Abstract
Neuromonitoring in thyroid surgery has been employed to make nerve identification easier and decrease the rates of laryngeal nerve injuries. Several individual randomized controlled trials (RCTs) have been published, which did not identify statistical differences in the rates of recurrent laryngeal nerve (RLN) or external branch of the superior laryngeal nerve (EBSLN) injuries. The objective of this report is to perform meta-analysis of the combined results of individual studies to measure the frequency of RLN and EBSLN injuries in patients who underwent thyroidectomy with routine neuromonitoring in comparison with common practice of search and identification. RCTs comparing routine neuromonitoring versus no use in patients who underwent elective partial or total thyroidectomy were evaluated. Outcomes measured were temporary and definitive palsy of the RLN and EBSLN. A systematic review and meta-analysis was done using random effects model. GRADE was used to classify quality of evidence. Six studies with 1,602 patients and 3,064 nerves at risk were identified. Methodological quality assessment showed high risk of bias in most items. Funnel plot did not reveal publication bias. The risk difference for temporary RLN palsy, definitive RLN palsy, temporary EBSLN palsy, and definitive EBSLN palsy were −2 % (95 % confidence interval −5.1 to 1); 0 % (−1 to 1); −9 % (−15 to −2) and −1 % (−4 to 2), respectively. Quality was rated low or very low in most outcomes due to methodological flaws. Meta-analysis did not demonstrate a statistically significant decrease in the risk of temporary or definitive RLN injury and definitive EBSLN injury with the use of neuromonitoring. The neuromonitoring group had a statistically significant decrease in the risk of temporary EBSLN injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, our group published a critical appraisal of the literature on the efficacy of neuromonitoring of the laryngeal nerves for prevention of nerve injury during thyroid surgery [1]. This review of individual studies concluded that the current literature on neuromonitoring has not proven that routine monitoring produces results superior to those obtained by traditional anatomical methods of nerve identification during thyroid surgery, although it may be helpful in difficult cases. The purpose of the present study is to evaluate the results of qualifying individual studies by meta-analysis of the combined results—to determine whether neuromonitoring offers a benefit with regard to minimizing laryngeal nerve injury during thyroid surgery.
The techniques of neuromonitoring began to be employed in the 1970s with the objective of facilitating nerve identification, and consequently, decreasing the rates of recurrent laryngeal nerve (RLN) and external branch of the superior laryngeal nerve (EBSLN) injuries below the levels attained with non-monitored dissection. The methods of neuromonitoring range from pressure measurements [2], intraoperative vocal cord movement visualization [3] and registry of effector muscle movement after stimulation [4] by the insertion of direct or indirect electrodes to observe the electromyographic (EMG) response after nerve stimulation [5–7]. Many authors have suggested that neuromonitoring should be routinely used in thyroid surgery [8, 9]. However, others have concluded that neuromonitoring does not offer advantages if the previously defined principles of surgical technique are followed [10, 11]. It may even be proposed that neuromonitoring could be detrimental by causing the surgeon to place unwarranted trust in the technology, not to mention added cost and time for equipment set up. Therefore, the use of neuromonitoring in thyroid surgery has become controversial. To solve this controversy, several randomized controlled trials (RCTs) have been performed [12–17]. These trials could not identify statistical differences in the rates of RLN or EBSLN injury between groups using neuromonitoring and those who underwent conventional thyroidectomy. However, these trials had small sample sizes, and did not offer a conclusive response.
Recently, Higgins et al. [18] performed a meta-analysis and concluded that there were no differences in complication rates between patients who underwent thyroidectomy with routine neuromonitoring in comparison with those who did not. However, the authors included case reports and other observational studies that are highly prone to bias, did not assess the quality of the studies reviewed, and only included one RCT, making their conclusions controversial. Neuromonitoring increases operative costs [19] primarily due to the cost of equipment and devices, and also can put surgeons who do not use it at legal risk, it is important to define clearly its utility in thyroid surgery [20].
Materials and methods
RCTs comparing the results in patients undergoing elective partial or total thyroidectomy for benign or malignant disease with or without routine neuromonitoring were evaluated. No limitations were placed regarding the number of patients randomized, source, or language of the article. The studies chosen included patients older than 18 years with a preoperative clinical diagnosis of benign (goiter, thyroiditis), indeterminate (follicular neoplasm) or malignant (papillary or follicular carcinoma) disease of the thyroid, scheduled for partial or total thyroidectomy and without previous nerve injury. Papers including patients with previous neck surgery or laryngeal nerve injury were excluded.
