Abstract
Current and emerging biomarkers in primary head and neck carcinoma have diagnostic, prognostic, and predictive value. Following histomorphological evaluation, immunohistochemical markers, and molecular methods may help to differentiate between numerous tumor types. Phenotypic markers which are related with squamous and neuroendocrine differentiation or viral infections are helpful in upper aerodigestive tract carcinomas. Additionally, recently described molecular alterations, such as NUT translocation or SMARCB1 and IDH2 mutations, aid in classifying them better. The prognostic importance of high-risk human papillomavirus positivity which is prominent in the oropharynx and is possible in some other sites in head and neck and the diagnostic utility of the detection of latent Epstein-Barr virus infection in nasopharyngeal carcinoma or lymphoepithelial carcinoma is notified. Immunohistochemical markers together with certain molecular alterations, especially specific translocations in salivary glands, are helpful for overcoming diagnostic difficulties and for increasingly guiding the utilization of targeted therapies. Hereby, this chapter reviews the latest developments in biomarkers in primary head and neck carcinomas with a focus on distinctive phenotypic and molecular aspects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Head and neck region is a complex area and consists of various organs and tissues. Primary carcinomas may arise from numerous tissues including mucosal surfaces, salivary glands, seromucous glands, and odontogenic tissues. Squamous cell carcinomas (SCC) constitute the vast majority of carcinomas in head and neck region except for salivary glands, followed by neuroendocrine carcinomas and undifferentiated carcinomas which are observed rarely. Squamous cell carcinomas may have distinct characteristics by locations in head and neck, such as high-risk human papilloma virus (HPV) positivity in oropharyngeal SCC associated with favorable prognostic, and latent Epstein-Barr virus (EBV) infection is mostly related with nasopharyngeal SCC. Salivary gland tumors consist of various types of tumors which frequently have overlapping histological features. Genomic alterations, especially specific chromosomal rearrangements provide new insights into the pathogenesis of salivary gland neoplasms.
Histochemical, immunohistochemical, and molecular biomarkers mainly help as diagnostic tools in head and neck carcinoma. The prognostic and predictive importance of biomarkers is also increasing, and targeted therapy approaches in several cancers are gradually increasing.
4.1 Nasal Cavity, Paranasal Sinuses and Skull Base
4.1.1 Squamous Cell Carcinoma
Squamous cell carcinomas (SCC) constitute the majority of malignancies in the nasal cavity, paranasal sinuses, and skull bases. However, it is observed at a lower ratio (60–75%) when compared with SCC frequency in other head and neck sites. They generally present with nonspecific symptoms so that the majority of sinonasal SCCs present at an advanced stage and the prognosis is mostly poor. Tobacco; industrial exposures, such as leather dust, wood dust, and solvents; sinonasal papillomas; and high-risk HPV infection are risk factors for malignant transformation. Among them, the incidence of high-risk HPV-driven tumors has increased over the past 3 decades [1].
SCC has keratinizing, non-keratinizing, spindle cell, papillary, and basaloid subtypes. Keratinizing SCC is the most common sinonasal SCC type which exhibits histological features with irregular cords and nests of eosinophilic tumor cells demonstrating variable degrees of keratinization and stromal reaction. Non-keratinizing SCC consists of anastomosing ribbons and nest of tumor cells with minimal or no evidence of keratinization or stromal desmoplasia.
4.1.1.1 Diagnostic Biomarkers
SCC is positive for p63, p40 and high-molecular-weight cytokeratins, such as CK5/6.
High-risk HPV positivity is detected in 9–32% of sinonasal SCCs (Table 4.1). HPV16 is the most common type, and less commonly HPV18, 31, 33 and rarely HPV35, 39, 45, and 82 are identified. Non-keratinizing, papillary, or basaloid histologic subtypes display a higher rate of HPV positivity. SCC in nasal cavity demonstrates slightly more HPV positivity than tumors in sinuses [1,2,3,4,5,6,7,8,9].
Nuclear p16 immunoexpression, frequently associated with cytoplasmic positivity in more than 70% of tumor cells, is seen in nearly 15% of sinonasal SCCs and is strongly associated with transcriptionally active high-risk HPV infection. In sinonasal SCC, the specificity and sensitivity of nuclear p16 expression in determining HPV positivity are 90–94% and 67–100%, respectively. Immunoexpression of p16 may be used as a reliable surrogate biomarker for demonstrating high-risk HPV status; nonetheless it should be noted that it has relatively weak positive predictive value and not yet approved by World Health Organization for sinonasal SCCs [2,3,4, 10].
HPV-negative SCCs are often associated with TP53 mutation and loss of CDKN2A/B, while HPV-positive SCCs mostly contain PIK3CA and PTEN gene alterations [11]. EGFR copy number gains are detected in up to half of sinonasal SCCs and mostly correlate with EGFR overexpression [2, 12]. High-risk HPV positivity and EGFR copy number gains are mutually exclusive. FGFR1 and SOX2 amplifications are also identified in a small subgroup of sinonasal SCC [9].
Oncocytic sinonasal papilloma-associated SCC often harbors KRAS mutations in codon 12, whereas inverted sinonasal papilloma-associated SCC contains EGFR mutations, mainly exon 20 insertions and occasionally exon 19 mutations [13,14,15,16].
4.1.1.2 Prognostic/Predictive Biomarkers
HPV positivity and p16 expression are favorable prognostic factors in sinonasal SCCs. HPV-positive SCC is mostly seen in patients who are younger and less likely to have a smoking history [2, 5, 6, 10, 17]. Routine HPV testing, as currently is recommended for oropharyngeal tumors, might be warranted in individuals with sinonasal SCC as well.
EGFR mutations may predict a response to therapy with EGFR tyrosine kinase inhibitors (TKI), while KRAS mutations are negative predictor of response to EGFR TKI. TKI treatment may become a potential option against sinonasal SCCs with EGFR gene alterations. However, inverted sinonasal papilloma-associated SCC displays mainly EGFR exon 20 mutations which are associated with reduced sensitivity to EGFR TKI [18].
Immune checkpoint inhibitors are approved by FDA as first-line treatment together with chemotherapy for advanced HNSCCs and monotherapy for HNSCCs which express PD-L1 with a combined positive score ≥ 1. Combined positive score is defined as the ratio of the number of PD-L1 positive cells including tumor and immune cells to the number of total tumor cells [19]. The membranous PD-L1 immunoexpression (>5%) is detected in 34% of sinonasal SCC tumor cells and 45% of non-tumoral immune cells. HPV-positive tumors display higher PD-L1 positivity than HPV-negative tumors, while expression of PD-L1 seems to be of no prognostic importance [20].
4.1.2 Lymphoepithelial Carcinoma
Lymphoepithelial carcinoma (LEC) is a squamous cell carcinoma variant which histomorphologically resembles non-keratinizing nasopharyngeal carcinoma, undifferentiated subtype (Fig. 4.1) [1].
4.1.2.1 Diagnostic Biomarkers
LECs in sinonasal region are usually positive for EBV-encoded small RNA (EBER) by in situ hybridization. LEC is diffusely positive for pancytokeratin and squamous markers like CK5/6, p63, and p40.
4.1.3 NUT Carcinoma
NUT (nuclear protein in testis) carcinoma is an uncommon and aggressive midline tumor. Sinonasal region is the most common localization of these tumors in head and neck. Histological features of NUT carcinoma include poorly differentiated morphology with nests of monotonous tumor cells and “abrupt” keratinization focus [1].
4.1.3.1 Diagnostic Biomarkers
NUT carcinoma is often positive for p40, p63, and CK5/6 and occasionally positive for CD34, neuroendocrine markers, p16, or TTF-1.
NUT carcinoma is characterized by NUTM1 gene translocations. The fusion partner is mostly BRD4 gene (70%) and occasionally other BET family genes such as BRD3, NSD3, and ZNF532. These alterations can be detected by molecular methods including ISH, PCR, or NGS approaches. It can be confirmed with >50% nuclear NUT expression by immunohistochemistry which has a specificity of 100% and sensitivity of 87% and accepted as a diagnostic ancillary test. Focal NUT expression (less than 50% of tumor cells) can be observed in germ cell tumors or poorly differentiated carcinomas [21].
4.1.3.2 Prognostic/Predictive Biomarkers
Several studies indicate that tumors with NUTM1-BRD4 translocation have worse clinical course than tumors with the fusion of NUTM1 and non-BRD4 partner genes [21]. Accordingly, molecular tests which reveal fusion partner of NUTM1 gene may provide prognostic information [21].
Targeting chimeric fusion protein with BET inhibitors (extra-terminal bromodomain), histone deacetylase inhibitors, or p300/CBP inhibitors represents potential but not fully understood therapeutic approaches in NUT carcinoma [22,23,24].
4.1.4 Sinonasal Undifferentiated Carcinoma
Sinonasal undifferentiated carcinoma (SNUC) is defined as an undifferentiated carcinoma without glandular or squamous features. SNUC is characterized by high-grade undifferentiated cells without viral etiologies. Focal neuroendocrine differentiation may be observed in some of SNUCs. SNUC is typically thought as a diagnosis of exclusion; however, it has recently begun to be debated whether it is a distinct tumor type after certain molecular features of SNUC have been elucidated. IDH2-mutant sinonasal carcinoma is mostly described as a part of SNUC category, as SMARCB1-deficient sinonasal carcinoma and SMARCA4-deficient sinonasal carcinomas are emerging novel and distinct entities that are classified within the SNUC category for the present [25].
4.1.4.1 Diagnostic Biomarkers
Immunohistochemically, SNUC is positive for epithelial differentiation immunohistochemical biomarkers, such as PANCK and EMA, focally CK7 and CK8, but usually negative or occasionally positive for markers of any other specific squamous or neuroendocrine markers. These phenotypic features of SNUC can be confused with some high-grade mesenchymal tumors with focal cytokeratin expression, including Ewing family tumors, synovial sarcoma and alveolar rhabdomyosarcoma, and other sinonasal carcinomas with poorly differentiated/undifferentiated foci [25].
IDH2 R172X mutations, including R172S, R172T, R172M, and R172G, are detected in 55 to 82% of SNUCs which mostly display hypermethylator phenotype. IDH2 or IDH1/2 expression by immunohistochemistry is relatively specific to SNUC among head and neck carcinomas but has limited sensitivity, as IDH2 R172T mutations cannot be detected with this method [25,26,27,28,29]. TP53, CDKN2A/2B, KIT, MYC, SETD2, and SPEN mutations are detected in IDH2-mutant SNUCs [28]. A small part of SCC, large-cell neuroendocrine carcinoma, and poorly differentiated carcinomas also display IDH2 R172X mutation, but these tumors may represent phenotypic variants of the SNUC [28, 30]. Additionally, the metastasis of other IDH2-positive carcinomas such as solid papillary breast carcinoma with reverse polarity or intrahepatic cholangiocarcinoma may be considered in the differential diagnosis with IDH2-mutant SNUC despite histological dissimilarity [27].
