphenoid air cell down to the cricoid cartilage level with the neck placed in a neutral position; the patients were asked to breathe quietly. The scan time was 4–5.4 s, depending on the patient's size. Imaging parameters were 100 kV and 240 mAs, and a dose reduction algorithm and both eye and thyroid gland radiation shields were used. From the 3-D volume, 2-mm-thick axial, sagittal and coronal slices were reformatted perpendicular to the pharyngeal airway. All images were viewed using standardised window settings. All the measurements were done using standard workstation software. The repeated CT study was performed 12 months later, and the parameters were measured similar to the baseline CT.
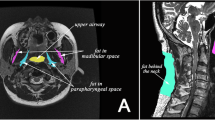
The parapharyngeal fat pad area was measured on the right at its maximum which, in most cases, was 5 mm below the hard palate. The measurement was conducted on the axial slices perpendicular to the pharyngeal airway, and the repeated 12-month fat pad area evaluation was performed at the same level (Fig. 1).
The densities of the right medial pterygoid and sternocleidomastoid muscle were measured in Hounsfield units (HU) using the free region of interest. In order to avoid beam hardening artefacts, the HU measurement of the pterygoid muscle was conducted at the upper third level of the tongue, and sternocleidomastoid muscle density was measured above the hyoid bone level.
The cross-sectional airway slices were captured as follows: the smallest diameter in nasopharynx (in millimetre) and area (in square millimetre) (PW1), the smallest diameter in oropharynx and area (PW2) and the smallest diameter in hypopharynx and area (PW3). The airway region boundaries were determined as follows: the nasopharynx extends from the base of the skull to the upper surface of the soft palate, and the oropharynx reaches from the level of hard and soft palate to the upper edge of the epiglottis (vallecula), while the superior boundary of the hypopharynx is at the level of vallecula with the inferior border being the lower level of the cricoid cartilage. Furthermore, the smallest diameter (in millimetre) of the whole pharyngeal airway was captured both anteroposteriorly and transversally as well as the minimal area (in square millimetre).
The position of the hyoid bone was determined with respect to its distance (in millimetre) to mandibular plane (a plane constructed from the menton to the gonion) (MPH) and to cervical tangent (a line across C3 and C4) (HVL). In addition, the distance (in millimetre) was measured between the posterior point of the mandibular symphysis and cervical tangent (GVL). Furthermore, the length and height of the tongue (TGL and TGH) and the soft palate thickness (in millimetre) and area (in square millimetre) were evaluated. These measurements were done on a midsagittal CT slices. The level of mandibular angulus was determined by multiplanar reconstruction and localization tool softwares.
Statistical analysis
Mean values and standard deviations were used to describe the baseline characteristics of the study groups. Student's t test was used in the comparison between the groups. Logistic regression analysis with a backward selection set-up procedure was used to evaluate the association between the group (0 = habitual snorers, 1 = OSA patients) and following craniofacial and oropharyngeal parameters (independent factors) MPH (in millimetre), HVL (in millimetre), GVL (in millimetre), the smallest diameter (in millimetre) of the whole pharyngeal airway both anteroposteriorly and transversally, pharyngeal fat pad area (in square millimetre) and soft palate area (in square millimetre). The model was adjusted for age and gender. The significance of changes in BMI, AHI, waist circumference and fat pad area in 12 months was analysed by using paired t test. The p values less than 0.05 were considered as statistically significant. All analyses were performed with the SPSS software 17.0 package (SPSS, Chicago, IL).
Results
Cross-sectional evaluation
Upper airway measurements in OSA patients and habitual snorers
The patients were stratified according to weight, and thus, there was no significant difference in BMI between the mild OSA patients and the habitual snorers. However, habitual snorers were on average 8 years younger than their OSA counterparts (Table 1).
There was no difference in mean upper airway dimensions between the groups neither in the volume of the tongue or soft palate. Only the distance from the hyoid to cervical (C3–C4) tangent was significantly longer in OSA patients compared to snorers (Table 3). However, the pharyngeal fat pad area was significantly larger in the OSA group than in the snorers (p = 0.002) (Table 3).
