Abstract
This study was conducted to investigate the presence of the accessory maxillary ostium and its effects on the maxillary sinus, and the concurrent occurrence of morphological variations of neighboring anatomical structures. This study was performed in a tertiary referral center. This is a cross-sectional retrospective study that evaluated coronal CTs of patients to determine the frequency of the accessory maxillary ostium and investigated any simultaneous morphological variations in neighboring anatomical structures. The presence of the accessory maxillary ostium (AMO) plus any concurrent morphological variations of neighboring structures were investigated in 377 patients, with 754 sides. AMO was found to be present in 19.1 % (72/377) of the patients. A concurrent mucus retention cyst was found to be statistically significant on both sides (right side: p = 0.00, left side: p = 0.00), as well as mucosal thickening (right side: p = 0.00, left side: p = 0.00), and maxillary sinusitis (right side: p = 0.04, left side: p = 0.03). No other concurrent variations of statistical significance were detected in the neighboring structures. Our study demonstrated that with the presence of AMO, the likelihood of encountering a mucus retention cyst (48.6 %) had an approximately threefold increase, and that of encountering mucosal thickening (43.0 %) and maxillary sinusitis (29.1 %) had a twofold increase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural ostium of the maxillary sinus is located anteriorly, has an oval form which extends transversely, and is not visible during routine nasal endoscopic examination. The maxillary sinus commonly opens to the anterior part of the posterior fontanelle, on the inferior part of the ethmoid infundibulum through its superomedially oriented ostium [1]. The accessory maxillary ostium is one of the anatomic variations which may have a role in the development of chronic maxillary sinusitis. Although some researchers argue that the accessory ostium develops after acute maxillary sinusitis, it is still not known whether AMO is a congenital or an acquired structure [2].
AMO is usually located on the posterior fontanelle of the lateral nasal wall, and should not be confused with the maxillary hiatus. The active mucociliary transport in the maxillary sinus is directed toward the natural ostium, and even when the natural ostium is blocked, AMO does not have a role in the physiological transport inside the maxillary sinus [1]. AMO is seen in 30 % of the patients with chronic maxillary sinusitis and in 10–20 % of healthy subjects [3, 4].
Between the uncinate process and the inferior concha there is a membranous area on the lateral nasal wall, covered only by mucoperiosteum. This area is named as fontanelle, and the ethmoid process of the inferior concha separates the membranous area into two parts, the anterior and the posterior fontanelles. The natural ostium of the maxillary sinus commonly opens to the anterior part of the posterior fontanelle. Accessory ostia are also most commonly encountered at the posterior fontanelle [5]. An ostium seen at the middle meatus during endoscopic examination is always an accessory ostium since the natural maxillary sinus ostium is located deep in the infundibulum and is hidden from view by the uncinate process [5].
The aim of our study was to investigate the frequency of AMO, its effect on the maxillary sinus and the concurrent presence of morphological variations in neighboring anatomical structures.
Material and method
This is a retrospective study, approved by the local Clinical Research Ethics Committee, evaluating axial paranasal sinus CT (Philips Brilliance 64-slice CT scanner, Philips Medical Imaging, The Netherlands) images, taken at 3 mm sections. The axial CT scanning was done on patients positioned supinely and the head position of those patients was adjusted in such a way that the hard palate was parallel to the floor, while the sagittal plane was perpendicular to the floor. All CT images were ordered for sinonasal, otologic and maxillofacial inquiries, between January and June 2013. Patients with maxillofacial trauma, nasal polyposis or sinus anomalies and those who had previous sinus surgery were excluded from the study.
Coronal sectional CTs of 377 patients were evaluated for the prevalence of accessory maxillary ostia and for any concurrent occurrences of morphological variants in neighboring anatomical structures and for the presence of mucus retention cyst and mucosal thickening.
The data were evaluated statistically by the Chi-square test. Differences at the level of p < 0.05 were accepted to be statistically significant.
Results
One hundred ninety-three (51.2 %) of the 377 patients (754 sides) were male and 184 were female (48.8 %). The age of cases ranged between 10 and 84 years old with the average age of 36.21 ± 15.50.
Accessory maxillary ostia (AMO) and accompanying morphological variants of neighboring structures were evaluated in 754 sides of the 377 patients. AMO was detected in a total of 72 (19.1 %) patients. In 7.2 % of the patients, AMO was located in the right side, in 3.7 % of the patients in the left side; in 8.2 % AMO was located bilaterally.
Mucus retention cyst (MRC) was detected at 21.8 %. Of 144 sides in 72 patients with AMO, MRC was encountered in 48.6 % (70/144) of the sides, with 52.8 % (38/72) located on the right and 44.4 % (32/72) on the left side. Of 610 sides in 305 patients without AMO, MRC was encountered in 15.5 % (95/610) of the patients, with 13.8 % (42/305) located on the right and 17.4 % (53/305) located on the left side. Simultaneous occurrence of AMO and MRC was found to be statistically significant on both sides (right side: p = 0.00, left side: p = 0.00) (Odds Ratio=5.15) (Table 1).