4.1.4.2 Prognostic/Predictive Biomarkers
SNUCs with IDH2 mutation have a trend of better prognosis than those without mutation, but it has not been fully validated yet [28, 30]. Targeting IDH2 mutation is a potential treatment approach for SNUC patients. Promising results with IDH2 inhibitor are demonstrated in IDH2-mutated acute myeloid leukemia, and accordingly it may be predicted similar outcomes in SNUC as well; however, there are limited number of clinical trials on this subject yet [27].
4.1.5 Neuroendocrine Carcinoma
Sinonasal neuroendocrine carcinoma (SNEC) is a rare and aggressive malignancy which includes small-cell and large-cell sinonasal neuroendocrine carcinomas. Histological features of SNEC resemble neuroendocrine carcinomas in other sites such as lung.
4.1.5.1 Diagnostic Biomarkers
SNEC stains for cytokeratins, EMA, and at least one of the neuroendocrine markers including chromogranin, synaptophysin, CD56, and neuron-specific enolase; the latter two are less specific [31,32,33]. It has recently demonstrated that insulinoma-associated protein 1 (INSM1) expression is a promising biomarker for the diagnosis of NEC, especially differentiating it from SNUC [34,35,36]. SNEC may also express p16, ASCL1, occasionally p63, TTF-1, and calretinin [32].
Some small-cell neuroendocrine carcinomas harbor ARID1A mutation [28].
4.1.6 Adenocarcinoma
Sinonasal adenocarcinoma is a group of malignancy that includes intestinal-type and non-intestinal-type adenocarcinoma. Each tumor type displays distinct morphologic and immunohistochemical features. Intestinal-type adenocarcinoma (ITAC) is an aggressive neoplasm that is associated with industrial wood and leather dust exposures. ITAC is morphologically similar to primary intestinal adenocarcinoma. Non-intestinal-type sinonasal adenocarcinoma (non-ITAC) is a heterogeneous group of tumors including low-grade non-ITAC, high-grade non-ITAC, and recently proposed sinonasal renal cell carcinoma-like adenocarcinoma. Low-grade non-ITAC has papillary/tubular histomorphology and has particularly good prognosis, so that it is crucial to distinguish this type of tumor from others. High-grade non-ITAC is a group of histologically diverse tumors which mostly display high mitotic index, frequent necrosis, and solid pattern with moderate amounts of glandular structures.
4.1.6.1 Diagnostic Biomarkers
Intestinal-type adenocarcinoma is consistently stained with markers of intestinal differentiation, including cytokeratin 20, CDX2, villin, and MUC2.
TP53, KRAS, BRAF mutations, EGFR amplification, and loss of CDKN2A are detected in some intestinal-type adenocarcinomas [37].
4.1.7 Emerging Entities
4.1.7.1 HPV-Related Multiphenotypic Sinonasal Cancer
HPV-related multiphenotypic sinonasal cancer (HMSC) is a distinct entity which is previously termed as HPV-related carcinoma with adenoid cystic features and classified in non-keratinizing SCC. HMSC has indolent clinical behavior characterized by frequent local recurrences and rare distant metastasis, and disease-specific mortalities have not been reported yet [38]. HMSC displays high-grade histological features, basaloid cells including necrosis and high mitotic rates, and adenoid cystic carcinoma-like cribriform areas [39].
4.1.7.1.1 Diagnostic Biomarkers
HPV positivity is basically detected in all cases, and high incidence of HPV type 33 (%84) is more noticeable than others including HPV type 35, 16, 56, and 82 [38, 40]. p16 expression is also observed in majority of HMSCs. However, a small subset of solid type adenoid cystic carcinomas in sinonasal site may also express p16, without HPV RNA ISH positivity. Accordingly, it is not recommended that p16 expression alone can be used as a surrogate for HPV testing to distinguish sinonasal adenoid cystic carcinoma from HMSC [41]. Unlike the characteristics of adenoid cystic carcinomas, MYB/NFIB or MYBL1/NFIB fusions are also not expected to be present in HMSCs.
HMSC is positive for PANCK, CK7, SOX-10, and LEF-1, p40, and a subset demonstrates expression of SMA, CK5/6, SMMS-1, and S100 [40].
4.1.7.2 Renal Cell-like Adenocarcinoma
Renal cell-like adenocarcinoma is a variant of non-intestinal-type adenocarcinoma (non-ITAC) that has similar features to clear cell renal cell carcinoma. A vast majority of these tumors are low-grade and display good clinical prognosis, but a rare case with lymph node metastasis has been reported [42].
Renal cell-like adenocarcinoma stains for CK7, CAIX (carbonic anhydrase IX), and EGFR and less consistently stains for SOX10 and S100, but it is negative for CK20, renal cell carcinoma antigen (RCCag), vimentin, and PAX8 [42].
4.1.7.3 SMARCB1-Deficient Sinonasal Carcinomas
SMARCB1-deficient sinonasal carcinoma is an uncommon and aggressive carcinoma which mostly involves paranasal sinuses and displays poorly differentiated histomorphology with monomorphic/basaloid cells. Tumor cells variably express p16, p40, CK5/6, synaptophysin, and chromogranin. Loss of nuclear expression of SMARCB1 (INI1) in tumor cells is useful for diagnosis [43, 44] (Fig. 4.2). A subset of sinonasal non-ITAC, renal medullary carcinoma and a subset of myoepithelial carcinoma of the soft tissue also display loss of SMARCB1 [45].
4.1.7.4 SMARCA4-Deficient Sinonasal Carcinoma
SMARCA4-deficient sinonasal carcinoma is a very rare poorly differentiated tumor in sinonasal region. The tumor consists of high-grade basaloid or epithelioid cells in nested and solid pattern with frequent necrosis. SMARCA4-deficient sinonasal carcinoma is positive for PANCK, focally neuroendocrine markers, and negative for CK5/6, p63, and p16. A distinguishing feature of these tumors is the loss of nuclear immunoexpression for SMARCA4 (BRG1), but it has been recently reported that majority of sinonasal teratocarcinosarcomas also show the loss of SMARCA4 [46,47,48].
4.1.8 Salivary Gland-Type Tumors of the Seromucous Glands
Minor salivary gland carcinomas of the sinonasal cavity are rare cancers. Adenoid cystic carcinoma, mucoepidermoid carcinoma, and polymorphous adenocarcinoma comprise the large majority of sinonasal malignant salivary gland tumors [49].
4.1.9 Metastatic Tumors to the Nasal Cavity
The sinonasal region is rare location for metastases. Metastatic tumors mostly originate from the kidney, breast, thyroid, and prostate. Renal cell-like adenocarcinoma may be confused with metastatic renal cell carcinoma, but the latter is positive for RCC-ab and PAX8 (Fig. 4.3). Differentiating ITAC from metastatic adenocarcinoma of the colon mostly depends on clinical history, colonoscopy, or imaging modalities. Colonic adenocarcinoma cases have lower expressions of CK7, MUC5, and chromogranin, and this feature may be helpful in differential diagnosis. Metastatic thyroid carcinoma expresses TTF-1, PAX8, and thyroglobulin, and metastatic prostatic carcinoma expresses PSA, PAP, EpCam, NKX3.1, and prostein. Metastatic breast carcinomas are positive for estrogen receptor, progesterone receptor, HER2, mammaglobin, gross cystic disease fluid protein-15 (GCDFP-15), and GATA-3. However, these biomarkers are also positive in salivary gland ductal carcinoma, a rare primary tumor at this location, and mostly expresses AR and Her2 [50]. Differential diagnosis from salivary gland-type tumors should be kept in mind; biomarkers of myoepithelial differentiation and GATA-3 positivity are in favor of primary sinonasal tumors [51,52,53,54,55].
4.2 Nasopharynx
4.2.1 Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma (NPC) is a malignant tumor arising from the surface mucosa of nasopharynx associated with frequently associated with EBV infection. NPC has a distinctive ethnic and geographic distribution with a particularly high prevalence in the Southern China, Southeast Asia, North Africa, and Arctic. Host genetic susceptibility genes (HLA-A*A2, B*46, B*17, A*0207 alleles and CYP2E1, CYP2A6, GSTM1, GSTT1, OGG1, XRCC1 gene polymorphisms, etc.), consumption of tobacco, alcohol, and salted-fermented foods are likely contributing factors for the disease.
Three histological variants of NPC encompass keratinizing squamous, non-keratinizing, and basaloid squamous. Keratinizing NPC shows evidence of keratinocytic differentiation in the form of intercellular junctions and variable keratin. Non-keratinizing NPC histologically consists of malignant cells that form syncytial or cohesive arranged irregular nests and are mixed with variable reactive lymphoplasmacytic infiltrate. Non-keratinizing NPC can exhibit differentiated and undifferentiated morphology. Other rare variant is basaloid type NPC that is histologically identical to other basaloid SCCs in other head and neck sites.
Non-keratinizing NPC is more common in endemic regions and closely related to EBV infection. EBV infection can be latent or lytic. In latent infection, EBV genome forms episomes and expresses EBV latent genes including LMP-1, LMP-2, EBNA1, EBNA2, EBERs, BARTs, and miR-BARTs. These genes are effectors of EBV-mediated malignant transformation and dysregulate mainly WNT/β-catenin, JAK/STAT, PI3K/Akt/mTOR, EGFR, MAPK, and NF-κB signaling pathways [56].
4.2.1.1 Diagnostic Biomarkers
Immunohistochemically, NPC expresses PANCK, p63, CK5/6, CK8, and CK19, and NPC is negative for CK7, CK14, and CK20 [57]. Undifferentiated morphology may require differential diagnosis with lymphoma, malignant melanoma, and neuroendocrine carcinoma leading to a larger panel of biomarkers. Loss of cytokeratin expression may be observed, and other epithelial markers should be applied. EBV infection is nearly detected in all non-keratinizing NPCs. Nuclear reaction for EBV EBER by in situ hybridization is the most reliable way to display EBV infection. Detecting LMP-1 expression by immunohistochemistry is also other method for it, but it has relatively low sensitivity and can detect only 50–80% of EBV infections in NPC [58]. High-risk HPV positivity is also detected in up to 10% of NPCs [59, 60].
Circulating cell-free EBV DNA in plasma is a useful tool for screening population for early asymptomatic NPC in endemic regions and for the early detection of tumor recurrence [61, 62].
4.2.1.2 Prognostic/Predictive Biomarkers
EBV infection in NPC is proposed as a favorable prognostic factor, although there are controversial results about the prognostic importance of EBV or HPV infections [59, 63, 64]. Several strategies targeting EBV are being developed such as DNAzymes against LMP-1 mRNA, adenovirus-based adoptive immunotherapy, and LMP-1-specific autologous CTLs [65]. Detectable circulating cell-free EBV DNA in plasma is also an indicator for unfavorable response to chemoradiotherapy/radiotherapy [66].
Nasopharyngeal carcinoma, especially undifferentiated non-keratinizing EBV positive type, is characterized by high PD-L1 expression in up to 90% of tumor cells and abundant infiltration of stromal lymphocytes that render it an attractive target for immunotherapy. Accordingly, in several early trials, immune checkpoint blockade therapies have showed promising clinical results, and phase III trials are still ongoing [67]. Thus far, the correlation between PD-L1 expression in tumor or immune cells and response rate to anti-PD-1 antibodies has not been found [68]. On the other hand, several studies show that high PD-L1 expression is associated with poor prognosis in NPC [69,70,71].