The multiple logistic regression analysis revealed that in overweight subjects with OSA, the pharyngeal fat pad area was larger, the distance between the posterior points of the symphysis to the cervical tangent was shorter, but the distance between the hyoid bone and the cervical tangent was longer than the corresponding parameter in the BMI-matched habitual snorers (Table 4).
Randomised, controlled 1-year follow-up
Association between OSA and weight reduction
The baseline demographics in OSA patients participating in the randomised study did not differ between the intervention and control groups (Table 2). At the follow-up, there were significant decreases in the BMI, waist circumference and pharyngeal fat pad area. Furthermore, a significant reduction in the AHI was found (Table 5). There were also some improvements observed in the control group.
Discussion
The present study provides evidence that there is more adipose tissue in the pharynx in OSA patients compared to the corresponding situation in habitual snorers. Furthermore, in the logistic regression model, besides the retrusive mandible and the low hyoid bone position, the pharyngeal fat pad area was found to be a significant risk factor for OSA. These results support the belief of the nature of OSA as a chronic, progressive disease and the harmful impact of obesity, even weight gain in this process. In the 1-year follow-up of a randomised, controlled trial in overweight patients with mild OSA, supervised lifestyle intervention with weight loss resulted in significant reductions in both central obesity and the amount of adipose tissue in the pharynx. In conjunction with this loss of the overall fat tissue, also a marked improvement of AHI was detected. Previous studies have revealed inconsistent effects of pharyngeal fat on upper airway [14, 15]. A recent study investigated the effect of weight loss on the volume of upper airways in obese men [9]. The main conclusion of the study was that weight loss improves AHI by especially reducing the airway length. Also in the study of Segal et al. [16], upper airway length is suggested to be of greater importance in the pathophysiology of OSAs. Unfortunately, airway length of the patients was not assessed in this study, but our present findings in a homogenous group of patients highlight the significance of oropharyngeal fat in narrowing of the airway and emphasise the beneficial effects of weight loss in reducing the excessive fat tissue in overweight patients with OSA.
The pathophysiology of OSA is complex and most likely multifactorial, consisting of a combination of predisposing anatomical factors (both soft and hard tissues) and impaired neuromuscular compensatory mechanisms [2]. Due to these factors, at some level, the airspace of the nasopharynx and oropharynx may become narrowed, thus increasing the risk of OSA in the sleeping position and the loss of neuromuscular compensation at the onset of sleep. Previously, it has been demonstrated that in patients with severe OSA, the retrolingual airspace or narrow retropalatal area may be the major sites of obstruction [17, 18]. Furthermore, it has been shown that the minimum upper airway area occurs at the end of expiration and enlarges during inspiration [19–21]. In many studies, hypopharyngeal airspace has been found to be larger in the OSA group compared to controls [17, 22, 23]. A gradual increase has been detected in the mean cross-sectional areas at the level of hypopharynx with the lowest values in the control group and increasing in patients with severe OSA [17]. The present results are in parallel to these findings since the distance from the hyoid bone to the cervical tangent (HVL) was significantly larger in OSA patients than that of habitual snorers. This measurement most likely reflects lower hyoid bone position (or perhaps low tongue position) which extends to a larger hypopharyngeal airway, but as found in the present study, the actual hypopharyngeal airway measurements did not differ between the groups.
It has also been proposed that the airway collapse during sleep is favoured by a narrow velopharynx associated with large hypopharynx [22]. Especially long soft palate in proportion to oropharyngeal airway length is suggested to increase the risk for OSA [24]. In the present study, no difference between the groups was found in the airway space at the velopharyngeal area, neither in soft palate thickness or area. However, it has been speculated that some heavy snorers may not suffer oropharyngeal collapse because the peak inspiratory suction pressure could already have declined at the level of the relatively narrow hypopharyngeal airways [22].