Mucosal thickening (MT) was detected at 25 %. Of 144 sides in 72 patients with AMO, MT was encountered in 43 % (62/144) of the sides with 41.7 % (30/72) located on the right and 44.4 % (32/72) on the left side. Of 610 sides in 305 patients without AMO, MT was encountered in 20.8 % (127/610) of the patients, with 17.7 % (54/305) located on the right and 23.9 % (73/305) located on the left side. Simultaneous occurrence of AMO and MT was found to be statistically significant on both sides (right side: p = 0.00, left side: p = 0.00) (Odds Ratio=2.87) (Table 1).
Maxillary sinusitis (MS) was detected at 14.5 %. Of 144 sides in 72 patients with AMO, MS was encountered in 29.1 % (42/144) of the sides, with 33.3 % (24/72) located on the right and 25 % (18/72) on the left side. Of 610 sides in 305 patients without AMO, MS was encountered in 11.1 % (68/610) of the patients, with 10.8 % (33/305) located on the right and 11.5 % (35/305) located on the left side. Simultaneous occurrence of AMO and maxillary sinusitis was found to be statistically significant on the right and left sides (right side: p = 0.04, left side: p = 0.03) (Odds Ratio=3.28) (Table 1).
Agger nasi cells (ANC) were detected at 62.8 %. Of 144 sides in 72 patients with AMO, ANC was encountered in 73.6 % (106/144) of the sides, with 72.2 % (52/72) located on the right and 75 % (54/72) on the left side. Of 610 sides in 305 patients without AMO, ANC was encountered in 60.3 % (368/610) of the patients, with 61.6 % (188/305) located on the right and 59 % (180/305) located on the left side. There was no statistical significance for the simultaneous occurrence of AMO and the agger nasi cell (right side: p = 0.27, left side: p = 0. 48) (Odds Ratio=1.83) (Table 1).
Haller cells (HC) were detected at 18.3 %. Of 144 sides in 72 patients with AMO, HC was encountered in 22.2 % (32/144) of the sides, with 25 % (18/72) located on the right and 19.4 % (14/72) on the left side. Of 610 sides in 305 patients without AMO, HC was encountered in 17.4 % (106/610) of the patients, with 18.7 % (57/305) located on the right and 16.1 % (49/305) located on the left side. There was no statistical significance for the simultaneous occurrence of AMO and the haller cell (right side: p = 0.11, left side: p = 0. 20) (Odds Ratio=1.35) (Table 1).
Septal deviation (SD) was detected at 47.7 %. Of 144 sides in 72 patients with AMO, SD was encountered in 60.45 % (43/72) of the sides, with 55.7 % (39/72) located on the right and 65.2 % (47/72) on the left side. Of 610 sides in 305 patients without AMO, SD was encountered in 43.9 % (134/305) of the patients, with 40.6 % (124/305) located on the right and 47.2 % (144/305) on the left side. The simultaneous occurrence of AMO and septal deviation was not found to be statistically significant on either side (right side: p = 0.37, left side: p = 0.42) (Odds Ratio=1.89) (Table 1).
Hypertrophy of inferior concha (HIC) was detected at 37.4 %. Of 144 sides in 72 patients with AMO, HIC was encountered in 37.5 % (54/144) of the sides, with 38.9 % (28/72) located on the right and 36.1 % (26/72) on the left side. Of 610 sides in 305 patients without AMO, HIC was encountered in 37.3 % (228/610) of the patients, with 33.4 % (102/305) located on the right and 41.3 % (126/305) located on the left side. Simultaneous occurrence of AMO and hypertrophy of inferior concha was found to be statistically significant on the left side, while no such significance was found for the right side (right side: p = 0.23, left side: p = 0.04) (Odds Ratio=1.00) (Table 1).
Pneumatization of the middle concha (PMC) was detected at 44.9 %. Of 144 sides in 72 patients with AMO, PMC was encountered in 47.9 % (69/144) of the sides with 45.8 % (33/72) located on the right and 50 % (36/72) on the left side. Of 610 sides in 305 patients without AMO, PMC was encountered in 44.3 % (270/610) of the patients with 42 % (128/305) located on the right and 46.6 % (142/305) located on the left side. There was no statistical significance for the simultaneous occurrence of AMO and the pneumatization of the middle concha (right side: p = 0.22, left side: p = 0. 38) (Odds Ratio=1.15) (Table 1).