4.2.2 Nasopharyngeal Papillary Adenocarcinoma
Nasopharyngeal papillary adenocarcinoma (NPPA) is a low-grade adenocarcinoma which is histologically characterized by papillary structures and cellular features akin to seen in papillary carcinoma of thyroid gland.
4.2.2.1 Diagnostic Biomarkers
NPPA stains for EMA, CK5/6, CK7, and vimentin. A subset of NPPA which expresses TTF-1 and CK19 is called as thyroid-like low-grade NPAC (Fig. 4.4). These tumors are negative for EBER, thyroglobulin, and CK20 and do not harbor BRAF mutations [72].
4.2.3 Metastatic Tumors to the Nasopharynx
Metastatic carcinomas in the nasopharynx are quite rare. The metastasis of the lung, breast, and kidney carcinomas is reported [73,74,75].
4.3 Larynx, Hypopharynx, and Oral Cavity
4.3.1 Squamous Cell Carcinoma
Larynx, hypopharynx, and oral cavity are the most common locations for squamous cell carcinomas in head and neck area. Cigarette and alcohol are main risk factors for development of SCC. Other risk factors include gastroesophageal reflux, diet, social-economic status, poor-fitting oral dentures, and betel chewing in certain geographic regions. Transcriptionally active high-risk HPV infection is an etiologic factor in some of the SCC at these sites, but the frequency is less than oropharyngeal region [76, 77].
The Cancer Genome Atlas Network study demonstrated that head and neck SCCs which are mainly located in oral cavity, oropharynx, hypopharynx, and laryngeal sites are associated with distinct molecular backgrounds. Human papilloma virus-associated SCCs are mostly located in oropharynx and display PIK3CA mutation, the loss of TRAF3, and E2F1 amplification. Smoking-related SCCs contain TP53 inactivating mutation, CDKN2A inactivation, and 3q26/28 and 11q13/2 amplifications. A subset of oral cavity SCCs that demonstrate HRAS or PIK3CA activating mutations, CASP8, NOTCH1, and TP53 inactivating mutations is associated with favorable clinical outcome. A subset of SCCs that are mostly at laryngeal sites displays NSD1 (chromatin modifier), AJUBA, and FAT1 (WNT pathway genes) loss-of-function alterations, and NFE2L2-activating mutations [78].
4.3.1.1 Diagnostic Biomarkers
Transcriptionally active high-risk HPV infection is identified in 4–15% of laryngeal SCCs, of 5% of hypopharyngeal SCCs, and 5–15% of oral cavity SCCs [76, 79,80,81,82]. While most of the HPV-positive SCCs in larynx, hypopharynx, and oral cavity share histologic features of oropharyngeal HPV-positive SCCs, a quarter of these tumors are recently called as warty variant SCCs which display exophytic growth, vigorous hyperkeratosis/parakeratosis, koilocytic atypia, and prominent nuclear pleomorphism. Warty variant SCC is similar to anogenital HPV-positive SCC and associated with good prognosis [83].
P16 expression is detected in 13–20% of laryngeal SCCs, 16–21% of hypopharyngeal SCCs, and 6–26% of oral cavity SCCs and is not well correlated with HPV status [82, 84, 85].
Laryngeal SCCs mainly harbor NSD1, AJUBA, FAT1, and NFE2L2 mutations [86].
4.3.1.2 Prognostic/Predictive Biomarkers
The prognostic role of high-risk HPV infection status or p16 expression has not fully been established in SCCs of larynx, hypopharynx, and oral cavity, despite that several studies show that either high-risk HPV positivity or p16 expression is related with improved prognosis [79, 80, 84, 85, 87,88,89].
A subgroup of oral cavity SCCs which display CASP8, NOTCH1, and TP53 mutations together with HRAS or PIK3CA mutations and infrequent copy number alterations demonstrates improved clinical courses [86]. In early glottic SCC, it has been reported that EGFR expression is an unfavorable prognostic factor [90].
Positive PD-L1 immunohistochemical expression defined by ≥1% tumor proportion score or ≥ 1 combined positive score renders head and neck SCCs potentially suitable for immunotherapies [19].
4.3.2 Neuroendocrine Carcinoma
The most common locations of neuroendocrine carcinomas in head and neck are the larynx, hypopharynx, and oral cavity [91]. Well-differentiated, moderately differentiated, and poorly differentiated neuroendocrine carcinomas are classified according to nuclear atypia of tumor cells and mitotic index.
4.3.2.1 Diagnostic Biomarkers
These tumors stain for cytokeratins, EMA, and neuroendocrine markers (e.g., chromogranin, synaptophysin, or CD56) and variably stain for TTF-1. Ki-67 proliferating index is not in routine for grading of neuroendocrine tumors of the head and neck region.
4.3.3 Metastatic Tumors to the Larynx, Hypopharynx, and Oral Cavity
Metastatic tumors which generally originate from the lung, breast, kidney, and skin may present in oral cavity, hypopharynx, and larynx. The gingiva and tongue are the most common locations for metastasis [92, 93].
The positivity of epithelial squamous cell differentiation markers, CK5/6, p40, p63, CK8, CK13, CK19, and EMA, is very frequent; besides CK4, CK7, CK10, CK14, P16, and NUT expressions may be observed in SCCs of the head and neck region [94]. These biomarkers are frequently positive in SCC of other sites, and in case of tumors without intramucosal carcinoma component rising suspicion of metastatic carcinomas, the biomarkers are not helpful about the primary site.
Minor salivary glands and seromucous glands give rise to salivary gland-type carcinomas. Oral cavity and oropharynx are the most frequent sites for this type of tumor if major salivary glands are excluded. p63 expression is an expected finding in salivary gland carcinomas with myoepithelial differentiation and mucoepidermoid carcinomas. p63 should be interpreted with caution as a squamous differentiation marker at the head and neck region, or other markers should be preferred. In cases with adenocarcinoma morphology without myoepithelial differentiation, metastatic tumors should be considered. The most frequent primary sites metastatic to the oral cavity are the lung, liver, breast, kidney, and colorectal in decreasing frequency, but rare sites like prostate should also be considered in the differential diagnosis (Fig. 4.5) [95].
4.4 Oropharynx
4.4.1 Squamous Cell Carcinoma
Oropharyngeal squamous cell carcinoma (OPSCC) mostly occurs in the base of tongue, tonsil, and adenoid of the oropharynx. OPSCC is classified according to high-risk HPV status, since it is a strong factor for favorable prognosis [96]. HPV-positive OPSCC generally occurs in young and nonsmoker patients and is presented as smaller primary tumors at advanced stage with nodal involvement. HPV-positive OPSCC arises from crypt epithelium and generally displays non-keratinizing morphology. HPV-negative OPSCC which is frequently associated with tobacco and alcohol consumption typically exhibits differentiated squamous features.
4.4.1.1 Diagnostic Biomarkers
HPV status can be detected by molecular methods including PCR and ISH or immunohistochemistry. p16 expression by immunohistochemistry is a surrogate biomarker for detecting transcriptionally active high-risk HPV. Overexpression of p16 occurs because of inactivation of tetinoblastoma (Rb) by HPV E7 early protein. p16 test may serve as a sufficient biomarker for determining HPV status in OPSCC. The nuclear and cytoplasmic strong immunoexpression of more than 70% of tumor cells is widely accepted as positive p16 staining result. The nuclear and cytoplasmic expression of more than 50% of tumor cells with >25% confluent staining may also be accepted. Molecular tests can be used following p16 test, if the clinical or histological features suggest otherwise. Among these molecular technics, HPV RNA-ISH seems to be the most effective method for determining transcriptionally active HPV status [97] (Fig. 4.6). The detection of cell-free HPV DNA in blood is recently described diagnostic tool for classifying OPSCC [98].
TRAF3, CYLD, PIK3CA, and E2F1 mutations are present in HPV-positive OPSCCs, while HPV-negative OPSCC displays CCND1, FADD, BIRC2, YAP1, and TP53 mutations [86].
4.4.1.2 Prognostic/Predictive Biomarkers
HPV status is an independent prognostic factor in OPSCC. At the same time, p16 expression in OPSCC is an independent prognostic factor regardless of the HPV status [99, 100]. TRAF3/CYLD mutations in HPV-positive OPSCCs are also associated with favorable clinical outcomes [101].
NOTCH1 mutation, SOX2 amplification, and HER3 expression are associated with poorer overall survival, while expression of estrogen receptor-α is associated with improved overall survival in HPV-positive OPSCC [102,103,104].
The tumor-infiltrating lymphocytes and high immunoexpression of PD-L1 more than 5% of intratumoral immune cells are favorable prognostic factors [105].
4.4.2 Metastatic Tumors to the Oropharynx
The most common primary sites of oropharyngeal metastases are the lung, kidney, prostate, breast, and female genital organs [106, 107].
4.5 Salivary Glands
Immunohistochemical expressions and molecular alterations are summarized in Tables 4.2–4.4.
4.5.1 Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland neoplasm. MEC is histologically composed of squamoid, mucin-producing, and intermediate cell types, which may form various patterns, including cystic and/or solid areas [108]. Several histopathological grading systems suggest stratifying MECs according to prognostic significance. At present, the WHO does not endorse a specific grading scheme. Currently, MECs are divided into three groups: low, intermediate, and high histological grade, with different clinical courses [109, 110].
4.5.1.1 Diagnostic Biomarkers
Lack of expression of myoepithelial markers and p63 and intracellular mucin positivity are typical features. CRTC1/3-MAML2 fusions are accepted as diagnostic markers for MECs. Molecular tests may especially help to diagnose variants of MECs, such as oncocytic or clear cell types or in cytology materials [110, 111].
4.5.2 Adenoid Cystic Carcinoma
Adenoid cystic carcinoma (AdCC) is a common, salivary gland malignancy. AdCC histologically consists of epithelial and myoepithelial cells. Various architectural morphologies, including cribriform, tubular, and solid patterns, can be seen. Local recurrences, metastases, and perineural invasion are eventually expected in most AdCCs [108, 112].
4.5.2.1 Diagnostic Biomarkers
MYB/MYBL1 rearrangement is highly specific for AdCC among salivary gland tumors, and it has been described in 29–86% of AdCCs [113,114,115,116]. A similar rearrangement involving MYBL1-NFIB fusion is another major molecular alteration which is reported in 9–14% of AdCCs [115, 117, 118]. MYB FISH is a useful diagnostic biomarker to distinguish AdCC from other types of salivary gland tumors [115, 119, 120]. Increased nuclear MYB expression has been observed in up to 85% of cases [121, 122]. MYB-NFIB translocation is not always correlated; generally, MYB immunoexpression is higher in AdCCs with the MYB-NFIB fusion [110, 120, 123].
4.5.2.2 Prognostic/Predictive Biomarkers
Activating mutations in NOTCH1 gene or loss-of-function mutations in SPEN gene seems to be associated with high-grade transformation [124].