In previous studies, especially mandibular deficiency has been found to be a significant predictor for SDB [25, 26]. A study in obese and non-obese patients with severe OSA demonstrated that irrespective of the BMI, the patients display aberrations of cervico-craniofacial and upper airway soft tissue morphology when compared with the controls [27]. However, when studying a large population of snorers with or without OSA, it was concluded that only in lean and young subjects, the upper airway abnormalities explain a major part of the variance in AHI and are likely to play an important physiopathogenic role [28]. In line with these findings, in our previous study, we observed that morphological deviations were related to mild SDB among non-obese patients, but not in obese patients [29]. In the present study, the multiple regression analysis showed that the small distance of the posterior point of the mandibular symphysis and the cervical line was associated with OSA. This indicates that in OSA patients, the mandible is more retrusive or more posteriorly rotated than in those with habitual snoring. In contrast to previous studies, we examined a homogenous study population, i.e. middle-aged, overweight patients with mild OSA.
The impact of mechanical factors on pharyngeal patency in patients with OSA is still poorly understood. Some of the anatomical predisposing factors for OSA, such as large tonsils, prominent uvula, enlarged tongue, mandibular retrognathia and nasal deformities, have been well documented [2, 30]. However, the single most important risk factor for OSA is obesity, and it has been estimated that at least two out of every three patients with OSA are obese [31]. The question arises: How does obesity contribute to airway collapse during sleep? It has been proposed that obesity can act in many different ways, e.g. narrowing of the upper airway structure [14], alterations in function (such as collapsibility) [32], reduced chest wall compliance, disturbances in the relationship between respiratory drive and load compensation [2, 33] as well as causing reductions in functional residual capacity and hypoxemia [34]. Clinical studies have demonstrated that mandibular advancement can enlarge the bony enclosure and lower pharyngeal collapsibility in lean, but not obese individuals [8]. The failure to reduce passive pharyngeal collapsibility in obese subjects may be due to the enlarged peripharyngeal fat pads, which may evoke a collapse of lateral pharyngeal structures. In fact, a recent study examining the mechanical parameters determining pharyngeal collapsibility found that collapsibility was caused primarily by the surrounding pressure [7]. Furthermore, recent studies have emphasised the importance of obesity on airway shape and pharyngeal function, rather than the pharyngeal structure alone [31, 35]. In addition to the localisation of excess adipose tissue, there is evidence that systemic inflammatory mediators related to obesity may have additional effects also on the pharyngeal neural and mechanical control mechanisms that mediate collapsibility and increase OSA susceptibility [8]. Previously, we have shown that even mild OSA can be associated with an activation of pro-inflammatory pathways, and furthermore, in conjunction with weight loss, the most sensitive marker of the pro-inflammatory state, IL-1 Ra, decreases in patients with mild OSA [36]. It has been suggested that in patients with OSA, less than 50 % of the overall response to weight loss may be attributable to reductions in passive mechanical properties with the remainder related to concomitant improvements in neuromuscular control of upper airways [8]. This could well explain the stability of the prolonged improvements in respiration during sleep encountered in the 2-year follow-up of a lifestyle intervention study, which demonstrated that although there had been a typical regaining of the weight loss, the improvement in nocturnal respiratory function was maintained and did not follow the trend of modest weight gain [37]. Therefore, it seems that both local, i.e. excessive pharyngeal adipose tissue and obesity, particularly central obesity, and systemic inflammations may increase OSA susceptibility.
The present study has some limitations, and the intervention was conducted in patients with mild OSA; therefore, the findings may not be directly generalisable to all OSA patients. Although these data are encouraging, they need to be replicated in a larger study. Due to the relatively small sample sizes, our study was not originally designed to compare the individual effects of different factors in determining pharyngeal collapsibility and, thus, on the pathogenesis of OSA. In the present study, OSA patients were on average 8 years older than those with habitual snoring. It has been shown that weight gain is a risk factor for snoring in all age groups, while smoking is mainly associated with snoring in men less than 60 years of age [38]. The natural history of OSA is that the disease tends to worsen, mostly due to weight gain, and therefore, overweight individuals with mild OSA may be considered to be at high risk for suffering from progression of the disease. How all these are related to the different fat deposits of body needs large, long-term studies. Furthermore, although the standard for diagnosing OSA has been based on in-laboratory polysomnography, the cardiorespiratory devices used in the present study are considered as reliable for diagnosing and monitoring the response to non-CPAP treatments for OSA with some limitations [39].