Interventions assessed were neuromonitoring by any method (direct or indirect electrodes) associated to searching and identification of the nerve versus searching and identification alone. Studies with continuous intraoperative monitoring were not included. Outcomes measured were temporary and definitive palsy of the RLN and EBSLN, detected clinically and/or by laryngoscopy and recorded as yes or no (primary outcome). As most studies reported patients and nerves at risk, we decided to collect information of events for both outcomes. Rate of RLN and EBSLN identification was a secondary outcome. All outcome measures were confined to 18 months of follow-up.
Search strategy
We (AS, AR) searched The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (2012), The National Library of Medicine (PubMed) (1966–December 2012), EMBASE (1980–December 2012) and The Latin American and Caribbean Health Sciences Library (LILACS) (1980–December 2012). The search strategy identified studies in all languages. When necessary, we translated non-English language papers for a full assessment. The search strategy for the review was constructed using a combination of MESH subject headings and text words (thyroid diseases, thyroid neoplasms, parathyroid, thyroidectomy, surgery, monitor, monitoring, neuromonitoring, and nerve), relating to the use of neuromonitoring in partial or total thyroidectomy. Authors of included trials were contacted to seek further information on any published, unpublished, and ongoing trials. We also checked the reference lists of all the identified trials for more relevant reports.
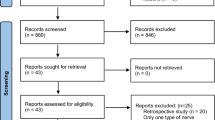
If inclusion criteria were not fulfilled, trials were excluded and reasons for exclusion were listed (Fig. 1). For RCT, methodological quality assessment was performed by two independent evaluators (AS, AR) including evaluation of selection bias (randomization, allocation concealment), performance and detection bias (blinding), attrition bias (lost to follow-up and intention to treat analysis), reporting bias (outcomes reporting), and each criterion was classified as high risk of bias, low risk of bias or unclear risk of bias, as recommended by Cochrane Collaboration [21]. Differences between evaluators were solved by consensus. We considered as low quality those studies which had at least one quality factor classified as high risk. As some data were lacking from the articles, we contacted authors of studies to get this information. Only Dionigi et al. [15] responded to the request.
Statistics
The statistical package Review Manager© (RevMan) (Version 5.2. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2012) was used. For effectiveness analysis and dichotomous outcomes, the impact of the intervention was expressed as risk difference (RD) with 95 % confidence interval (CI). A Mantel–Haenszel random effects model was used. The Chi square test for heterogeneity was used to provide an indication of between-study heterogeneity, and the degree of heterogeneity observed was quantified using the I 2 statistic. Sensitivity analysis was performed using trials with high methodological quality. Causes of heterogeneity, where heterogeneity was found, (Chi squared test P < 0.10 or I 2 > 50 %) were explained subjectively. Funnel plot [22] was used to assess publication bias. The GRADEpro© software and the GRADE Handbook [23, 24] were used to classify quality of evidence. The importance of the outcomes was scored with the mean of independent assessment of authors. An evidence profile and a summary of findings table were built.
Results
477 references were reviewed and only six studies were identified by the primary search (Fig. 1) [12, 16, 25]. The agreement for searching between two evaluators was good (kappa = 0.91). Inclusion criteria were similar for all studies: candidates for total or partial thyroidectomy. Barczyński et al. [13] only included patients with goiter while the other studies also included patients with malignancies. Most patients had a small thyroid (goiter <than 100 ml [13], thyroid volume <25 ml or nodule <35 mm [15], or candidates with mini-incision thyroidectomy [12]) and were euthyroid. Those patients expected to have a difficult thyroidectomy (large goiter, thyroiditis, hyperthyroidism, etc.) were excluded, except for Sari et al. [25] and Barczyński et al. [13, 16]. Dionigi et al. [15] only included patients who underwent a minimally invasive thyroidectomy and Barczyński et al. [16] included patients with central neck dissection. Barczyński et al. [16] and Lifante et al. [12] used muscle inserted electrodes while the other studies used endotracheal tube embedded electrodes. Most studies selected temporary or definitive RLN and/or EBSLN palsy as the primary outcome. Barczyński et al. [13, 16] added anatomical variations and Barczyński et al. [13], Dionigi et al. [15], Khaled et al. [14] and Lifante et al. [12] added subjective vocal scale assessment. Other characteristics of studies are shown in Table 1.