Discussion
Accessory maxillary ostium may be present at varying degrees. Several cadaver and patient studies reported that the prevalence of accessory maxillary ostium in human beings is in the range of 0–43 % [6, 7]. The accessory is located at 5–10 mm superior to the attachment point of the inferior concha and it often opens to the lateral nasal wall and rarely to the infundibulum. Its size is in the range of 1–10 mm [8]. In a study conducted by Balasubramanian in the year 2012, the accessory ostium was located in the posterior side and as an elliptical shape, which can easily be seen in routine endoscopic nasal examination [9].
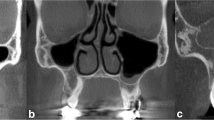
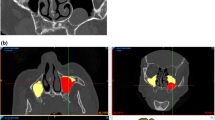
Genc et al. investigated the development of accessory ostium in the year 2008 and demonstrated that accessory maxillary ostium developed following experimentally induced sinusitis [2]. The incidence of accessory ostium appears to be higher in patients with a history of infundibular obstruction or maxillary sinus infection. This in turn suggests that accessory ostium emerges as a result of a pathological situation and it then stays open. Rarely, the natural ostium itself opens directly to the middle meatus, i.e., the free boundary posterior to the uncinate process [10]. Accessory maxillary ostium causes the mucus circulation, which is from the natural ostium and sinus towards the nasal cavity, to be redirected into the sinus, i.e., the re-entry of mucus drained through the natural ostium to the maxillary sinus through accessory ostium. Therefore, chronic maxillary sinus infection was blamed in its pathogenesis [11]. Our study also supports this result. While the incidence of mucosal thickening was observed to be 20.8 % in total in patients without AMO, it was identified to have been 43 % in patients with AMO, i.e., its incidence had increased more than two times (right side: p = 0.00, left side: p = 0.00). Maxillary sinusitis was observed to have an incidence rate of 11.1 % in total among patients without AMO whereas it was 29.1 % among patients with AMO, i.e., its incidence has more than doubled (right side: p = 0.04, left side: p = 0.03) (Figs. 1, 2).
Mucus retention cyst is commonly located within the maxillary sinus, and the imaging studies performed find it in approximately 9–22 % in the general population. It is thought to arise out of the obstruction of seromucous gland canals on the sinus surface. Therefore, the obstruction of the canals results in the growth of a retention cyst encapsulated in serous or mucous fluid-containing epithelium. They rarely reach a size at which they cause bone erosion or obstruct the sinus ostium resulting in symptoms. In the CT scan, they appear as well-delineated hypodense masses [12]. They may result in symptoms, such as headache, facial pain in the sinus areas and symptoms related to postnasal drainage and nasal drainage. They generally do not require treatment unless they are symptomatic [13]. Wang et al. reported 52.5 % nasal obstruction, 35.7 % nasal discharge and 2.5 % headache in patient populations with maxillary retention cysts [14]. Busaba and Kieff reported the symptoms of facial pain or pressure in the sinus site in all retention cyst patients [15]. Albu demonstrated in his study that there were no associations between retention cyst size and facial pain and pressure, nasal obstruction, nasal discharge and antral size [12].
Although the etiology of maxillary cyst is not definitively known, allergy, barotrauma, dental diseases, rhinitis and sinusitis play a role in the development of cysts [15]. Harar et al. concluded that chronic sinusitis played an important role in the development of maxillary mucosal retention cysts [16]. On the other hand, another study demonstrated that chronic sinusitis, allergy, asthma and recently recovered upper respiratory tract infection and dental infection were not associated with the high prevalence of retention cysts [17]. In another study, no correlations could be identified between ostiomeatal complex obstruction and growth of retention cysts [18].
In our study, mucus retention cyst was observed to have an incidence rate of 15.5 % in patients without AMO while it was identified that its incidence was 48.6 % among patients with AMO, i.e., it had almost tripled (right side: p = 0.00, left side: p = 0.00). This result indicates that there might have been an increased combination of retention cyst, mucosal thickening and accessory ostium that develop as complications following maxillary sinusitis. However, mucosal thickening occurs in paranasal sinuses following infection apart from maxillary sinuses, whereas the growth of retention cysts is observed very rarely [14, 19].
Another potential mechanism influencing the growth of retention cysts is the possibility that accessory maxillary ostium distorts the mucociliary activity by leading it back into the sinus, thereby paving the way for the obstruction of seromucous glands in the sinus and increasing retention cyst growth. Behrbohm and Sydow demonstrated that retention cysts larger than 1.5 cm delayed mucus transportation within the maxillary sinus; therefore, they played a role in chronic sinus etiology and the mucociliary clearance was changed with the removal of the cyst [20]. When combined with the result of our study, it is concluded that reduced mucociliary activity increases retention cyst growth while the growth of retention cyst reduces mucociliary activity; hence, a vicious cycle is created.
Our finding of pneumatization of the middle concha is also supported by the study of Stallmann et al. who also demonstrated the rate of pneumatization of the middle concha as 44 % [21].