The present study demonstrates that the volume of parapharyngeal fat area may play an important role both in the pathophysiology and the evolution of OSA in overweight patients. In addition to an improvement of central obesity and a reduction in the chronic systemic inflammation, the beneficial effect of weight loss in overweight patients with mild OSA may partly result from a reduction in the pharyngeal fat pad.
References
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnoea. A population health perspective. Am J Respir Crit Care Med 165:1217–1239
Young T, Skatrud J, Peppard PE (2004) Risk factors for obstructive sleep apnoea in adults. JAMA 291:2013–2016
Fisher D, Pillar G, Malhotra A, Peled N, Lavie P (2002) Long-term follow-up of untreated patients with sleep apnoea syndrome. Respir Med 96:337–343
Dávila-Cervantes A, Dominiguez-Cherit G, Borounda D, Gamino R, Vargas-Vorackova F, Gonzáles-Barranco J, Herrera MF (2004) Impact of surgically-induced weight loss on respiratory function: a prospective analysis. Obes Surg 14:1389–1392
Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P (2004) A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med 5:125–231
Tuomilehto HP, Seppä JM, Partinen MM, Peltonen M, Gylling H, Tuomilehto JO, Vanninen EJ, Kokkarinen J, Sahlman JK, Martikainen T, Soini EJ, Randell J, Tukiainen H, Uusitupa M; Kuopio Sleep Apnea Group (2009) Lifestyle intervention with weight reduction. First-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 179:320–327
Oliven A, Kaufman E, Kaynan R, Oliven R, Steinfeld U, Tov N, Odeh M, Gaitini L, Schwarz AR, Kimmel E (2010) Mechanical parameters determining pharyngeal collapsibility in patients with sleep apnea. J Appl Physiol 109:1037–1044
Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL (2010) Obesity and upper airway control during sleep. J Appl Physiol 108:430–435
Sutherland K, Lee RWW, Philips CL, Dungan G, Yee BJ, Magnussen JS, Grunstein RR, Cistulli PA (2011) Effect of weight loss on upper airway size and facial fat in men with obstructive sleep apnea. Thorax 66:797–803
Vgontzas AN (2008) Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem 114:211–223
Bonsignore MR, McNicholas WT, Montserrat JM, Eckel J (2012) Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J 39:746–767
Berger G, Berger R, Oksenberg A (2009) Progression of snoring and obstructive sleep apnoea: the role of increasing weight and time. Eur Respir J 33:338–345
American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689
Horner RL, Shea A, Mclvor J, Guz A (1989) Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. Q J Med S 72:719–735
Schwab RJ, Gupta KB, Gefler WB, Metzger LJ, Hoffman EA, Pack AI (1995) Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 152:1673–1689
Segal Y, Malhotra A, Pillar G (2008) Upper airway length may be associated with severity of obstructive sleep apnea syndrome. Sleep Breath 12:311–316
Yucel A, Unlu M, Haktanir A, Acar M, Fidan F (2005) Evaluation of the upper airway cross-sectional area changes in different degrees of severity of obstructive sleep apnea syndrome: cephalometric and dynamic CT study. Am J Neuroradiol 26:2624–2629
Barkdull GC, Kohl CA, Patel M, Davidson TM (2008) Computed tomography imaging of patients with obstructive sleep apnea. Laryngoscope 118:1486–1492
Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI (1993) Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis 148:1385–1400
Morrell MJ, Arabi Y, Zahn B, Badr S (1998) Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med 158:1974–1981
Bhattacharyya N, Blake SP, Fried MP (2000) Assessment of the airway in obstructive sleep apnea syndrome with 3-dimensional airway computed tomography. Otolaryngol Hear Neck Surg 123:444–449
Polo OJ, Tafti M, Fraga J, Porkka KVK, Dejean Y, Billiard M (1991) Why don't all heavy snorers have obstructive sleep apnea? Am Rev Respir Dis 143:1288–1293
Caballero P, Alvarez-Sala R, Garcia-Rio F, Prados C, Hernan MA, Villamor J, Alvarez-Sala JL (1998) CT in the evaluation of the upper airway in healthy subjects and in patients with obstructive sleep apnea syndrome. Chest 113:111–116