Methodological quality
Studies included were parallel RCTs. Random sequence generation was considered to have high risk of bias in Dionigi et al. [15] (used times of admission as randomization criteria) and Lifante et al. [12] (the randomization method was not reported and imbalance between groups was found (total thyroidectomy rate for intervention 59 vs. control 32 %). Allocation concealment was considered to have high risk of bias in Dionigi et al. [15] (already reported randomization method and imbalance) and unclear risk of bias in Khaled et al. [14], Lifante et al. [12] and Sari et al. [25] (method not reported). Blinding of outcome assessment was considered to have unclear risk of bias in Khaled et al. [14] and Sari et al. [25] (there was no report of independent assessment of outcomes). Incomplete outcome data were considered to have a high risk of bias in Lifante et al. [12] (the study protocol excluded all patients who experienced a postoperative transient or permanent laryngeal nerve palsy) and Sari et al. [25] (were excluded due to lack of signal) and unclear risk of bias in the others (the experimental branch uses the device to affect the continuity of surgery and data by group not reported for some outcomes in Khaled et al. [14]). Selective reporting was considered to have high risk of bias in Khaled et al. [14] (RLN injuries were not reported) and Lifante et al. [12] (there is no report of rate of EBSLN injuries and patients with RLN injuries were excluded) (Table 2).
Funnel plot using the most common reported outcome (RLN palsy) did not show publication bias, but the number of trials is small. Funnel plots for other outcomes had fewer trials and showed similar results (Fig. 2).
Outcomes
The six studies recruited a total of 1,602 patients: 804 in the neuromonitoring group and 798 in the visual identification group; and assessed 3,064 nerves at risk: 1,523 in the neuromonitoring group and 1,541 in the visual identification group. However, due to design and reporting, not all studies offered data for all outcomes. Even more, when extracting data for nerves at risk comparisons, we had to make adjustments in the number reported by authors. In Barczyński et al. [16] the number of nerves at risk in the neuromonitoring group was not 1,000 as reported, but 952 because they mention sensitivity of the test and reported that in cases of loss of signal the procedure was stopped, avoiding the exploration of the contralateral nerve, and therefore, decreasing the number of nerves at risk. In Sari et al. [25], the number of nerves at risk reported by the authors was 210, but they excluded 20 nerves at risk because of no acoustic signal. Therefore, the total number of nerves at risk increased to 230. In Dionigi et al. [15] the same occurred with a change from 55 to 54 nerves at risk in the neuromonitoring group. In Barczyński et al. [13] we used 105 patients instead of 101 and in Sari et al. [25] we used 120 and 111 instead of 123 and 114, assuming intention to treat analysis and excluding patients with previous palsy. We made other adjustments in the events of temporary and definitive palsy, because Barczyński et al. [13] reported each outcome assuming they were independent, but it is clear that all definitive palsies initially corresponded to temporary palsies that did not recover. Therefore, the real number of temporary palsies corresponds to the overall reported by the authors.
Analysis for number of patients
Recurrent laryngeal nerve palsy
1,513 patients were assessed for this outcome in four studies. Temporary RLN palsy occurred in 4.2 % of patients in the monitoring group vs. 7.7 % in the visualization group. The RD was −2 % (95 % CI −5.1 to 1), non-statistically significant and without heterogeneity (I 2 = 44 %). Definitive RLN palsy occurred in 1 % of patients in the neuromonitoring group vs. 1.6 % in the visualization group [RD 0 % (−1 to 1)], non-statistically significant and without heterogeneity (I2 = 0 %) (Fig. 3a, b).
External branch of the superior laryngeal nerve
Temporary palsy was reported by one study in 210 patients. 2.9 % of patients suffered temporary palsy in the neuromonitoring group vs. 11.4 % in the visualization group [RD −9 % (−15 to −2)] (p = 0.01). The heterogeneity was not evaluable. Definitive palsy was reported by 3 studies in 324 patients. 0.6 % of patients suffered definitive palsy in the neuromonitoring group vs. 1.8 % in the visualization group [RD −1 % (−4 to 2)], non-statistically significant and without heterogeneity (I 2 = 0 %) (Fig. 4).
Analysis for number of nerves at risk
Recurrent laryngeal nerve palsy
2,912 nerves were assessed for this outcome in four studies. Temporary RLN palsy occurred in 2.2 % of nerves in the monitoring group vs. 3.9 % in the visualization group. The RD was −1 % (95 % CI −2 to 1), non-statistically significant and without heterogeneity (I 2 = 31 %). Definitive RLN palsy occurred in 0.5 % of nerves in the neuromonitoring group vs. 0.8 % in the visualization group [RD 0 % (−1 to 0)], non-statistically significant and without heterogeneity (I 2 = 0 %) (Fig. 5a, b).
External branch of the superior laryngeal nerve
Temporary palsy was reported in 420 nerves by one study. 1.4 % of nerves suffered temporary palsy in the neuromonitoring group vs. 5.7 % in the visualization group [RD −4 % (−8 to −1)] (p = 0.02). The heterogeneity was not evaluable. Definitive palsy was reported by 3 studies in 616 nerves. 0.3 % of nerves suffered definitive palsy in the neuromonitoring group vs. 0.9 % in the visualization group [RD 0 % (−2 to 1)], non-statistically significant and without heterogeneity (I 2 = 0 %) (Fig. 4).
