Abstract
It is important to detect novel predictive biomarkers of cervical lymph node metastasis (CLNM) in papillary thyroid carcinoma (PTC) to help the surgeons in making early decision of performing central lymph node dissection and aggressive management strategies in selected high-risk patients, thus improving their prognosis. Zinc finger protein 703 (ZNF703) is a member of the neutrophil extracellular trap (NET) transcription factors family which has roles in proliferation and invasion of cancer cells. SMAD4 is a protein that has a role in cellular processes including cell proliferation, invasion, and metastasis through many genes’ transcription. In this study, we aimed to assess the expression of ZNF703 and SMAD4 in PTC and evaluated the correlation between its expression, clinicopathological features of PTC cases, and prognostic parameters of patients to evaluate their roles in PTC progression. This is a retrospective study which included 40 cases with PTC. For immunohistochemistry, tissues stained their paraffin blocks with ZNF703 and SMAD4. We followed patients to detect disease progression and recurrence. Positive ZNF703 expression and negative SMAD4 expression were associated with higher incidence of CLNM, advanced stage and large tumor size, higher incidence of disease progression, recurrence, unfavorable PFS, and unfavorable OS rates. The higher ZNF703 expression and the lower SMAD4 expression were significantly increased in PTC patients with cervical LNM compared with those without. ZNF703 over expression and downregulation SMAD4 expression was significantly increased in PTC patients. Elevated expression of ZNF703 in tumor tissue with CLNM can be considered a predictive factor for the development of metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidence of malignant thyroid tumors becomes the 4th commonest cancer worldwide [1], and the 5th commonest cancer in Egypt [2]. Papillary thyroid carcinoma (PTC) forms the commonest histopathological subtype of these cancers [3]. Cells of PTC have a higher ability of lymphatic vessels penetration which lead to occurrence of cervical lymph node metastasis (CLNM) which was found in 12.3–64.1% of PTC patients at the time of disease diagnosis and worsening patients’ outcome [4]. Moreover, one of the most important causes of PTC progression is occurrence of distant metastasis [3].
Role of radiological evaluation CLNM assessment is limited and less sensitive [5]. It is important to detect novel predictive biomarkers of CLNM and distant metastasis in PTC to help the surgeons in making early decision of performing central lymph node dissection and aggressive management strategies in selected high-risk patients, thus improving their prognosis [3, 4].
Zinc finger protein 703 (ZNF703) is a member of the neutrophil extracellular trap (NET) transcription factors family which has roles in regulation of many developmental processes [1]. Abnormal ZNF703 expression was found in many malignant tumors and it was incriminated in occurrence of nodal metastasis and tumor progression [6, 7].
SMAD4 is a protein that has a role in controlling cell cycle and apoptosis through many genes transcription [8]. SMAD4 protein has tumor suppressor roles in many malignant tumors and loss of its function is associated with their progression [9,10,11].
ZNF703 was related to proliferation, apoptosis, and invasion of PTC cells by controlling levels of E2F1, MMP9, and p27 expression in PTC cells [1].
SMAD4 expression in thyroid cells increased TGF-β signaling, resulting in mesenchymal conversion, E-cadherin loss, and EMT [8, 9].
However, detailed roles of ZNF703 and SMAD4 expression in PTC are unclear. Their predictive roles in lymph node metastasis and association with disease recurrence, progression, and patient’s survival have not been reported yet.
In this study, we aimed to assess the expression of ZNF703 and SMAD4 in PTC and evaluated the correlation between its expression, clinicopathological features of PTC cases, and prognostic parameters of patients to evaluate their roles in PTC progression (Figs. 1 and 2).