MYB activation may represent a potential therapeutic target for AdCCs. Potential approaches include using short interfering RNA-mediated silencing of gene expression, targeting downstream effectors of MYB, and blocking protein-protein interactions in transcription complexes [125, 126]. Additionally, Notch signaling pathway inhibitors (including gamma-secretase inhibitors and Notch receptors targeting antibodies) and PI3K/IGF/FGFR1 pathway inhibitors are potential therapeutic targets in a subset of AdCC. Partial response to Notch signaling pathway inhibitors has been reported in several case reports [127,128,129]. NICD (Notch intracellular domain) expression by immunohistochemistry is a sensitive tool for detecting NOTCH1 mutations [127].
4.5.3 Acinic Cell Carcinoma
Acinic cell carcinoma (AciCC) is a low-grade carcinoma, composed of ductal and acinar cells with basophilic cytoplasm.
4.5.3.1 Diagnostic Biomarkers
AciCC displays PAS-positive, diastase-resistant zymogen granules, and it is positive for amylase, DOG-1, and CD117.
A recurrent translocation between the SCPP gene cluster and NR4A3 fusion by t(4;9)(q13;q31) is recently reported in 84% of AciCCs [130]. SCPP gene cluster contains several genes, such as STATH, HTN3, and HTN1. The nuclear NR4A3 expression is a reliable biomarker with high specificity (100%) and sensitivity (98%) for the diagnosis of AciCC [130].
Additionally, MSANTD3 rearrangements are described in 4–15% of AciCCs, and particularly MSANTD3-HTN3 translocation has been identified in around 4–8% of AciCCs [131, 132].
4.5.4 Secretory Carcinoma
Secretory carcinoma (SC) (previously known as mammary analog secretory carcinoma) is a low-grade salivary gland carcinoma. This entity is recognized as SC due to different morphological, immunohistochemical, and molecular features from AciCC [108, 133, 134].
4.5.4.1 Diagnostic Biomarkers
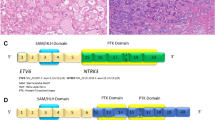
MUC4, S100, mammaglobin, GCDFP-15, GATA-3, and CD117-positive SC harbors the ETV6-NTRK3 fusion gene due to t (12;15)(p13;q25) translocation, unlike AciCC [133]. A small subset of SC harbors ETV6 translocations with unknown partners other than NTRK3 designated ETV6-X fusions [135, 136].
4.5.4.2 Prognostic/Predictive Biomarkers
NTRK3 fusion is both a diagnostic and predictive biomarker. ETV6-RET, ETV6-MAML3, and ETV6-MET translocations have recently been reported in subsets of SCs and observed to be related with aggressive biological features [137,138,139,140].
4.5.5 Polymorphous Adenocarcinoma
Polymorphous adenocarcinoma (PAC) is a rare salivary gland tumor that generally has a good prognosis and is predominantly seen in minor salivary glands. The PAC-classic variant and the PAC- cribriform adenocarcinoma of minor salivary glands (CAMSG) variant are defined within the PAC spectrum in the new WHO classification [110, 141, 142].
4.5.5.1 Diagnostic Biomarkers
PRKD1 E710D hotspot point mutations have been identified in more than 70% of classic variant PACs [143, 144]. Rearrangements in PRKD1, PRKD2, or PRKD3 genes rather than point mutations have been noted in about 80% of CAMSG-variant PACs [145]. ARID1A and DDX3X are identified as the partner genes of PRKD1 rearrangement [142].
4.5.5.2 Prognostic/Predictive Biomarkers
Although the knowledge about the prognostic and clinical significance of PRKD mutation is limited, the PRKD1 mutation may be associated with good, metastasis-free survival. Fusion-positive PACs are usually located in the base of the tongue and histologically show papillary pattern. They have a high risk of nodal metastasis [146].
4.5.6 Clear Cell Carcinoma
Clear cell carcinoma (CCC) is a low-grade salivary gland tumor, with clear cells, with or without hyalinization and typically arising in minor salivary glands, [108, 147].
4.5.6.1 Diagnostic Biomarkers
CCC is positive for p63 and PANCK and negative for S100, smooth muscle actin, calponin, and GFAP.
The EWSR1-ATF1 fusion is a major molecular aberration in 80–90% of CCCs [148]. EWSR1-ATF1 fusion is specific for CCCs among salivary gland malignancies; however, it should be noted that EWSR fusion with other genes may be detected in other salivary gland tumors.
4.5.7 Basal Cell Adenocarcinoma
Basal cell adenocarcinoma (BCAC) is a rare, low-grade, salivary gland malignancy with an indolent clinical course and is formed by nests and glandular structures, consisting of basal and myoepithelial neoplastic cells [1].
4.5.7.1 Diagnostic Biomarkers
Molecular alterations in the CYLD gene are identified in BCACs, notably the membranous type. These BCACs may present as a sporadic tumor or in the setting of a hereditary syndrome-related tumor, such as Brooke-Spiegler syndrome [149, 150].
Molecular alterations involving the PIK3CA and NFKBIA genes have also been reported in BCACs [151].
4.5.8 Intraductal Carcinoma
Intraductal carcinoma is (IC) a rare, low-to-intermediate-grade, recently described malignant salivary gland neoplasm which exhibits histologic features that are similar to mammary atypical ductal hyperplasia and ductal carcinoma in situ. IC tumors are histologically grouped in three patterns: apocrine, intercalated duct, and hybrid type [108, 152].
4.5.8.1 Diagnostic Biomarkers
ICs harbor RET gene rearrangements. NCOA4-RET fusion has been identified in 47% of intercalated duct type ICs, whereas TRIM27-RET fusion is generally observed in a subset of apocrine or hybrid type ICs [152,153,154,155,156].
4.5.9 Salivary Duct Carcinoma
SDC is a high-grade malignant neoplasm that mostly arises from the parotid gland. SDC is one of the most aggressive salivary gland tumors and shares similar microscopic features with high-grade mammary ductal carcinoma [108].
4.5.9.1 Diagnostic Biomarkers
The AR immunoexpression has been detected in more than 70% of SDCs and is seen in almost all apocrine variant SDCs [157, 158]. The amplification of ERBB2 (also known as HER2) is identified in approximately one-third of SDCs [159]. The most frequent gene alterations in SDCs are observed in TP53 (about two-thirds of SDCs), followed by the PIK3CA and H-RAS genes. Mutations of the KIT, EGFR, BRAF, N-RAS, AKT1, FBXW7, ATM, and NF1 genes, loss of heterozygosity of CDKN2A/p16, and loss or mutation of PTEN have also been identified in a subset of SDC [159,160,161,162,163,164,165,166,167,168,169]. Gene fusions involving PLAG1 and HMGA2 have been detected in SDC ex-PA [110, 162].
4.5.9.2 Prognostic/Predictive Biomarkers
TP53 mutations and ERBB2 amplification are related with an unfavorable prognosis in SDCs and are also more commonly observed in SDCs ex-PA than de novo counterparts [165].
AR immunoexpression appears to be both a diagnostic and predictive biomarker in SDCs [170]. Androgen deprivation therapy (ADT) seems to be an potential option with promising results for tumors with AR expression (Fig. 4.7) [171].
SDC cases with ERBB2 amplification seems to have benefitted from anti-ERBB2 treatment [172].
4.5.10 Myoepithelial Carcinoma
MC is an uncommon malignant salivary gland tumor, fully composed of myoepithelial cells with an infiltrative pattern.
4.5.10.1 Diagnostic Biomarkers
Myoepithelial markers and increased ki67 expression may help in the diagnosis of malignant myoepithelioma (Fig. 4.8). EWSR1 gene rearrangement has been identified in approximately one-third of MCs, which have predominantly clear cell morphology and aggressive clinical behavior [173, 174]. However, it is recently reported that none of MCs with EWSR1 rearrangements identified with FISH had an EWSR1 fusion transcript by sequencing methods, and this type of EWSR1 abnormality in MCs may represent a passenger mutation with minor effect [175].
4.5.11 Epithelial-Myoepithelial Carcinoma
EMC is a rare, biphasic tumor with low malignant potential.
4.5.11.1 Diagnostic Biomarkers
HRAS mutations are the leading molecular aberrations in EMCs, including p.Q61R and p.Q61K, which have been reported in 33% to 83% of EMCs [176, 177]. In addition, PIK3CA, CTNNB1, and/or AKT1 mutations may accompany HRAS mutations [176, 178, 179].
4.5.12 Sebaceous Carcinoma
Sebaceous adenocarcinoma (SAC) is a malignant tumor consisting of sebaceous cells.
4.5.12.1 Diagnostic Biomarkers
SAC is stained with EMA, antiadipophilin, CA15–3, and AR but is negative with BerEP4.
4.5.13 Lymphoepithelial Carcinoma
Lymphoepithelial carcinoma (LC) is an uncommon malignant tumor that occurs in salivary glands as well as in other organs. LCs are composed of undifferentiated malignant epithelial cells and non-tumoral lymphoid stroma [108].
4.5.13.1 Diagnostic Biomarkers
LCs are often associated with the Epstein-Barr virus (EBV). EBV can be detected using in situ hybridization for Epstein-Barr virus-encoded small RNA (EBER) and by PCR to detect latent membrane protein-1 (LMP-1) (Fig. 4.9) [180].
4.5.14 Oncocytic Carcinoma
Oncocytic changes may be seen in different types of salivary gland tumors like AciCC and MEC. Oncocytic carcinoma is a malignant epithelial neoplasm composed of oxyphilic cells and does not have histopathological features of other salivary gland tumor types. Mitochondrial antigen and BSND staining may help in identifying oncocytes [181, 182].
4.5.15 Carcinoma ex-Pleomorphic Adenoma
Carcinoma ex-pleomorphic adenoma (Ca-ex-PA) arises within a preexisting PA as a malignant proliferation that is often a high-grade carcinoma such as SDC but may also be any other malignant salivary gland tumor.
4.5.15.1 Diagnostic Biomarkers
The detection of PLAG1 or HMGA2 rearrangements appears to be useful to distinguish Ca-ex-PA from its de novo counterparts [183, 184]. TP53 mutations and/or HER2 amplification may help to differentiate Ca-ex-PA from pleomorphic adenoma.
4.5.16 Emerging Entities
4.5.16.1 Microsecretory Carcinoma
Microsecretory carcinoma (MsC) is a recently proposed distinct low-grade salivary gland tumor type which harbors a novel gene fusion: MEF2C-SS18. MsCs display unique histologic features including intercalated duct-like cells, infiltrative microcysts, tubules and cords, intraluminal secretions, and fibromyxoid stroma. Tumor cells are S100 and p63 positive but p40 negative [185, 186].
4.5.17 Metastatic Tumors to the Salivary Glands
Salivary glands appear as one of the most frequently metastasized regions in head and neck. The most frequent salivary gland site is the parotid gland. Common primary sites for metastatic carcinomas include other head and neck regions, skin, lung, kidney, prostate, uterus, ovary, breast, and colorectum [187, 188]. Frequent histologic subtypes include squamous cell carcinomas arising from other head and neck regions or skin and malignant melanoma, renal cell carcinoma, lung carcinoma, and breast carcinoma [189, 190]. Lack of expression of myoepithelial markers in any tumor at the salivary gland may raise a suspicion of the possibility of metastatic carcinoma.