Nerve identification
Recurrent laryngeal nerve
2,912 nerves were assessed for this outcome in four studies. RLN was identified in 99.8 % of nerves in the monitoring group vs. 99.5 % in the visualization group. The RD was 0 % (0–1), non-statistically significant and without heterogeneity (I 2 = 0 %).
External branch of the superior laryngeal nerve
712 nerves were assessed for this outcome in three studies. EBSLN was identified in 69.0 % of nerves in the monitoring group vs. 28.9 % in the visualization group. The RD was 38 % (18–58), statistically significant (p = 0.03) but with high heterogeneity (I 2 = 87 %). The heterogeneity is solved by the exclusion of Dionigi et al. [15] and we believe that the low rate of EBSLN identification is due to the lack of routine searching for it in the minimally invasive thyroidectomy technique.
GRADE analysis
The means of importance ratings for temporary and definitive palsy of the RLN and temporary and definitive palsy of EBSLN were 6.4, 9, 4.8 and 7.4, respectively. The numbers were rounded to the next decimal to categorize it in the Grade© software. Finally, definitive palsy of any nerve was considered critical and temporary palsy of any nerve was considered important from a patient-centered perspective. The GRADE evidence profile is shown in Tables 3 and 4. Quality was rated low or very low in most outcomes due to methodological flaws and most outcomes did not show a statistically significant difference.
Discussion
There are two major technical challenges in thyroid surgery: the first, to preserve the laryngeal nerves and second, to preserve viable parathyroid gland. Since Kocher′s description of the modern thyroidectomy, surgeons have tried to decrease the complications associated with the injury of these anatomical structures. The most important step made in the field of nerve preservation was described by Lahey and Hoover [26] in 1938 with the demonstration that routine identification of the RLN significantly decreased the number of injuries. Later, Cernea et al. [27] in his study of the anatomy of the EBSLN in relation to the upper pole and superior thyroid artery clearly elucidated the expected risk of injuries and the techniques to avoid it during thyroidectomy. The rates of definitive nerve injuries after these modifications have reached as low as 0.5 % in specialized centers, which have been maintained over time [28–33].
The introduction of nerve monitoring in thyroid surgery is recent. Although many device developments were made in the 1970s, neuromonitoring, as known today, has been introduced into clinical practice only in the last two decades. At the beginning, neuromonitoring for laryngeal nerves was performed using visual detection of muscle movement after stimulation [34], pressure monitors placed in the vocal cords [2], and direct electrodes on the effector muscles [35]. The most common method in current use is a special endotracheal tube with electrodes embedded on it that register effects of stimulation in the vocal cords [36]. However, it should be realized that conventional intraoperative nerve monitoring can only predict RLN palsy after the damage has been done. Other strategies, such as continuous vagus nerve stimulation, could detect early changes in EMG response that indicate imminent danger to functional integrity of the RLN, but this method has not been widely assessed [37].
Neuromonitoring has been widely adopted in Europe, especially in Germany. There are many non-randomized trials that assess its utility with conflicting results [38, 39]. The largest non-randomized multicenter trial conducted in Germany with more than 16,000 patients reported that the device could help in decreasing the risk of nerve injury [9]. However, all these trials are prone to bias because of the observational design. In the absence of randomization, it has been demonstrated that results are overestimated and prone to selection bias [40, 41]. This can be explained because neuromonitoring is used in more challenging cases where its effects could be greater or because patients compared are not equivalent in initial relevant patient characteristics. Other factors that lead to difficulty in the interpretation of these results, specifically in a multicenter trial, are the case mix of patients in different centers [42] (reference vs. community), with different surgeons (high volume vs. low volume) and the lack of a standard method of using the device and assessing the results. In order to overcome these difficulties, some RCTs have been undertaken. However, sample sizes in these studies have been small, with a consequent lack of power to detect clinically significant differences. The only available way to solve this last problem is to conduct a systematic review and meta-analysis.
The present study included six studies with more than 1,600 patients and 3,000 nerves at risk. However, not all studies assessed all outcomes, so the numbers for the evaluation of each outcome are less.
Temporary and definitive RLN palsy was the most frequent outcome evaluated. With regard to outcomes, considering either patients or nerves at risk, it was not possible to identify a statistically significant RD between groups. The difference in the risk of temporary injury was 2 and 1 %, respectively, when either patients or nerves at risk were counted. For definitive injury, the RD was near 0 % between either groups, regardless of whether patients or nerves at risk were considered. For EBSLN palsy, the only statistically significant RD was found in the frequency of temporary palsy (9 % for patient comparison vs. 4 % for nerves at risk). However, when assessing definitive injuries the comparisons did not show statistically significant RDs (1 % for patients and 0 % for nerves at risk).