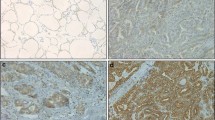
Immunohistochemical expression of ZNF703 in papillary thyroid carcinoma (PTC) tissues. A Strong diffuse nuclear expression in high-grade conventional PTC with positive cervical lymph node metastases × 400. B Moderate diffuse nuclear expression in high-grade PTC (follicular variant) with positive cervical lymph nodes metastases × 400. C Weak nuclear expression in low-grade PTC (follicular variant) with negative cervical lymph nodes metastases × 400. D Negative nuclear expression in low-grade conventional PTC with negative cervical lymph nodes metastases × 400
Immunohistochemical expression of SMAD4 in papillary thyroid carcinoma (PTC) tissues. A Diffuse positive nuclear expression in adjacent non-neoplastic thyroid tissues to PTC × 400. B Moderate diffuse nuclear expression in low-grade PTC (follicular variant) with positive cervical lymph nodes metastases × 400. C Negative nuclear expression in high-grade conventional PTC with positive cervical lymph nodes metastases × 400
Material and Methods
This is a retrospective study which included 40 paraffin blocks with PTC. Patients were surgically operated in the Department of General Surgery, Faculty of Medicine, Zagazig University Hospitals.
Performed surgical procedures:
Total thyroidectomy was performed in cases of clinical absence of cervical lymph nodes metastases, subtotal thyroidectomy was performed in cases with small-sized tumors and micro-carcinoma while total thyroidectomy with block neck dissection was performed in cases with clinical evidence of cervical lymph nodes metastases.
Surgically removed tissues were sent to Pathology Department, Faculty of Medicine, Zagazig University, where they have been processed, diagnosed, subtyped, and staged with other clinicopathological features (Table 1). Follow-up and survival date were collected from Clinical Oncology and Nuclear Medicine Department, Faculty of Medicine, Zagazig University (Table 2). The study was performed in the period from July 2015 to July 2020. The mean follow-up of cases is 30 months ranging from 10 to 40. The study was approved by the Ethical Committee of Faculty of Medicine, Zagazig University.
Inclusion Criteria
Cases with a sure diagnosis of PTC, cases with complete clinical and follow-up data, cases with sufficiency histopathological specimens in the paraffin blocks.
Histopathology slides have been reviewed by two pathologists.
Exclusion Criteria
Cases with doubtful PTC diagnosis or with other histopathological types of thyroid malignancy and cases with incomplete data were excluded.
Included cases are classified according to WHO guidelines [12].
Immunohistochemistry
For immunohistochemistry, we used streptavidin–biotin technique as previously detailed [13]. Slides are with primary monoclonal rabbit anti-ZNF703 antibody (1:50, ABCAM Technology) and primary monoclonal mouse anti-SMAD4 antibody (1:100, Santa Cruz Biotechnology).
Evaluation of ZNF703 and SMAD4 Expression in the Studied Samples
We evaluated both ZNF703 and SMAD4 expression in the nuclei of PTC cells.
We evaluated their expression by combined assessment of the stain intensity and extent in tumor cells.
Stain extent was classified into four classes: 0 if the markers were found in ≤ 10% of nuclei of the tumor cells, 1 if the markers were found in 11–30% of nuclei of the tumor cells, 2 if the markers were found in 31–50% of nuclei of the tumor cells, and 3 if the markers were found in > 50% of nuclei of the tumor cells. Stain intensity was classified into three classes: 0 if no stain was detected; 1 in the case of faint weak stain, 2 in the case of moderate yellowish-brown stain, and 3 in the case of strong brown stain.
To reach the final stain score of both ZNF703 and SMAD4 expression, we evaluate the sum of scores of intensity and extent, reaching values from 0 to 7 and to make statistical analysis, we consider 2 as the cut point where values ≥ 2 were considered positive expression while values below 2 were considered negative for both markers [1, 8].
Sections from adenocarcinoma of the colon and from normal colonic mucosa were used as positive control for ZNF703 and SMAD4 respectively. Negative controls were done by removal of the primary antibodies replacing them by phosphate-buffered saline.