Although there are several studies about maxillary sinus anatomy and its variations, there are no clinical studies investigating whether these variations have an association with accessory maxillary ostium or not. In that respect, our study brings a novel approach to the literature.
Conclusion
It was identified that the presence of accessory maxillary ostium is associated with an approximate threefold increase in the incidence of mucus retention cysts and a nearly twofold increase in mucosal thickening and maxillary sinusitis incidence. With respect to the growth of retention cysts, the distortion of mucociliary activity secondary to accessory ostium and the consequent obstruction of seromucous glands in the sinus account for the combination of accessory maxillary ostium and retention cysts.
Abbreviations
- AMO:
-
Accessory maxillary ostium
- ANC:
-
Agger nasi cells
- CT:
-
Computed tomography
- HC:
-
Haller cells
- HIC:
-
Hypertrophy of inferior concha
- MRC:
-
Mucus retention cyst
- MS:
-
Maxillary sinusitis
- MT:
-
Mucosal thickening
- PMC:
-
Pneumatization of middle concha
- SD:
-
Septal deviation
References
Stammberger H (1990) Functional endoscopic sinus surgery. The Messerklinger technique, vol 247. B.C. Decker, Philadelphia, pp 63–76
Genc S, Ozcan M, Titiz A, Unal A (2008) Development of maxillary accessory ostium following sinusitis in rabbits. Rhinology 46:121–124
Joe JK, Ho SY, Yanagisawa E (2000) Documentation of variations in sinonasal anatomy by intraoperative nasal endoscopy. Laryngoscope 110:229–235
Jones NS (2002) CT of the paranasal sinuses: a review of the correlation with clinical, surgical and histopathological findings. Clin Otolaryngol Allied Sci 27:11–17
Kane KJ (1997) Recirculation of mucus as a cause of persistent sinusitis. Am J Rhinol 11:361–369
Kuma RH, Kaka RS (2001) Accessory maxillary ostia: topography and clinical application. J Anat Soc India 50:3–5
Jog M, McGarry GW (2003) How frequent are accessory sinus ostia? J Laryngol Otol 117:270–272
Youngs R, Evans K, Watson M (2005) The paranasal sinuses. A handbook of applied surgical anatomy. Taylor & Francis, London
Balasubramanian T (2012) Advanced anatomy of lateral nasal wall: for the endoscopic sinus surgeon. Otolaryngol Online J Rhinol 2:4–9
Zinreich SJ (1998) Functional anatomy and computed tomography imaging of the paranasal sinuses. Am J Med Sci 316:2–12
Matthews BL, Burke AJ (1997) Recirculation of mucus via accessory ostia causing chronic maxillary sinus disease. Otolaryngol Head Neck Surg 117:422–423
Albu S (2010) Symptomatic maxillary sinus retention cysts: should they be removed? Laryngoscope 120:1904–1909
Hadar T, Shvero J, Nageris BI et al (2000) Mucus retention cyst of the maxillary sinus: the endoscopic approach. Br J Oral Maxillofac Surg 38:227–229
Wang JH, Yong JJ, Lee BJ (2007) Natural course of retention cysts of the maxillary sinus: long-term follow-up results. Laryngoscope 117:341–344
Busaba NY, Kieff D (2002) Endoscopic sinus surgery for inflammatory maxillary sinus disease. Laryngoscope 112:1378–1384
Harar RP, Chadha NK, Rogers G (2007) Are maxillary mucosal cysts a manifestation of inflammatory sinus disease? J Laryngol Otol 121:751–754
Kanagalingam J, Bhatia K, Georgalas C et al (2009) Maxillary mucosal cyst is not a manifestation of rhinosinusitis: results of a prospective three-dimensional CT study of ophthalmic patients. Laryngoscope 119:8–13
Bhattacharyya N (2000) Do maxillary sinus retention cysts reflect obstructive sinus phenomena? Arch Otolaryngol Head Neck Surg 126:1369–1371
Hong SL, Cho KS, Roh HJ (2014) Maxillary sinus retention cysts protruding into the inferior meatus. Clin Exp Otorhinolaryngol 7:226–228
Behrbohm H, Sydow K (1991) Nuclear medicine studies of the regenerative behavior of paranasal sinus mucosa after functional endoscopic ethmoid bone surgery [in German]. HNO 39:173–176
Stallman JS, Lobo JN, Som PM (2004) The incidence of concha bullosa and its relationship to nasal septal deviation and paranasal sinus disease. AJNR Am J Neuroradiol 25:1613–1618
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source, financial disclosures
None.
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Yenigun, A., Fazliogullari, Z., Gun, C. et al. The effect of the presence of the accessory maxillary ostium on the maxillary sinus. Eur Arch Otorhinolaryngol 273, 4315–4319 (2016). https://doi.org/10.1007/s00405-016-4129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4129-8