Some comments should help to interpret these results. First, even with this large number of patients included, it is possible that lack of power is still present in this analysis. As the rate of temporary and definitive RLN palsy is low, a higher sample size might be necessary. A sample size calculation made with actual results, with an α error of 0.05 and a power of 80 % shows that for RLN temporary palsy, the number of patients included is enough to be confident of their results, but not for definitive palsy comparisons, where the calculated sample size needed is around 4,500 patients or 9,000 nerves at risk. In the case of definitive injury of EBSLN, the numbers required for confidence will be 1,100 patients or 6,000 nerves at risk. It should be realized that increase of sample size will only increase the precision of the pooled result, but probably will not change the overall value of the RD. Rather than a question of sample size, the important question will depend on the relevance of finding a clinically significant result in comparison with a statistically significant result. With an expected RD of 1 % in the rate of temporary RLN palsy or 0.5 % in the risk of definitive RLN palsy, the real effect on patients and the health care system is negligible. The calculation of the number needed to treat in this scenario shows that 100–200 thyroidectomies must be monitored to avoid one definitive nerve palsy. The costs of monitoring, electrodes and stimulating tips for each thyroidectomy must be weighed against the possible avoidance of one definitive nerve palsy in 100–200 cases. The concept of quality of life in this issue has been previously addressed showing that an early correction of vocal fold paralysis recovers quality of life scores to pre-injury values [43–45]. Even, upper aerodigestive symptoms (voice and swallowing symptoms) after thyroidectomy have been evaluated in patients with normal vocal fold mobility who did and who did not have intraoperative neuromonitoring. The proportion of patients who reported aerodigestive symptoms was 39 and 45 %, respectively, with no statistical differences between both groups [46]. Also, neuromonitoring was not correlated with non-recurrent nerve injury-related changes in voice after thyroidectomy using multi-dimensional voice measurements (negative vocal outcomes between neuromonitored and non-monitored patients at 6 months [14 vs. 7 %, p = 0.42)] [47].
Second, the basal risk of injury is an important factor to consider when deciding whether to use neuromonitoring. As can be seen in this study, control groups had a risk of temporary and definitive RLN palsy of 7 and 1.6 %, respectively. As our results showed, the expected decrease of injuries in absolute numbers is less than 2 % for temporary and 1 % for definitive injury. Therefore, if an institution or a center has a rate equal to or less than 1 %, it is clear that utility of neuromonitoring will be insignificant. Some authors have suggested that neuromonitoring should be useful in low-volume centers [48], but data do not support this assumption, because most studies have been made in high-volume centers. The discussion about the use of neuromonitoring for low-volume centers or surgeons obscures the real problem in these settings. If meticulous nerve visualization and a standardized surgical technique have shown progressive and sustained decrease in nerve palsy, the solution is to follow these simple surgical principles in a standard way, to specialize surgeons in the procedure and to strengthen remission of patients to high-volume centers that have shown low risk of complication, instead of believing that the use of a technology itself can reduce a surgical complication [49]. Others have suggested its use in high-risk patients [50], but most patients included in this meta-analysis correspond to low risk patients. Up to now, there is not a RCT for high-risk patients.
Another consideration is the relationship between the rate of temporary and definitive palsy. As can be seen in the results, the rate of temporary RLN palsy is four times greater than the rate of definitive palsy in the neuromonitoring group and three times greater in the case of EBSLN. This means that most temporary palsies will resolve in the postoperative period without any clinical intervention. An important question is whether the use of neuromonitoring decreases the number of temporary palsies that do not end up becoming a definitive palsy.
The discussion of proxy outcomes has been extensively discussed in the literature [51]. These have been defined as outcomes that occur in a causal way between an intervention and a clinical patient oriented result, and are used as proxy of this final result. The most common proxy variables are test results. But in thyroid surgery, temporary palsy has been recognized as a proxy outcome, since the frequency is always greater than the frequency of definitive palsy, and most of them resolve without further treatment. This notion is supported by the long duration of 6–12 months generally required to consider a temporary palsy versus a definitive one. Therefore, the effect of neuromonitoring on the frequency of temporary palsy should be carefully considered, since it most often does not translate into a definitive palsy. This is also shown in this study, where the outcome of nerve identification was analyzed. More EBSLN were identified with neuromonitoring, a statistically significant result, but the frequency of definitive palsy did not change. Some authors have suggested that neuromonitoring will decrease the time required for nerve identification [15], but this decrease should also be compared with the costs of using the device, as well as the time required for set up. Time reduction of about 5–7 min would not be sufficiently cost-effective to justify its routine use.