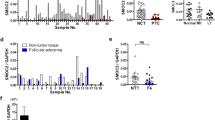
We focused on relations of ZNF703 and SMAD4 expression, pathological findings, disease progression, recurrence, progression-free survival, and overall survival rates (Figs. 3 and 4).
Statistical Analysis
Statistical analysis was performed using the SPSS program (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as the medians with interquartile ranges and categorical variables as numbers with percentages. The Wilcoxon signed-rank test and Fisher’s exact test were used to evaluate the clinicopathological variables associated with marker expressions. Cox regression analyses were used to quantify the independent risk factors for progression of PTC cases. All p values were two sided. A p value of < 0.05 was considered significant.
Results
Expression of ZNF703 in Cells of PTC and Association with Clinicopathological Findings (Table 1)
Positive ZNF703 expression was positively associated with higher incidence of cervical LNM (P < 0.001), advanced stage (P < 0.001), and tumor diameter (P = 0.011).
We found no significant associations between ZNF703 expression, patients’ age or gender, or PTC histopathological subtype.
Expression of ZNF703 in Cells of PTC and Association with Prognostic and Follow-up Findings (Tables 2, 3)
Positive ZNF703 expression was positively associated with higher incidence of disease progression, recurrence, unfavorable PFS, and unfavorable OS rates (P = 0.01).
Expression of SMAD4 in Cells of PTC and Association with Clinicopathological Findings (Table 1)
Positive SMAD4 expression was negatively associated with higher incidence of cervical LNM (P = 0.008), advanced stage (P = 0.04), and tumor diameter (P = 0.014).
We found no significant association between SMAD4 expression, patients’ age or gender, or PTC histopathological subtype.
Expression of SMAD4 in Cells of PTC and Association with Prognostic and Follow-up Findings (Table 2, 3)
Positive SMAD4 expression was negatively associated with; higher incidence of disease progression, recurrence, unfavorable PFS, and unfavorable OS rates (P = 0.03).
Univariate and Multivariate Cox Regression Analyses of Different Prognostic Factors for Disease-Free Survival (Table 5)
Univariate analysis showed that lymph node involvement (P = 0.019), tumor size (p = 0.02), higher expressions of ZNF703 (p = 0.02), downregulation SMAD4 (P = 0.05), and advanced stage (P = 0.001) were the risk factors for progression in patients with PTC, while multivariate analysis revealed that higher ZNF 703 expression and LN metastasis were the only independent risk factors for progression in patients with PTC (P = 0.048).
Discussion
Detecting accurate and sensitive predictive factors for cervical LNM is a very essential aim in improving management of PTC and patient’s outcome, as most of established prognostic clinicopathological parameters were not found to be accurate predictors of cervical LNM (Table 4) [14].
The recently described oncogene; ZNF703, is a transcription factor which regulated many genes expression; those genes have roles in transcriptional regulation, modulation of stem cells, proliferation, and invasion of cancer cells [15].
In the current study, we assessed the expression of ZNF703 in PTC cases that showed high positive expression was positively associated with unfavorable pathological, prognostic parameters of patients. Our results were in agreement with [1] which suggested that increased ZNF703 expression is associated with disease progression and poor prognosis of PTC (Table 5).
Moreover, our results are consistent with results of [16], who demonstrated that high expression of ZNF703 was positively associated with unfavorable pathological parameters of PTC as large tumor size and positive lymph nodes status.
Additionally, increased expression of ZNF703 was found to be related to bad clinical, pathological, and prognostic parameters of many tumors as in breast cancer [17], gastric cancer [18], and cholangiocarcinoma [19]. All these results collectively showed that ZNF703 expression has transforming and oncogenic roles in many tumors [9]
Wang et al. [15] explained the role of ZNF703 in oral squamous cell carcinoma progression by that it promotes proliferation, invasion, and metastasis of malignant cells through activation of PI3K/AKT/GSK‑3β signaling pathway.
Moreover, ZNF703 inhibits transforming growth factor beta (TGF-β) receptor activity, decreased E-cadherin expression, and increased expression of pro-migratory P120 catenin, which lead to increasing cell proliferation and decreasing intercellular adhesion which lead to malignant invasion and metastasis [20].