Primary or metastatic squamous cell carcinomas similarly express p63, p40, and CK5/6. Primary SCC of the salivary gland is quite rare. Primary SCC is a diagnosis of exclusion of metastatic tumors. The distinction between metastatic and primary salivary gland SCCs cannot be made by histology or immunohistochemistry. Before accepting as primary salivary gland SCC, any possible primary sites should be searched, and potential partially sampled mucoepidermoid carcinoma should be excluded (Fig. 4.10).
Malignant melanoma is positive for S100, HMB45, MART1, and MITF. SOX10 is also useful for diagnosis of malignant melanoma, but the latter is also a marker for salivary gland tumors arising from intercalated ducts or acini (Fig. 4.11).
Renal cell carcinoma expresses CD10, PAX8, and RCC Ag, while primary salivary gland carcinomas are stained with CK7. Clear cell renal cell carcinoma displays cytoplasmic clearing due to glycogen and lipid content and may mimic salivary gland tumors with clear cell components, such as mucoepidermoid carcinoma, clear cell carcinoma, and epithelial-myoepithelial carcinoma.
Estrogen receptor, progesterone receptor, GATA-3, mammaglobin, and GCDFP-15 are commonly utilized biomarkers in the diagnosis of metastatic carcinoma of the breast, but salivary gland tumors express GATA-3, while salivary duct carcinoma or secretory carcinoma of salivary glands may also express mammaglobin and GCDFP-15. Additionally, secretory carcinoma and salivary duct carcinoma share similar phenotypic and molecular features with counterparts in breast, secretory carcinoma of breast, and luminal androgen receptor type/molecular apocrine type of ductal carcinomas.
4.6 Odontogenic and Maxillofacial Bone Tumors
4.6.1 Ameloblastic Carcinoma
Ameloblastic carcinoma (AC) is an uncommon primary epithelial odontogenic malignant neoplasm which combines the histological features of ameloblastoma and cytologic atypia, poor differentiation, and high mitotic index.
4.6.1.1 Diagnostic Biomarkers
BRAF V600E mutations are detected in 40–60% of AC, as seen in other ameloblastic tumors, such as ameloblastomas, ameloblastic fibromas, and ameloblastic fibro-odontomas [191, 192].
SOX2 immunoexpression and higher Ki67 proliferative index in AC are more frequently detected in ameloblastic carcinoma than ameloblastoma.
4.6.1.2 Prognostic/Predictive Biomarkers
BRAF V600E mutation was associated with the aggressive behavior of AC [192].
4.6.2 Sclerosing Odontogenic Carcinoma
Sclerosing odontogenic carcinoma (SOC) is a locally aggressive and infiltrative odontogenic carcinoma.
4.6.2.1 Diagnostic Biomarkers
The tumor cells stain with PANCK, CK5/6, CK14, p63, and CK19 but are negative for CK7 and CK20 [193, 194].
4.6.3 Clear Cell Odontogenic Carcinoma
Clear cell odontogenic carcinoma (CCOC) is an odontogenic carcinoma which shares similar histological and immunohistochemical features with clear cell carcinoma of salivary glands. The tumor is composed of vacuolated and clear cells.
4.6.3.1 Diagnostic Biomarkers
CCOC harbors EWSR1 translocations, mainly partner with ATF1; such tumors may be interpreted as an odontogenic analogue of clear cell carcinoma of salivary glands [195,196,197].
CCOC is positive for CK14, CK19, and pancytokeratin and negative for vimentin, S100, desmin, SMA, HMB45, alpha-1-antichymotrypsin, CD10, CD31, CD45, and GFAP.
4.6.4 Ghost Cell Odontogenic Carcinoma
Ghost cell odontogenic carcinoma is an odontogenic carcinoma which is composed of ghost cell keratinization and a dentinoid formation.
4.6.5 Metastatic Tumors to Maxillofacial Bone
The jawbones are frequently metastasized site due to their rich vascularization and high bone marrow content. The most common primary sites are the lung, kidney, prostate uterus, breast, colorectal, and ovary [198].
4.7 Ear
4.7.1 Squamous Cell Carcinoma
Squamous cell carcinoma is an external auditory canal or middle ear located malignant tumor which exhibit histological features that are no different from conventional SCC at any other site.
4.7.2 Ceruminous Adenocarcinoma
Ceruminous adenocarcinoma is a malignant tumor that arises from ceruminous glands of external auditory canal. Ceruminous adenocarcinoma is a heterogenous group of tumors involving adenocarcinoma, NOS, adenoid cystic carcinoma, and mucoepidermoid carcinoma. The latter two tumors show similar features those seen in salivary glands. Ceruminous adenocarcinoma has dual expression of luminal CK7 and CD117 and basal cell p63, S100, and CK5/6 expressions and low Ki67 index in most of the cases [199, 200].
4.7.3 Aggressive Papillary Tumor
Aggressive papillary tumor is a locally aggressive intermediate-grade tumor which composed of papillary pattern lined by two-layer cells. A subset of tumor is seemed to be related with von Hippel-Lindau syndrome.
4.7.4 Endolymphatic Sac Tumor
Endolymphatic sac tumor is a low-grade tumor that arises from endolymphatic sac of temporal bone. Tumor is composed of papillary or cystic architecture lined by usually one layer cell. This tumor shows a very high association with von Hippel-Lindau syndrome. Frequently CK5/6, CK8/18, EMA, synaptophysin, S100, and PAX8 positivity is observed [199, 201].
Questions
-
1.
Which of the following squamous cell carcinoma location at head and neck area is the second highest for HPV positivity rate?
-
(A)
Oropharynx.
-
(B)
Larynx.
-
(C)
Oral cavity.
-
(D)
Sinonasal cavity.
-
(E)
Hypopharynx.
-
(A)
-
2.
Which of the genetic alterations are characteristic for the sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma and oncocytic sinonasal papilloma, respectively?
-
(A)
KIT-NF1.
-
(B)
EGFR-KRAS.
-
(C)
KRAS-BRAF.
-
(D)
BRAF-KIT.
-
(E)
ALK-EGFR.
-
(A)
-
3.
Which of the tumor type shares phenotypic similarities with salivary duct carcinoma?
-
(A)
Mammary ductal carcinoma.
-
(B)
Clear cell renal cell carcinoma.
-
(C)
Colonic adenocarcinoma.
-
(D)
Pancreatic ductal adenocarcinoma.
-
(E)
Thyroid papillary carcinoma.
-
(A)
-
4.
Which salivary gland tumor type shares histological and genetic similarities with clear cell odontogenic carcinoma?
-
(A)
Acinic cell carcinoma.
-
(B)
Secretory carcinoma.
-
(C)
Clear cell carcinoma.
-
(D)
Mucoepidermoid carcinoma.
-
(E)
Basal cell adenocarcinoma.
-
(A)
-
5.
Which genetic aberration is frequently detected in ameloblastic carcinoma?
-
(A)
EGFR mutation.
-
(B)
BRAF mutation.
-
(C)
KRAS mutation.
-
(D)
KIT mutation.
-
(E)
GNAQ mutation.
-
(A)
-
6.
Which of the following immunohistochemical marker is helpful for detecting Notch pathway mutations in adenoid cystic carcinoma?
-
(A)
LEF-1.
-
(B)
MYB.
-
(C)
PLAG1.
-
(D)
Pan-TRK.
-
(E)
NICD.
-
(A)
-
7.
Which of the following immunohistochemical markers have predictive value in salivary duct carcinoma?
-
(A)
ER-PR.
-
(B)
EGFR-HER2.
-
(C)
AR-ER.
-
(D)
ER-HER2.
-
(E)
AR-HER2.
-
(A)
-
8.
Which of the following salivary gland tumor harbors NTRK gene rearrangement?
-
(A)
Acinic cell carcinoma.
-
(B)
Secretory carcinoma.
-
(C)
Clear cell carcinoma.
-
(D)
Mucoepidermoid carcinoma.
-
(E)
Basal cell adenocarcinoma.
-
(A)
-
9.
Which of the following gene is not often mutated in HPV-positive oropharyngeal squamous cell carcinoma?
-
(A)
TRAF3.
-
(B)
CYLD.
-
(C)
YAP1.
-
(D)
PIK3CA.
-
(E)
E2F1.
-
(A)
-
10.
Which of the following gene rearrangements present in a salivary gland carcinoma ex-pleomorphic adenoma?
-
(A)
CTRC1, CRTC3.
-
(B)
MYB, NFIB.
-
(C)
PRKD1, PRKD2.
-
(D)
PLAG1, HMGA2.
-
(E)
RET, MET.
-
(A)
Answers
1-D, 2-A, 3-B, 4-C, 5-B, 6-E, 7-E, 8-B, 9-C, 10-D.
References
Stelow EB, Bishop JA. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the nasal cavity, paranasal sinuses and skull base. Head Neck Pathol. 2017;11(1):3–15.
Jiromaru R, Yamamoto H, Yasumatsu R, Hongo T, Nozaki Y, Hashimoto K, et al. HPV-related sinonasal carcinoma: clinicopathologic features, diagnostic utility of p16 and Rb immunohistochemistry, and EGFR copy number alteration. Am J Surg Pathol. 2020;44(3):305–15.
Larque AB, Hakim S, Ordi J, Nadal A, Diaz A, Del Pino M, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27(3):343–51.
Laco J, Sieglová K, Vošmiková H, Dundr P, Němejcová K, Michálek J, et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Arch. 2015;467(4):405–15.
Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185.
Oliver JR, Lieberman SM, Tam MM, Liu CZ, Li Z, Hu KS, et al. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer. 2020;126(7):1413–23.
Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal carcinoma: systematic review and meta-analysis. Hum Pathol. 2013;44(6):983–91.
Lewis JS, Westra WH, Thompson LD, Barnes L, Cardesa A, Hunt JL, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014;8(3):241–9.
Elgart K, Faden DL. Sinonasal squamous cell carcinoma: etiology, pathogenesis, and the role of human papilloma virus. Curr Otorhinolaryngol Rep. 2020:1–9.
Cohen E, Coviello C, Menaker S, Martinez-Duarte E, Gomez C, Lo K, et al. P16 and human papillomavirus in sinonasal squamous cell carcinoma. Head Neck. 2020;42:2021.
Chung CH, Guthrie V, Masica D, Tokheim C, Kang H, Richmon J, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015;26(6):1216–23.
López F, Llorente JL, Oviedo CM, Vivanco B, Marcos C, García-Inclán C, et al. Gene amplification and protein overexpression of EGFR and ERBB2 in sinonasal squamous cell carcinoma. Cancer. 2012;118(7):1818–26.
Udager AM, McHugh JB, Betz BL, Montone KT, Livolsi VA, Seethala RR, et al. Activating KRAS mutations are characteristic of oncocytic sinonasal papilloma and associated sinonasal squamous cell carcinoma. J Pathol. 2016;239(4):394–8.
Udager AM, Rolland DC, McHugh JB, Betz BL, Murga-Zamalloa C, Carey TE, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75(13):2600–6.
Maisch S, Mueller SK, Traxdorf M, Weyerer V, Stoehr R, Iro H, et al. Sinonasal papillomas: a single centre experience on 137 cases with emphasis on malignant transformation and EGFR/KRAS status in “carcinoma ex papilloma”. Ann Diagn Pathol. 2020;151504
Wang H, Li H, Hu L, Zhou J, Zhai C, Wang D, et al. EGFR and KRAS mutations in Chinese patients with sinonasal inverted papilloma and oncocytic papilloma. Histopathology. 2019;75(2):274–81.