A final comment regarding methodological quality is also necessary. In general, the quality of published studies is low. This is due to weaknesses in randomization, allocation concealment and outcome reporting. The first issue has been clearly studied and has shown that results are prone to bias [40, 41]. Regarding outcomes reporting, an important weakness was identified in this systematic review. It has been demonstrated that intention to treat analysis is important to overcome the problems derived from lack of long-term follow-up or losses during a study. In most trials, especially when nerves at risk were analyzed, authors used the total number of nerves, but they did not consider that this total is affected by the use of the device. Guidelines on neuromonitoring [52] strongly recommend stopping the surgery when the first dissected nerve loses its EMG signal, to avoid a contra-lateral dissection with the consequent risk of bilateral nerve palsy. Therefore, if this recommendation is followed (and this is not always the fact), the total number of nerves at risk will be reduced by the use of the device. In other words, the device will determine the final number of nerves at risk. Only Barczyński et al. [16] reported sensitivity and specificity of neuromonitoring, which allows one to make an adjustment for the total number of nerves at risk. The other studies did not do this, so numbers included in the analysis are at high risk of bias. On the other hand, some studies excluded patients with loss of signal, changing the analysis from intention-to-treat to per-protocol analysis. Finally, some studies, particularly those exploring the use of neuromonitoring in EBSLN palsy did not report the rate of RLN palsy. This is an example of selective reporting bias, because it appears very improbable not to have data about RLN palsy in a study assessing the EBSLN function. We made every effort to adjust the analysis to compensate for these weaknesses, but we could not get any additional information from the authors. So the low methodological quality of evidence made the recommendation of its use weak. The use of the GRADE methodology as a more objective tool of assessment, that besides methodological quality, also introduces other factors such as inconsistency, imprecision and indirectness, showing that most evidence available for each outcome selected was classified as low or very low quality. This fact and the results obtained from the meta-analysis make it difficult to support the routine use of neuromonitoring in thyroidectomy. Furthermore, there is no high-quality evidence to support a staged thyroidectomy when a loss of signal from intraoperative nerve monitoring is observed after first-side dissection of the RLN. In a series of 295 patients, loss of signal on the first side was noted in 16 procedures. The contralateral thyroidectomy was completed and, at retesting, 15 of 16 initially silent nerves recovered an EMG signal. In no patient was the signal lost on the contralateral side. Therefore, there was a 90 % chance of intraoperative signal recovery [53].
In conclusion, in six RCTs of low methodological quality, neuromonitoring could not demonstrate a statistically significant decrease in the risk of temporary or definitive RLN injury and definitive EBSLN injury. For temporary EBSLN injury, neuromonitoring showed a statistically significant decrease in the risk. With this data it is not possible to recommend its routine use and more studies focused on high-risk patients (reoperation) should be done.
Even with extensive meta-analysis and review of the literature, at this time, it is difficult to predict the impact of RLN monitoring on nerve injuries overall. The studies may be difficult to interpret occasionally because every patient may not undergo late fiber optic laryngoscopy especially if the voice has improved. Nerve injury rarely occurs due to transection of the nerve but occurs more commonly due to thermal injury with electrocautery, especially, if there is any bleeding around the nerve or in the region of the Berry’s ligament. This may be almost impossible to improve whether one uses a nerve monitor or not. The loss of signal may jeopardize the exploration of the other side, which probably can be performed with safety. However, the general recommendation is to avoid contralateral surgery with loss of signal on one side.
What is lacking in the literature is information regarding the risk stratification of the surgical difficulty, such as larger tumor, gross extrathyroidal extension, adherence of the nerve to the tumor, extent of paratracheal nodal disease and any anatomical variations. The issues related to superior laryngeal nerve monitoring and injury are more complex as to the difficulty in evaluating objectively the nerve injury in the post operative period. There is no unified test which confirms EBSLN injury, either temporary or permanent. So the information in relation to EBSLN monitoring and injury needs to be taken with some skepticism. Clearly, RLN monitoring depends upon the surgeon’s practice, experience, expertise, level of comfort, and the ease of availability of the instrumentation. Younger surgeons are probably using nerve monitoring more often than their senior colleagues. This is clearly related to individual practice of surgery. One thing becomes very clear from the entire literature that nerve monitoring is probably helpful in reoperative surgery, especially, if there is recurrent disease in the tracheo-esophageal groove or recurrent disease near the cricoid cartilage. Most surgeons will agree that nerve monitors in these difficult situations are more helpful than in the initial surgical procedures.