Shigeta Y, Ogawa T, Tomoko I, Clark GT, Enciso R (2010) Soft palate length and upper airway relationship in OSA and non-OSA subjects. Sleep Breath 14:353–358
Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA (1995) The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest 108:375–381
Hou HM, Hägg U, Sam K, Rabie ABM, Wong RWK, Lam B, Ip MS (2006) Dentofacial characteristics of Chinese obstructive sleep apnea patients in relation to obesity and severity. Angle Orthod 76:962–969
Tangugsorn V, Krogstad O, Espeland L, Lyberg T (2000) Obstructive sleep apnoea: multiple comparisons of cephalometric variables of obese and non-obese patients. J Craniomaxillofac Surg 28:204–212
Mayer P, Pepin JL, Veale D, Ferretti G, Daschaux C, Levy P (1996) Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur Respir J 9:1801–1809
Pahkala R, Puustinen R, Tuomilehto H, Ahlberg J, Seppä J (2011) Risk factors for sleep-disordered breathing: the role of craniofacial structure. Acta Odontol Scand 69:137–143
Chi L, Comyn FL, Mitra N, Reilly MP, Wan F, Maislin G, Chmiewski L, Thorne-FitzGerald MD, Victor UN, Pack AI, Schwab RJ (2011) Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J 38:348–358
Pillar G, Shehadeh N (2008) Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care 31:303–309
Schwarz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL (1991) Effects of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 144:494–498
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study on the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
Hlavac MC, Catcheside PG, McDonald R, Eckert DJ, Windler S, McEvoy RD (2006) Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep 29:624–631
Tsai HH, Ho CY, Lee PL, Tan CT (2009) Sex differences in anthropometric and cephalometric characteristics in severity of obstructive sleep apnea syndrome. Am J Orthod Dentofacial Orthop 135:155–164
Sahlman J, Seppä J, Herder C, Peltonen M, Peuhkurinen K, Gylling H, Vanninen E, Tukiainen H, Punnonen K, Partinen M, Uusitupa M, Tuomilehto H (2012) Effect of weight loss on inflammation in patients with mild obstructive sleep apnea. Nutr Metab Cardiovasc Dis 22:583–590
Tuomilehto H, Gylling H, Peltonen M, Martikainen T, Sahlman J, Kokkarinen J, Randel J, Tukiainen H, Vanninen E, Partinen M, Tuomilehto H, Uusitupa M, Seppä J; Kuopio Sleep Apnea Group (2010) Sustained improvement in mild obstructive sleep apnea by diet- and physical activity-based lifestyle intervention: postinterventional follow-up. Am J Clin Nutr 92:688–696
Lindberg E, Taube A, Janson C, Gislason T, Svärdsudd K, Boman G (1998) 10-year follow-up of snoring in men. Chest 144:1048–1055
Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R (2007) Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. The diagnosis of obstructive sleep apnea in adult patients. Portable monitoring task force of the American Academy of Sleep Medicine. J Clin Sleep Med 3:737–747
Acknowledgments
We express our special thanks to Taina Poutiainen, a specially trained nurse, and Riitta Myllykangas, a dental hygienist, for their contributions to this study. In addition, warm thanks go to Veli-Matti Vartiainen, oral radiologist, and Anna Sutela, neuroradiologist, of the Department of Clinical Radiology, Kuopio University Hospital and University of Eastern Finland, for the demonstrative MRI figure. The members of Kuopio Sleep Apnea Group Matti Pukkila, Tatu Kemppainen, Tomi Laitinen, Tiina Lyyra-Laitinen, Ritva Vanninen, Heimo Viinamäki, Keijo Peuhkurinen, Kari Punnonen, Kati Venäläinen, Erkki Soini and Janne Martikainen are also cordially acknowledged. This study was supported by grants from the Hospital District of Northern Savo, Kuopio University Hospital, the Finnish Cultural Foundation and the Pulmonary Association Heli.
Conflict of interest
We declare that we have no conflicts of interest.
Role of funding sources
The funding sources had no role in the study design or in the collection, analysis or interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pahkala, R., Seppä, J., Ikonen, A. et al. The impact of pharyngeal fat tissue on the pathogenesis of obstructive sleep apnea. Sleep Breath 18, 275–282 (2014). https://doi.org/10.1007/s11325-013-0878-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-013-0878-4