Kılıç S, Kılıç SS, Kim ES, Baredes S, Mahmoud O, Gray ST, et al., editors. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. International Forum of Allergy & Rhinology; 2017.: Wiley Online Library.
Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019;4(1):1–10.
Cohen EE, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184.
Riobello C, Vivanco B, Reda S, López-Hernández A, García-Inclán C, Potes-Ares S, et al. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck. 2018;40(4):818–27.
French CA. NUT carcinoma: clinicopathologic features, pathogenesis, and treatment. Pathol Int. 2018;68(11):583–95.
Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord J-P. Rouge TdLM, et al. clinical response of carcinomas harboring the BRD4–NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6(5):492–500.
Zhang X, Zegar T, Lucas A, Morrison-Smith C, Knox T, French CA, et al. Therapeutic targeting of p300/CBP HAT domain for the treatment of NUT midline carcinoma. Oncogene. 2020;39(24):4770–9.
Napolitano M, Venturelli M, Molinaro E, Toss A. NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther. 2019;12:3235.
Agaimy A, Franchi A, Lund VJ, Skálová A, Bishop JA, Triantafyllou A, et al. Sinonasal undifferentiated carcinoma (SNUC): from an entity to morphologic pattern and Back again—a historical perspective. Adv Anat Pathol. 2020;27(2):51–60.
Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol. 2017;30(5):650–9.
Dogan S, Frosina D, Fayad M, de Oliveira TB, Alemar B, Rosenblum M, et al. The role of a monoclonal antibody 11C8B1 as a diagnostic marker of IDH2-mutated sinonasal undifferentiated carcinoma. Mod Pathol. 2019;32(2):205–15.
Dogan S, Chute DJ, Xu B, Ptashkin RN, Chandramohan R, Casanova-Murphy J, et al. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol. 2017;242(4):400–8.
Mito JK, Bishop JA, Sadow PM, Stelow EB, Faquin WC, Mills SE, et al. Immunohistochemical detection and molecular characterization of IDH-mutant sinonasal undifferentiated carcinomas. Am J Surg Pathol. 2018;42(8):1067–75.
Riobello C, López-Hernández A, Cabal VN, García-Marín R, Suárez-Fernández L, Sánchez-Fernández P, et al. IDH2 mutation analysis in undifferentiated and poorly differentiated sinonasal carcinomas for diagnosis and clinical management. Am J Surg Pathol. 2020;44(3):396–405.
Babin E, Rouleau V, Vedrine P, Toussaint B, De Raucourt D, Malard O, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol. 2006;120(4):289–97.
Montone KT. The differential diagnosis of Sinonasal/nasopharyngeal neuroendocrine/Neuroectodermally derived Tumors. Arch Pathol Lab Med. 2015;139(12):1498–507.
Bell D, Hanna EY, Weber RS, DeMonte F, Triantafyllou A, Lewis JS Jr, et al. Neuroendocrine neoplasms of the sinonasal region. Head Neck. 2016;38(S1):E2259–E66.
Rooper LM, Bishop JA, Westra WH. INSM1 is a sensitive and specific marker of neuroendocrine differentiation in Head and neck tumors. Am J Surg Pathol. 2018;42(5):665–71.
Mahalakshmi B, Baskaran R, Shanmugavadivu M, Nguyen NT, Velmurugan BK. Insulinoma-associated protein 1 (INSM1): a potential biomarker and therapeutic target for neuroendocrine tumors. Cell Oncol. 2020:1–10.
Lechner M, Liu J, Lund VJ. Novel biomarkers in sinonasal cancers: from bench to bedside. Curr Oncol Rep. 2020;22(10):1–10.
García-Inclán C, López F, Pérez-Escuredo J, Cuesta-Albalad MP, Vivanco B, Centeno I, et al. EGFR status and KRAS/BRAF mutations in intestinal-type sinonasal adenocarcinomas. Cell Oncol. 2012;35(6):443–50.
Ward ML, Kernig M, Willson TJ. HPV-Related Multiphenotypic Sinonasal Carcinoma: A Case Report and Literature Review. The Laryngoscope. 2020.
Bishop JA, Westra WH. Human papillomavirus-related multiphenotypic sinonasal carcinoma: an emerging tumor type with a unique microscopic appearance and a paradoxical clinical behaviour. Oral Oncol. 2018;87:17–20.
Rupp NJ, Camenisch U, Seidl K, Rushing EJ, Anderegg N, Broglie MA, et al. HPV-related multiphenotypic sinonasal carcinoma: four cases that expand the morpho-molecular spectrum and include occupational data. Head Neck Pathol. 2020;14(3):623–9.
Antony VM, Kakkar A, Sikka K, Thakar A, Deo SV, Bishop JA, et al. p16 Immunoexpression in sinonasal and nasopharyngeal adenoid cystic carcinomas: a potential pitfall in ruling out HPV-related multiphenotypic sinonasal carcinoma. Histopathology. 2020;77(6):989–93.
Kusafuka K, Onitsuka T, Terada T. Sinonasal renal cell-like adenocarcinoma with EGFR overexpression of the maxillary sinus: report of a high-grade case and a review of the literature. Human Pathol: Case Reports. 2020;21:200390.
Agaimy A, Hartmann A, Antonescu CR, Chiosea SI, El-Mofty SK, Geddert H, et al. SMARCB1 (INI-1)-deficient Sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol. 2017;41(4):458–71.
Bell D, Hanna EY, Agaimy A, Weissferdt A. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Arch. 2015;467(6):649–56.
Shah AA, Jain D, Ababneh E, Agaimy A, Hoschar AP, Griffith CC, et al. SMARCB1 (INI-1)-deficient adenocarcinoma of the sinonasal tract: a potentially under-recognized form of sinonasal adenocarcinoma with occasional yolk sac tumor-like features. Head Neck Pathol. 2020;14(2):465–72.
Agaimy A, Weichert W. SMARCA4-deficient sinonasal carcinoma. Head Neck Pathol. 2017;11(4):541–5.
Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma : a series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol. 2020;44(5):703–10.
Rooper LM, Uddin N, Gagan J, Brosens LAA, Magliocca KR, Edgar MA, et al. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol. 2020;44(10):1331–9.
Hay AJ, Migliacci J, Karassawa Zanoni D, McGill M, Patel S, Ganly I. Minor salivary gland tumors of the head and neck—Memorial Sloan Kettering experience: incidence and outcomes by site and histological type. Cancer. 2019;125(19):3354–66.
Agaimy A, Mueller SK, Bishop JA, Chiosea SI. Primary and secondary/metastatic salivary duct carcinoma presenting within the Sinonasal tract. Head Neck Pathol. 2021:1–11.
López F, Devaney KO, Hanna EY, Rinaldo A, Ferlito A. Metastases to nasal cavity and paranasal sinuses. Head Neck. 2016;38(12):1847–54.
Weng B, Wang Q, San Lin YL. Nasal cavity metastasis of breast cancer: a case report and review of the literature. Int J Clin Exp Pathol. 2014;7(10):7028.
Terada T. Renal cell carcinoma metastatic to the nasal cavity. Int J Clin Exp Pathol. 2012;5(6):588.
Azarpira N, Ashraf MJ, Khademi B, Asadi N. Distant metastases to nasal cavities and paranasal sinuses case series. Indian J Otolaryngol Head Neck Surg. 2011;63(4):349–52.
Cathro HP, Mills SE. Immunophenotypic differences between intestinal-type and low-grade papillary sinonasal adenocarcinomas: an Immunohistochemical study of 22 cases utilizing CDX2 and MUC2. Am J Surg Pathol. 2004;28(8):1026–32.
Richardo T, Prattapong P, Ngernsombat C, Wisetyaningsih N, Iizasa H, Yoshiyama H, et al. Epstein-Barr virus mediated signaling in nasopharyngeal carcinoma carcinogenesis. Cancers. 2020;12(9):2441.
Franchi A, Moroni M, Massi D, Paglierani M, Santucci M. Sinonasal undifferentiated carcinoma, nasopharyngeal-type undifferentiated carcinoma, and keratinizing and nonkeratinizing squamous cell carcinoma express different cytokeratin patterns. Am J Surg Pathol. 2002;26(12):1597–604.
Zhao Y, Wang Y, Zeng S, Hu X. LMP1 expression is positively associated with metastasis of nasopharyngeal carcinoma: evidence from a meta-analysis. J Clin Pathol. 2012;65(1):41.
Wotman M, Oh EJ, Ahn S, Kraus D, Costantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705–10.
Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34(2):213–8.
Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, et al. Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377(6):513–22.
Lo YM, Chan LY, Chan AT, Leung SF, Lo KW, Zhang J, et al. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res. 1999;59(21):5452–5.
Ruuskanen M, Irjala H, Minn H, Vahlberg T, Randen-Brady R, Hagström J, et al. Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: a nationwide study in Finland. Head Neck. 2019;41(2):349–57.
Kano M, Kondo S, Wakisaka N, Moriyama-Kita M, Nakanishi Y, Endo K, et al. The influence of human papillomavirus on nasopharyngeal carcinoma in Japan. Auris Nasus Larynx. 2017;44(3):327–32.
Cao Y. EBV based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precision Oncol. 2017;1(1):1–5.
Leung SF, Chan KC, Ma BB, Hui EP, Mo F, Chow KC, et al. Plasma Epstein-Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol. 2014;25(6):1204–8.
Le QT, Colevas AD, O’Sullivan B, Lee AW, Lee N, Ma B, et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J Natl Cancer Inst. 2019;111(7):655–63.
Chen Y-P, Chan AT, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
Zhou Y, Shi D, Miao J, Wu H, Chen J, Zhou X, et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci Rep. 2017;7:43627.
Zheng L, Cao C, Cheng G, Hu Q, Chen X. Cytomembranic PD-L1 expression in locoregionally advanced nasopharyngeal carcinoma. Onco Targets Ther. 2017;10:5483–7.
Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189–202.
Takakura H, Hamashima T, Tachino H, Nakazato A, Minato H, Sasahara M, et al. Clinicopathological features of thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report and review of the literature. Front Surg. 2020;7
fienol COfiKUN H, ÇANDIR Ö. Breast carcinoma metastatic to nasopharynx. Turkish J Pathol. 2006;22(3):196–9.
Iqneibi S, Nazzal J, Amoudi R, Owda B, Al-Ibraheem A, Yaser S, et al. Metastatic pulmonary adenocarcinoma to the nasopharynx at first clinical presentation: A case report and review of literature. SAGE Open Med Case Rep. 2020;8:2050313X20939826.
Wong RH, Gary MT, Ng CS, Wan IY, Underwood MJ, Yim AP. Solitary nasopharyngeal metastasis from lung primary: a long-term survivor after radiotherapy. Ann Thorac Surg. 2011;92(1):e13–e4.
Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403.
Delagranda A, Leterme G, Chirpaz E, Ferdynus C, Fernandez C, Rubin F. Epidemiological features of cancers of the oral cavity, oropharynx, hypopharynx and larynx cancer in Réunion Island. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(3):175–81.