References
Sanabria A, Silver CE, Suárez C et al (2013) Neuromonitoring of the laryngeal nerves in thyroid surgery: a critical appraisal of the literature. Eur Arch Otorhinolaryngol (in press)
Engel PM, Büter HA, Page PS, Mos A (1981) A device for the location and protection of the recurrent laryngeal nerve during operations upon the neck. Surg Gynecol Obstet 152:825–826
Hillermann CL, Tarpey J, Phillips DE (2003) Laryngeal nerve identification during thyroid surgery—feasibility of a novel approach. Can J Anaesth 50:189–192
James AG, Crocker S, Woltering E, Ferrara J, Farrar W (1985) A simple method for identifying and testing the recurrent laryngeal nerve. Surg Gynecol Obstet 161:185–186
Flisberg K, Lindholm T (1969) Electrical stimulation of the human recurrent laryngeal nerve during thyroid operation. Acta Otolaryngol Suppl 263:63–67
Dimov RS, Mitov FS, Deenichin GP, Ali MM, Doikov IJ, Yovchev IJ (2001) Stimulation electromyography as a method of intraoperative identification of the recurrent laryngeal nerve in thyroid surgery. Folia Med (Plovdiv) 43:17–20
Rea JL (1992) Postcricoid surface laryngeal electrode. Ear Nose Throat J 71:267–269
Dralle H, Sekulla C, Lorenz K et al (2008) Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 32:1358–1366
Thomusch O, Sekulla C, Timmermann W et al (2003) Intraoperative neuromonitoring in thyroid surgery - Results of the German prospective multicentre study. Eur Surg 35:240–245
Chan WF, Lang BH, Lo CY (2006) The role of intraoperative neuromonitoring of recurrent laryngeal nerve during thyroidectomy: a comparative study on 1000 nerves at risk. Surgery 140:866–872
Witt RL (2005) Recurrent laryngeal nerve electrophysiologic monitoring in thyroid surgery: the standard of care? J Voice 19:497–500
Lifante JC, McGill J, Murry T, Aviv JE, Inabnet WB 3rd (2009) A prospective, randomized trial of nerve monitoring of the external branch of the superior laryngeal nerve during thyroidectomy under local/regional anesthesia and IV sedation. Surgery 146:1167–1173
Barczyński M, Konturek A, Stopa M, Honowska A, Nowak W (2012) Randomized controlled trial of visualization versus neuromonitoring of the external branch of the superior laryngeal nerve during thyroidectomy. World J Surg 36:1340–1347
Khaled AO, Irfan M, Baharudin A, Shahid H (2012) Comparing the morbidity of external laryngeal nerve injury in thyroid surgery with and without identifying the nerve using intraoperative neuromonitoring. Med J Malaysia 67:289–292
Dionigi G, Boni L, Rovera F, Bacuzzi A, Dionigi R (2009) Neuromonitoring and video-assisted thyroidectomy: a prospective, randomized case-control evaluation. Surg Endosc 23:996–1003
Barczyński M, Konturek A, Cichon S (2009) Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 96:240–246
Ulmer C, Rieber F, Zopf W et al (2011) Effectiveness of continuous intraoperative neuromonitoring (CIONM) based on a new vagus nerve stimulation electrode-A randomized controlled trial. Langenbecks Arch Surg 396:1285–1286
Higgins TS, Gupta R, Ketcham AS, Sataloff RT, Wadsworth JT, Sinacori JT (2011) Recurrent laryngeal nerve monitoring versus identification alone on post-thyroidectomy true vocal fold palsy: a meta-analysis. Laryngoscope 121:1009–1017
Beccagutti G, Grifi M, Pantaleoni M, Dionigi G (2010) The impact of neuromonitoring on thyroid surgery costs. Value Health 13:A289
Wolf G (2003) Neuromonitoring is not a standard procedure in thyroid surgery. Eur Surg 35:236–239
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions, version 5.1.0. John Wiley & Sons, Chichester
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Schünemann H, Brozek J, Oxman A (2009) GRADE handbook for grading quality of evidence and strength of recommendation, version 3.2. The GRADE Working Group
Sari S, Erbil Y, Sümer A et al (2010) Evaluation of recurrent laryngeal nerve monitoring in thyroid surgery. Int J Surg 8:474–478
Lahey FH, Hoover WB (1938) Injuries to the recurrent laryngeal nerve in thyroid operations: their management and avoidance. Ann Surg 108:545–562
Cernea CR, Ferraz AR, Furlani J et al (1992) Identification of the external branch of the superior laryngeal nerve during thyroidectomy. Am J Surg 164:634–639
Zambudio AR, Rodríguez J, Riquelme J, Soria T, Canteras M, Parrilla P (2004) Prospective study of postoperative complications after total thyroidectomy for multinodular goiters by surgeons with experience in endocrine surgery. Ann Surg 240:18–25