Furthermore, high expression of ZNF703 altered phosphorylation of retinoblastoma-associated protein (RB1), decreased P27kip1 protein expression, and upregulating E2F1 and cyclin E1 expression which induces cells to escape from restriction point in the G1 phase increasing cell proliferation [20].
To clarify roles of ZNF703 in PTC progression, we assessed the expression of SMAD4 protein that plays an essential role in TGF-β signal transduction and was found to be related to cell proliferation and migration [21].
We showed that SMAD4 was downregulated in PTC tissues and its low expression was associated with unfavorable pathological parameters and dismal outcome which was consistent with results of [22]. Our results observed low SMAD4 expression was found in PTC patients with cervical LNM in comparison with those with negative cervical LNM.
Ormanns et al. [23] reported similar results in pancreatic cancer that loss of SMAD4 expression was related to poor prognosis and it is deleted in most cases of pancreatic cancer and called DPC4 (deleted in pancreatic cancer).
SMAD4 loss leads to inactivation of TGF-β signaling pathway and mediates malignant invasion [20] and increase rate of growth of PTC cancer cells [24]. Mutations of SMAD4 were frequently detected in PTC [25].
We found a significant positive correlation between the high ZNF703 expression, low SMAD4 expression, and cases of PTC with positive CLNM than those without. So, our results provide strong evidences for a critical role of ZNF703 and SMAD4 in mediating pathway in lymph node metastasis of PTC (Table 6). Thus, ZNF307 and SMAD4 might be beneficial in prediction of cervical LNM and progression in PTC [25].
Conclusion
We concluded that the overexpression of ZNF703 and lower expression of SMAD4 are significantly associated with progression, CLNM, and poor prognosis in PTC patients mainly through impairment of the TGF-β-SMAD-dependent pathway. Thus, ZNF703 and SMAD4 proteins may be considered potential therapeutic targets for metastatic PTC.
Limitations of the Study
The current study included little number of patients as it was performed in a single center and we used only a single method for assessment of tissue protein expression of ZNF703 and SMAD4 in PTC which is immunohistochemistry.
Recommendations
Future reports are needed to further investigate levels of ZNF703 and SMAD4 by mRNA analysis and a western blot for a more accurate evaluation of their roles. Moreover, we recommend evaluation of marker expression on larger numbers of patients.
References
Yang X, Liu G, Zang L et al (2020) ZNF703 is overexpressed in papillary thyroid carcinoma tissues and mediates K1 cell proliferation. Pathol Oncol Res 26:355–364
Gohar MG, Mohamed A E, Al-Azzouny H A. Risk of hypocalcemia after total thyroidectomy and bilateral central neck dissection in patients with well differentiated thyroid carcinoma, Al-Azhar International Medical Journal. 2020; 6(1),4:139–143
Wang Q, Shanga J, Zhanga Y et al (2020) MiR-451a restrains the growth and metastatic phenotypes of papillary thyroid carcinoma cells via inhibiting ZEB1. Biomedicine & Pharmacotherapy 127:109901
Cheng X, Xu S, Pan J et al (2019) MKL1 overexpression predicts poor prognosis in patients with papillary thyroid cancer and promotes nodal metastasis. Journal of Cell Science 132:231399
Lee Y, Kim J-H, Baek JH et al (2018) Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck 40:2137–2148
Li K, Wang J, Han J et al (2016) Overexpression of ZNF703 facilitates tumorigenesis and predicts unfavorable prognosis in patients with cholangiocarcinoma. Oncotarget 7:76108–76117
Hao W, Xubin D, Jinshan Z et al (2017) Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signaling in oral squamous cell carcinoma. Cell Physiol Biochem 44:920–934