Network CGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576.
Davidson SM, Ko HC, Harari PM, Wieland AM, Chen S, Baschnagel AM, et al. Impact of HPV status on the prognostic potential of the AJCC staging system for larynx cancer. Otolaryngol Head Neck Surg. 2018;159(3):456–65.
Hughes RT, Beuerlein WJ, O'Neill SS, Porosnicu M, Lycan TW, Waltonen JD, et al. Human papillomavirus-associated squamous cell carcinoma of the larynx or hypopharynx: clinical outcomes and implications for laryngeal preservation. Oral Oncol. 2019;98:20–7.
Fusconi M, Campo F, Gallo A, Zambetti G, Martellucci S, Seccia A, et al. Laryngeal cancer, HPV DNA vs E6/E7 mRNA test: a systematic review. J Voice. 2017;31(2):248.e1-.e5
Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930.
Rooper LM, Windon MJ, Hernandez T, Miles B, Ha PK, Ryan WR, et al. HPV-positive squamous cell carcinoma of the larynx, oral cavity, and hypopharynx: clinicopathologic characterization with recognition of a novel warty variant. Am J Surg Pathol. 2020;44(5):691–702.
Meshman J, Wang P-C, Chin R, John MS, Abemayor E, Bhuta S, et al. Prognostic significance of p16 in squamous cell carcinoma of the larynx and hypopharynx. Am J Otolaryngol. 2017;38(1):31–7.
Vitzthum LK, Mell LK. The role of p16 as a biomarker in nonoropharyngeal head and neck cancer. Oncotarget. 2018;9(70):33247.
Lawrence MS, Sougnez C, Lichtenstein L, Cibulskis K, Lander E, Gabriel SB, et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82.
Sánchez Barrueco A, González Galán F, Lora Pablos D, Villacampa Aubá JM, Ballestín Carcavilla C, Cenjor Español C, et al. HPV in larynx squamous cell carcinoma: new serotypes and survival study within 10-year follow-up. Otolaryngol Head Neck Surg. 2017;156(4):677–82.
Abrahão R, Anantharaman D, Gaborieau V, Abedi-Ardekani B, Lagiou P, Lagiou A, et al. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in Europe: the ARCAGE study. Int J Cancer. 2018;143(1):32–44.
Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519–25.
Demiral A, Sarıoglu S, Birlik B, Sen M, Kınay M. Prognostic significance of EGF receptor expression in early glottic cancer. Auris Nasus Larynx. 2004;31(4):417–24.
Pointer KB, Ko HC, Brower JV, Witek ME, Kimple RJ, Lloyd RV, et al. Small cell carcinoma of the head and neck: an analysis of the National Cancer Database. Oral Oncol. 2017;69:92–8.
Kaugars GE, Svirsky JA. Lung malignancies metastatic to the oral cavity. Oral Surg Oral Med Oral Pathol. 1981;51(2):179–86.
Markman R-L, Giuliano-Augusto-Belizario Rosa LC, Simonato L-E, Brandão T-B. Tongue metastasis of cutaneous melanoma: report of two cases and literature review. J Clin Exp Dent. 2018;10(11):e1130.
Tuffaha MS, Guski H, Kristiansen G. Immunohistochemistry in tumor diagnostics. Immunohistochem Tumor diagnostics. Springer; 2018. p. 1–9.
Liu Y, Vargo RJ, Bilodeau EA. Analytic survey of 57 cases of oral metastases. J Oral Pathol Med. 2018;47(3):275–80.
Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365.
Craig SG, Anderson LA, Moran M, Graham L, Currie K, Rooney K, et al. Comparison of molecular assays for HPV testing in oropharyngeal squamous cell carcinomas: a population-based study in Northern Ireland. Cancer Epidemiol Prev Biomarkers. 2020;29(1):31–8.
Wuerdemann N, Jain R, Adams A, Speel E-JM, Wagner S, Joosse SA, et al. Cell-free HPV-DNA as a biomarker for oropharyngeal squamous cell carcinoma—a step towards personalized medicine? Cancers. 2020;12(10):2997.
Lewis JS Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:8.
Oguejiofor KK, Hall J, Mani N, Douglas C, Slevin NJ, Homer J, et al. The prognostic significance of the biomarker p16 in oropharyngeal squamous cell carcinoma. Clin Oncol. 2013;25(11):630–8.
Hajek M, Sewell A, Kaech S, Burtness B, Yarbrough WG, Issaeva N. TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2017;123(10):1778–90.
Dogan S, Xu B, Middha S, Vanderbilt CM, Bowman AS, Migliacci J, et al. Identification of prognostic molecular biomarkers in 157 HPV-positive and HPV-negative squamous cell carcinomas of the oropharynx. Int J Cancer. 2019;145(11):3152–62.
Kwon S, Ahn S-H, Jeong W-J, Jung YH, Bae YJ, Paik JH, et al. Estrogen receptor α as a predictive biomarker for survival in human papillomavirus-positive oropharyngeal squamous cell carcinoma. J Transl Med. 2020;18(1):240.
Qian G, Jiang N, Wang D, Newman S, Kim S, Chen Z, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer. 2015;121(20):3600–11.
Solomon B, Young RJ, Bressel M, Urban D, Hendry S, Thai A, et al. Prognostic significance of PD-L1+ and CD8+ immune cells in HPV+ oropharyngeal squamous cell carcinoma. Cancer Immunol Res. 2018;6(3):295–304.
Shin S-J, Roh J-L, Choi S-H, Nam SY, Kim SY, Kim SB, et al. Metastatic carcinomas to the oral cavity and oropharynx. Korean J Pathol. 2012;46(3):266–71.
Bayram Şahin CD, Çelik M, Öztürk E, Güneş S, Kıyak ÖE. Atypical metastasis to the Head and neck region: an analysis of 11 patients. Turkish Arch Otorhinolaryngol. 2018;56(4):210.
El-Naggar AKCJ, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. 4th ed. Lyon: IARC; 2017.
Seethala RR, Stenman G. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the salivary gland. Head Neck Pathol. 2017;11(1):55–67.
Toper MH, Sarioglu S. Molecular pathology of salivary gland neoplasms: diagnostic, prognostic, and predictive perspective. Adv Anat Pathol. 2021; Publish Ahead of Print
Luk PP, Wykes J, Selinger CI, Ekmejian R, Tay J, Gao K, et al. Diagnostic and prognostic utility of mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):530–41.
Carlson J, Licitra L, Locati L, Raben D, Persson F, Stenman G. Salivary gland cancer: an update on present and emerging therapies. Am Soc Clin Oncol Educ Book. 2013;33:257–63.
Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106(44):18740–4.
Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016;6(2):176–87.
Mitani Y, Liu B, Rao PH, Borra VJ, Zafereo M, Weber RS, et al. Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res. 2016;22(3):725–33.
Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17(22):7003–14.
Rettig EM, Talbot CC Jr, Sausen M, Jones S, Bishop JA, Wood LD, et al. Whole-genome sequencing of salivary gland adenoid cystic carcinoma. Cancer Prev Res (Phila). 2016;9(4):265–74.
Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48(3):265–72.
Rettig EM, Tan M, Ling S, Yonescu R, Bishop JA, Fakhry C, et al. MYB rearrangement and clinicopathologic characteristics in head and neck adenoid cystic carcinoma. Laryngoscope. 2015;125(9):E292–E9.
de Almeida-Pinto YD, Costa S, de Andrade BAB, Altemani A, Vargas PA, Abreu LG, et al. t(6;9)(MYB-NFIB) in head and neck adenoid cystic carcinoma: a systematic review with meta-analysis. Oral Dis. 2019;25(5):1277–82.
Brill LB, Kanner WA, Fehr A, Andrén Y, Moskaluk CA, Löning T, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–76.
Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao Y-J, et al. Novel chromosomal rearrangements and break points at the t (6; 9) in salivary adenoid cystic carcinoma: association with MYB–NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17(22):7003–14.
Broz M, Steiner P, Salzman R, Hauer L, Starek I. The incidence of MYB gene breaks in adenoid cystic carcinoma of the salivary glands and its prognostic significance. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(3):417–22.
Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48(3):265.
Liu X, Xu Y, Han L, Yi Y. Reassessing the potential of Myb-targeted anti-cancer therapy. J Cancer. 2018;9(7):1259.
Wysocki PT, Izumchenko E, Meir J, Ha PK, Sidransky D, Brait M. Adenoid cystic carcinoma: emerging role of translocations and gene fusions. Oncotarget. 2016;7(40):66239.
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol. 2017;35(3):352–60.
Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C, et al. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 2014;4(10):1154–67.
Ferrarotto R, Heymach JV. Taking it up a NOTCH: a novel subgroup of ACC is identified. Oncotarget. 2017;8(47):81725–6.
Haller F, Skálová A, Ihrler S, Märkl B, Bieg M, Moskalev EA, et al. Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol. 2019;43(9):1264–72.
Andreasen S, Varma S, Barasch N, Thompson LD, Miettinen M, Rooper L, et al. The HTN3-MSANTD3 fusion gene defines a subset of acinic cell carcinoma of the salivary gland. Am J Surg Pathol. 2019;43(4):489–96.
Barasch N, Gong X, Kwei KA, Varma S, Biscocho J, Qu K, et al. Recurrent rearrangements of the Myb/SANT-like DNA-binding domain containing 3 gene (MSANTD3) in salivary gland acinic cell carcinoma. PloS One. 2017;12:2.
Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608.
Griffith C, Seethala R, Chiosea SI. Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Arch. 2011;459(1):117.
Ito Y, Ishibashi K, Masaki A, Fujii K, Fujiyoshi Y, Hattori H, et al. Mammary analogue secretory carcinoma of salivary glands. Am J Surg Pathol. 2015;39(5):602–10.
Taverna C, Baněčková M, Lorenzon M, Palomba A, Franchi A, Skalova A, et al. MUC4 is a valuable marker for distinguishing secretory carcinoma of the salivary glands from its mimics. Histopathology. 2020;
Skalova A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: report of 4 cases Harboring ETV6-X gene fusion. Am J Surg Pathol. 2016;40(1):3–13.
Skalova A, Vanecek T, Martinek P, Weinreb I, Stevens TM, Simpson RHW, et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation: report of 10 cases. Am J Surg Pathol. 2018;42(2):234–46.
Guilmette J, Dias-Santagata D, Nosé V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019;83:50–8.
Rooper LM, Karantanos T, Ning Y, Bishop JA, Gordon SW, Kang H. Salivary secretory carcinoma with a novel ETV6-MET fusion: expanding the molecular spectrum of a recently described entity. Am J Surg Pathol. 2018;42(8):1121–6.
Tasoulas J, Tsourouflis G, Klijanienko J, Theocharis S. Polymorphous adenocarcinoma of the salivary glands: an overview of immunohistochemical features and insights on molecular pathology. Histol Histopathol. 2019:18089.
Xu B, Barbieri A, Bishop J, Chiosea S, Dogan S, Di Palma S, et al. Histologic Classification and Molecular Signature of Polymorphous Adenocarcinoma (PAC) and Cribriform Adenocarcinoma of Salivary Gland (CASG): An International Interobserver Study. Am J Surg Pathol. 2020;44:545.
Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–9.
Wysocki PT, Westra WH, Sidransky D, Brait M. Advancing toward a molecular characterization of polymorphous low grade adenocarcinoma. Oral Oncol. 2017;74:192.
Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosom Cancer. 2014;53(10):845–56.
Sebastiao APM, Xu B, Lozada JR, Pareja F, Geyer FC, Paula ADC, et al. Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Mod Pathol. 2020;33(1):65–73.
Daniele L, Nikolarakos D, Keenan J, Schaefer N, Lam AK-Y. Clear cell carcinoma, not otherwise specified/hyalinising clear cell carcinoma of the salivary gland: the current nomenclature, clinical/pathological characteristics and management. Crit Rev Oncol Hematol. 2016;102:55–64.
Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;50(7):559–70.
Kazakov DV, Benkova K, Michal M, Vanecek T, Kacerovska D, Skalova A. Skin type spiradenoma of the parotid gland with malignant transformation: report of a case with analysis of the CYLD gene. Hum Pathol. 2009;40(10):1499–503.
Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10(2):125–30.
Jo VY, Sholl LM, Krane JF. Distinctive patterns of CTNNB1 (β-catenin) alterations in salivary gland basal cell adenoma and basal cell adenocarcinoma. Am J Surg Pathol. 2016;40(8):1143–50.
Palicelli A. Intraductal carcinomas of the salivary glands: systematic review and classification of 93 published cases. APMIS 2019.
Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol. 2018;42(4):442–52.
Skalova A, Vanecek T, Uro-Coste E, Bishop JA, Weinreb I, Thompson LDR, et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol. 2018;42(11):1445–55.
Lu H, Graham RP, Seethala R, Chute D. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2020;14(1):239–45.
Skálová A, Ptáková N, Santana T, Agaimy A, Ihrler S, Uro-Coste E, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors. Am J Surg Pathol. 2019;43(10):1303–13.
Xu B, Dogan S, Al Rasheed MRH, Ghossein R, Katabi N. Androgen receptor immunohistochemistry in salivary duct carcinoma: a retrospective study of 188 cases focusing on tumoral heterogeneity and temporal concordance. Hum Pathol. 2019;93:30–6.
Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M, et al. Salivary duct carcinoma. Am J Surg Pathol. 2015;39(5):705–13.
Nardi V, Sadow PM, Juric D, Zhao D, Cosper AK, Bergethon K, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment. Clin Cancer Res. 2013;19(2):480–90.
Griffith CC, Seethala RR, Luvison A, Miller M, Chiosea SI. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am J Surg Pathol. 2013;37(8):1201–7.
Qiu W, Tong G-X, Turk AT, Close LG, Caruana SM, Su GH. Oncogenic PIK3CA mutation and dysregulation in human salivary duct carcinoma. Biomed Res Int. 2014;2014
Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee K-W, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22(18):4623–33.
Wang K, Russell JS, McDermott JD, Elvin JA, Khaira D, Johnson A, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. 2016;22(24):6061–8.
Luk PP, Weston JD, Yu B, Selinger CI, Ekmejian R, Eviston TJ, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(S1):E1838–E47.
Shimura T, Tada Y, Hirai H, Kawakita D, Kano S, Tsukahara K, et al. Prognostic and histogenetic roles of gene alteration and the expression of key potentially actionable targets in salivary duct carcinomas. Oncotarget. 2018;9(2):1852.
Saintigny P, Mitani Y, Pytynia KB, Ferrarotto R, Roberts DB, Weber RS, et al. Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: implications for targeted therapy. Cancer. 2018;124(18):3693–705.
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE, et al. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39(6):744–52.
Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16(8):2266–74.
Dogan S, Ng CK, Xu B, Kumar R, Wang L, Edelweiss M, et al. The repertoire of genetic alterations in salivary duct carcinoma including a novel HNRNPH3-ALK rearrangement. Hum Pathol. 2019;88:66–77.
Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11(3):288–94.
Jaspers HC, Verbist BM, Schoffelen R, Mattijssen V, Slootweg PJ, van der Graaf WT, et al. Androgen receptor–positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473–e6.
Hanna GJ, Bae JE, Lorch JH, Haddad RI, Jo VY, Schoenfeld JD, et al. The benefits of adjuvant Trastuzumab for HER-2-positive salivary gland cancers. Oncologist. 2020;
Skálová A, Weinreb I, Hyrcza M, Simpson RH, Laco J, Agaimy A, et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol. 2015;39(3):338–48.
Ni H, Zhao P-Y, Wang X-T, Xia Q-Y, Wang X, Wu N, et al. EWSR1 rearrangement is present in a subset of myoepithelial tumors of salivary glands with variable morphology and does not correlate with clinical behavior. Ann Diagn Pathol. 2017;28:19–23.
Skálová A, Agaimy A, Vanecek T, Banecková M, Laco J, Ptáková N, et al. Molecular Profiling of Clear Cell Myoepithelial Carcinoma of Salivary Glands With EWSR1 Rearrangement Identifies Frequent PLAG1 Gene Fusions But No EWSR1 Fusion Transcripts. Am J Surg Pathol. 2020; Publish Ahead of Print
Urano M, Nakaguro M, Yamamoto Y, Hirai H, Tanigawa M, Saigusa N, et al. Diagnostic significance of HRAS mutations in epithelial-myoepithelial carcinomas exhibiting a broad histopathologic spectrum. Am J Surg Pathol. 2019;43(7):984–94.
Chiosea SI, Miller M, Seethala RR. HRAS mutations in epithelial–myoepithelial carcinoma. Head Neck Pathol. 2014;8(2):146–50.
El Hallani S, Udager AM, Bell D, Fonseca I, Thompson LD, Assaad A, et al. Epithelial-myoepithelial carcinoma: frequent morphologic and molecular evidence of preexisting pleomorphic adenoma, common HRAS mutations in PLAG1-intact and HMGA2-intact cases, and occasional TP53, FBXW7, and SMARCB1 alterations in high-grade cases. Am J Surg Pathol. 2018;42(1):18.
De Cecio R, Cantile M, Fulciniti F, Botti G, Foschini M, Losito N. Salivary epithelial-myoepithelial carcinoma: clinical, morphological and molecular features. Pathologica. 2017;109(1):1–8.
Mozaffari HR, Ramezani M, Janbakhsh A, Sadeghi M. Malignant salivary gland tumors and Epstein-Barr virus (EBV) infection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18(5):1201.
Shinmura K, Kato H, Kawanishi Y, Kamo T, Inoue Y, Yoshimura K, et al. BSND is a novel immunohistochemical marker for oncocytic salivary gland tumors. Pathol Oncol Res. 2018;24(2):439–44.
Giordano G, Gabrielli M, Gnetti L, Ferri T. Oncocytic carcinoma of parotid gland: a case report with clinical, immunohistochemical and ultrastructural features. World J Surg Oncol. 2006;4(1):54.
Katabi N, Ghossein R, Ho A, Dogan S, Zhang L, Sung Y-S, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015;46(1):26–33.
Bahrami A, Dalton JD, Shivakumar B, Krane JF. PLAG1 alteration in carcinoma ex pleomorphic adenoma: immunohistochemical and fluorescence in situ hybridization studies of 22 cases. Head Neck Pathol. 2012;6(3):328–35.
Bishop JA, Weinreb I, Swanson D, Westra WH, Qureshi HS, Sciubba J, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43(8):1023–32.
Kawakami F, Nagao T, Honda Y, Sakata J, Yoshida R, Nakayama H, et al. Microsecretory adenocarcinoma of the hard palate: a case report of a recently described entity. Pathol Int. 2020;70(10):781–5.
Ogunyemi O, Rojas A, Hematpour K, Rogers D, Head C, Bennett C. Metastasis of genitourinary tumors to the head and neck region. Eur Arch Otorhinolaryngol. 2010;267(2):273–9.
Horáková M, Porre S, Tommola S, Baněčková M, Skálová A, Kholová I. FNA diagnostics of secondary malignancies in the salivary gland: bi-institutional experience of 36 cases. Diagn Cytopathol. 2020;49:241.
Nuyens M, Schüpbach J, Stauffer E, Zbären P. Metastatic disease to the parotid gland. Otolaryngol Head Neck Surg. 2006;135(6):844–8.
Pastore A, Ciorba A, Soliani M, Di Laora A, Valpiani G, Bianchini C, et al. Secondary malignant tumors of the parotid gland: not a secondary problem. J BUON. 2017;22:513–8.
Oh K-Y, Cho S-D, Yoon H-J, Lee J-I, Hong S-D. Discrepancy between immunohistochemistry and sequencing for BRAF V600E in odontogenic tumours: Comparative analysis of two VE1 antibodies. J Oral Pathol Med.
Niu Z, Li Y, Chen W, Zhao J, Zheng H, Deng Q, et al. Study on clinical and biological characteristics of ameloblastic carcinoma. Orphanet J Rare Dis. 2020;15(1):316.
O'Connor R, Khurram S, Singh T, Jones K. Sclerosing odontogenic carcinoma–what we know so far. Oral Surg. 2019;12(2):133–8.
Wood A, Young F, Morrison J, Conn BI. Sclerosing odontogenic carcinoma presenting on the hard palate of a 43-year-old female: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(6):e204–e8.
Santana T, de Andrade FL, de Sousa Melo MC, da Rocha GBL, Trierveiler M. Clear cell odontogenic carcinoma harboring the EWSR1–ATF1 fusion gene: report of a rare case. Head Neck Pathol. 2019:1–5.
Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37(7):1001–5.
Tanguay J, Weinreb I. What the EWSR1-ATF1 fusion has taught us about hyalinizing clear cell carcinoma. Head Neck Pathol. 2013;7(1):28–34.
Capodiferro S, Limongelli L, Mastropasqua MG, Favia G, Lajolo C, Colella G, et al. Metastatic tumors of the oro-facial tissues: clear cell renal cell carcinoma. A clinico-pathological and immunohistochemical study of seven cases. J Clin Med. 2020;9(4):1151.
Thompson LD. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumours of the ear. Head Neck Pathol. 2017;11(1):78–87.
Crain N, Nelson BL, Barnes EL, Thompson LD. Ceruminous gland carcinomas: a clinicopathologic and immunophenotypic study of 17 cases. Head Neck Pathol. 2009;3(1):1–17.
Thompson LD, Magliocca KR, Andreasen S, Kiss K, Rooper L, Stelow E, et al. CAIX and pax-8 commonly immunoreactive in endolymphatic sac tumors: a clinicopathologic study of 26 cases with differential considerations for metastatic renal cell carcinoma in von Hippel-Lindau patients. Head Neck Pathol. 2019;13(3):355–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Toper, M.H., Sarioglu, S., Skálová, A. (2022). Biomarkers in Head and Neck Carcinomas. In: Sarioglu, S., Sagol, O., Aysal, A. (eds) Biomarkers in Carcinoma of Unknown Primary. Springer, Cham. https://doi.org/10.1007/978-3-030-84432-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-84432-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84431-8
Online ISBN: 978-3-030-84432-5
eBook Packages: MedicineMedicine (R0)