Filho JG, Kowalski LP (2004) Postoperative complications of thyroidectomy for differentiated thyroid carcinoma. Am J Otolaryngol 25:225–230
Karamanakos SN, Markou KB, Panagopoulos K et al (2010) Complications and risk factors related to the extent of surgery in thyroidectomy. Results from 2,043 procedures. Hormones (Athens) 9:318–325
Reeve TS, Delbridge L, Crummer P (1986) Total thyroidectomy in the management of differentiated thyroid cancer: a review of 258 cases. Aust N Z J Surg 56:829–833
Bhattacharyya N, Fried MP (2002) Assessment of the morbidity and complications of total thyroidectomy. Arch Otolaryngol Head Neck Surg 128:389–392
Chaudhary IA, Samiullah Masood R, Majrooh MA, Mallhi AA (2007) Recurrent laryngeal nerve injury: an experience with 310 thyroidectomies. J Ayub Med Coll Abbottabad 19:46–50
Randolph GW, Kobler JB, Wilkins J (2004) Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg 28:755–760
Maloney RW, Murcek BW, Steehler KW, Sibly D, Maloney RE (1994) A new method for intraoperative recurrent laryngeal nerve monitoring. Ear Nose Throat J 73:30–33
Dimov RS, Doikov IJ, Mitov FS, Deenichin GP, Yovchev IJ (2001) Intraoperative identification of recurrent laryngeal nerves in thyroid surgery by electrical stimulation. Folia Med (Plovdiv) 43:10–13
Schneider R, Randolph GW, Sekulla C et al (2012) Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck. [Epub ahead of print]
Atallah I, Dupret A, Carpentier AS, Weingertner AS, Volkmar PP, Rodier JF (2009) Role of intraoperative neuromonitoring of the recurrent laryngeal nerve in high-risk thyroid surgery. J Otolaryngol Head Neck Surg 38:613–618
Chiang FY, Lee KW, Chen HC et al (2010) Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 34:223–229
Moher D, Pham B, Jones A et al (1998) Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352:609–613
Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273:408–412
Duclos A, Voirin N, Touzet S et al (2010) Crude versus case-mix-adjusted control charts for safety monitoring in thyroid surgery. Qual Saf Health Care 19:e17
Spector BC, Netterville JL, Billante C, Clary J, Reinisch L, Smith TL (2001) Quality-of-life assessment in patients with unilateral vocal cord paralysis. Otolaryngol Head Neck Surg 125:176–182
Hogikyan ND, Wodchis WP, Terrell JE, Bradford CR, Esclamado RM (2000) Voice-related quality of life (V-RQOL) following type I thyroplasty for unilateral vocal fold paralysis. J Voice 14:378–386
Billante CR, Spector B, Hudson M, Burkard K, Netterville JL (2001) Voice outcome following thyroplasty in patients with cancer-related vocal fold paralysis. Auris Nasus Larynx 28:315–321
Silva IC, Netto ID, Vartanian JG, Kowalski LP, Carrara-de-Angelis E (2012) Prevalence of upper aerodigestive symptoms in patients who underwent thyroidectomy with and without the use of intraoperative laryngeal nerve monitoring. Thyroid 22:814–819
Stevens K, Stojadinovic A, Helou LB et al (2012) The impact of recurrent laryngeal neuromonitoring on multi-dimensional voice outcomes following thyroid surgery. J Surg Oncol 105:4–9
Poveda MCD, Dionigi G, Sitges-Serra A et al (2011) Intraoperative monitoring of the recurrent laryngeal nerve during thyroidectomy: a standardized approach (Part 1). World J Endocr Surg 3:144–150
Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R (1998) The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg 228:320–330
Chiang FY, Lu IC, Tsai CJ, Hsiao PJ, Hsu CC, Wu CW (2011) Does extensive dissection of recurrent laryngeal nerve during thyroid operation increase the risk of nerve injury? Evidence from the application of intraoperative neuromonitoring. Am J Otolaryngol 32:499–503
Ciani O, Buyse M, Garside R et al (2013) Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. BMJ 346:f457
Randolph GW, Dralle H, Abdullah H et al (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 121(Suppl 1):S1–16
Sitges-Serra A, Fontané J, Dueñas JP et al (2013) Prospective study on loss of signal on the first side during neuromonitoring of the recurrent laryngeal nerve in total thyroidectomy. Br J Surg 100:662–666
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com).
Rights and permissions
About this article
Cite this article
Sanabria, A., Ramirez, A., Kowalski, L.P. et al. Neuromonitoring in thyroidectomy: a meta-analysis of effectiveness from randomized controlled trials. Eur Arch Otorhinolaryngol 270, 2175–2189 (2013). https://doi.org/10.1007/s00405-013-2557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-013-2557-2