Ritterhouse LL, Wu EY, Kim WG et al (2019) Loss of SMAD4 protein expression in gastrointestinal and extra-gastrointestinal carcinomas. Histopathology 75:546–551
Singhi AD, Foxwell TJ, Nason K et al (2015) Smad4 loss in esophageal adenocarcinoma is associated with an increased propensity for disease recurrence and poor survival. Am J Surg Pathol 39:487–495
Kozak MM, von Eyben R, Pai J et al (2015) Smad4 inactivation predicts for worse prognosis and response to fluorouracil-based treatment in colorectal cancer. J Clin Pathol 68:341–345
Davison JM, Hartman DA, Singhi AD et al (2014) Loss of SMAD4 protein expression is associated with high tumor grade and poor prognosis in disseminated appendiceal mucinous neoplasms. Am J Surg Pathol 38:583–592
DeLellis RA, Lloyd RV, Heitz PU et al (2004) Pathology and genetics of tumors of endocrine organs World Held Organization Clasifications of tumors. IARC press, Lyon
Metgud R, Astekar MS, Soni A et al (2013) Conventional xylene and xylene-free methods for routine histopathological preparation of tissue sections. Biotech Histochem 88:235–241
Liu W, Cheng R, Su Y et al (2017) Risk factors of central lymph node metastasis of papillary thyroid carcinoma: a single-center retrospective analysis of 3273 cases. Medicine 96:8365
Wang H, Deng X, Zhang J et al (2017) Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signaling in oral squamous cell carcinoma. Cell Physiol Biochem 44(3):920–934
Zhang X, Liu L, Deng X et al (2019) MicroRNA 483–3p targets Pard3 to potentiate TGF-β1-induced cell migration, invasion, and epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Oncogene 38(5):699–715
Zhang X, Mu X, Huang O et al (2013) Luminal breast cancer cell lines overexpressing ZNF703 are resistant to tamoxifen 17 through activation of Akt/mTOR signaling. PLoS One 8:72053
Yang G, Ma F, Zhong M et al (2014) ZNF703 acts as an oncogene that promotes progression in gastric cancer. Oncol Rep 31:1877–1882
Du X, Li Q, Yang L et al (2020) SMAD4 activates Wnt signaling pathway to inhibit granulosa cell apoptosis. Cell Death Dis 11:373
Li T, Zhao N, Lu J et al (2019) Epigallocatechin gallate (EGCG) suppresses epithelial-mesenchymal transition(EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways. Bioengineered 10(1):282–291
Ivanova K, Manolova I, Ignatova MM et al (2018) Immunohistochemical expression of TGF-Β1, SMAD4, SMAD7, TGFβRII and CD68-Positive TAM densities in papillary thyroid cancer. Open Access Maced J Med Sci. 6(3):435–441
Ormanns S, Haas M, Remold A et al (2017) The impact of SMAD4 loss on outcome in patients with advanced pancreatic cancer treated with systemic chemotherapy. Int J Mol Sci 18(5):1094
Xu CB, Liu SB, Li JQ et al (2019) MicroRNA-539 functions as a tumor suppressor in papillary thyroid carcinoma via the transforming growth factor β1/ Smads signaling pathway by targeting secretory leukocyte protease inhibitor. J Cell Biochem. 120:10830–10846
Ullah I, Sun W, Tang L et al (2018) Roles of Smads family and alternative splicing variants of Smad4 in different cancers. J Cancer 9(21):4018–4028
Rendueles AR, Rodrigues JS, Garcia-Rendueles MER et al (2017) Rewiring of the apoptotic TGF-beta-SMAD/NF kappa B pathway through an oncogenic function of p27 in human papillary thyroid cancer. Oncogene 36:652–666
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, A.H., Harb, O.A., Shorbagy, S.E. et al. Prognostic Roles of ZNF703 and SMAD4 Expression in Patients with Papillary Thyroid Cancer and Association with Nodal Metastasis. Indian J Surg Oncol 13, 169–177 (2022). https://doi.org/10.1007/s13193-022-01519-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-022-01519-5