Abstract
The Vibrant Soundbridge is a semi-implantable middle ear hearing device used in the rehabilitation of adults with sensorineural hearing loss. In order to evaluate the long-term effects of the implanted part of the device, audiological data from 39 patients implanted over several implant sites across France were collected and analyzed retrospectively. The mean follow-up time was 16 months; 25 patients had a follow-up period of over 1 year. Surgery was uneventful in all cases. The present study of the 39 implanted patients with a mid- to long-term follow-up found a statistically significant modification of hearing thresholds (pre- versus postoperative) for frequencies of 0.5 and 4 kHz. However, the shift of threshold was rather limited (2.79 and 3.34 dB, respectively), and this variation was not statistically different from the evolution of the opposite non-operated ear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Vibrant Soundbridge is a semi-implantable middle ear hearing device intended for use in patients with a mild to severe sensorineural hearing loss who desire an alternative to an acoustic hearing aid. The internal transducer is directly coupled to the incus in order to address some of the known shortcomings of conventional hearing aids (i.e., occlusion of the ear canal by the ear mold, physical limitations of the output transducer and reduction of distortion). The electromagnetic transducer (Floating Mass Transducer: FMT) comprises a mobile magnet in a small titanium case (2 mm long by 1.5 mm diameter, weight: 25 mg) with a coil. Electric current through the coil produces vibration of the magnet. The capabilities of the FMT, measured on temporal bones, show that the output passband ranges from 500 Hz to 10 kHz with a maximum output level comparable to that produced by sounds around 110 dB SPL [1, 2]. Clinical results have demonstrated a measurable benefit from the Vibrant Soundbridge over conventional hearing aids and a greater ease in communication for the majority of implanted patients [6]. Despite the presence of demonstrable clinical benefits, questions have been raised about the potential side effects related to the implant and long-term use of the device, especially with reference to the impact of the FMT upon hearing thresholds. To date, no short-term effect because of surgery or the presence of the FMT has been reported [6, 8]. Clinical studies on the long-term effect of the FMT are rare [8] and to date have reported on the observations of only a small number of patients with no greater than 12 months post-surgery follow-up. Potential long-term side effects may occur as a consequence of cochlear trauma because of overstimulation of the ossicular chain, change of middle ear impedance resulting from the physical weight of the FMT, modification of the incus because of the method of fixation or middle ear aeration.
The aim of the this paper was to evaluate the long-term effects of the presence of the FMT in the middle ear by examining the available audiological data for retrospectively implanted patients. Audiological data from repeated measures for 39 patients implanted over several implant sites across France are analyzed and reported. Included in the analysis are data for a subgroup of 12 patients with at least 18 months post-surgery experience and assessments at similar test intervals.
Subjects and methods
Device description
The Vibrant Soundbridge consists of two main subsystems: the implanted part called the VORP and the external amplification system called the audio processor. The VORP consists of an implanted receiver unit (a demodulator circuit filters the modulated signal to the appropriate drive signal for the transducer), a conductor link and a floating mass transducer. The audio processor consists of four functional components: a microphone, a sound processing system, a modulator circuit and a battery. It is designed to be worn under the hair, behind and above the external ear. The audio processor is held in place and centered over the internal receiver with magnets. The external audio processor houses an omni-directional microphone, a transmitting coil, a standard 675 battery, a modulator circuit and a signal processor (initially analogic followed by two digital versions).
Study design
Retrospective audiological data for repeated measures from 39 patients implanted in several tertiary referral hospitals across France were collected. Pure-tone thresholds for air and bone conduction were measured in both ears preoperatively and at one or more postoperative test intervals of 2, 6, 12, 18 and 24 months postoperatively. Pure-tone audiometry was carried out using standard procedures and equipment. In addition, although not reported in this paper, impedance tympanometry and stapedial reflex measurements were performed in some clinics for a subgroup of the patients.
Air conduction thresholds were tested for the following frequencies: 500, 1,000, 2,000 and 4,000 Hz. Bone conduction thresholds were recorded for the frequencies: 500, 1,000, 2,000 and 4,000 Hz. Patient acted as their own control for intra-ear measurements. In addition, the patient’s contralateral ear served as a control for inter-ear measures over time. A Student’s t-test for paired samples was performed for comparison of air conduction thresholds between the preoperative baseline and the last postoperative test interval values for the following frequencies: 500, 1,000, 2,000 and 4,000 Hz. Twenty-four months post-surgery, data were available for a subgroup of eight patients (patients 1–4, 11, 13, 16, 17 and 24); 12 to 24 months post-surgery, data were available for a subgroup of 17 patients (patients 6–8, 12–15, 19–22, 25–27, 29, 38 and 39); 6 months post-surgery, data was available for a subgroup of 7 patients (patients 5, 9, 23, 28, 30, 31 and 37), with the remaining 7 patients having had a follow-up time of 3 months (patients 10, 18 and 32–36).
Subjects
The implanted patient group fulfilled the criteria for implantation, namely, symmetrical sensorineural hearing loss with normal middle ear function and no retrocochlear pathology. Preoperative hearing thresholds (for the frequencies: 500, 1,000, 2,000 and 4,000 Hz) ranged from 32.5 to 80 dB HL (mean hearing loss: 54.6 dB HL, standard deviation: 12.9 dB HL). Hearing thresholds at 0.5 kHz were below 65 dB HL, except for in one subject (no. 21). Surgery was uneventful in all cases.
Results
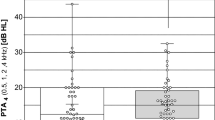
Figure 1 shows the modification of air conduction hearing thresholds between the preoperative and last postoperative evaluation. The pure-tone average at each test interval was calculated as the sum of the pure-tone thresholds for the main speech frequencies (500, 1,000, 2,000 and 4,000 Hz) divided by 4. The calculated difference values ranged between −2.5 dB to 8.75 dB for the patient group. The majority of patients (85%, 33/39) displayed difference values between ±5 dB, which is not considered as being clinically significance. Finally, seven patients had an improvement of pure-tone thresholds postoperatively. After 3 months, no significant evolution of hearing thresholds was noted. Table 1 shows the modification of thresholds for 500, 1,000, 2,000 and 4,000 Hz. After surgery, the mean deterioration varied from 1.61 dB at 2 kHz to 3.34 dB at 4 kHz. The shift of the pure tone air conduction threshold was statistically significant only for the frequencies of 500 Hz (Δ=2.79 dB, P=0.008) and 4,000 Hz (Δ=3.34 dB, P=0.002). Further analysis of the change in hearing thresholds at these frequencies was performed by subtracting the preoperative airbone gap from the postoperative airbone gap in the implant ear. The difference value for the implant ear was then compared to that obtained for the contralateral non-implanted ear for each patient at each of these frequencies. A Student’s t-test for paired samples resulted in a P value of 0.06, suggesting no clinical significant inter-aural difference was observed at 4,000 Hz. For the frequency of 500 Hz, a Student’s t-test for paired samples was also performed and reached a 0.12 P value.
Discussion
Since the introduction of the Vibrant Soundbridge device, many clinical studies have been performed to demonstrate the benefits of the device for the hearing impaired adult [5, 6, 9]. The positive results of these studies have led to the development of this technique as a treatment option in the rehabilitation of adults with sensorineural hearing loss, with approximately 1,000 implanted patients worldwide to date.
However, the long-term effects from the presence of a mobile device clipped to the ossicular chain must also be evaluated. Potential long-term effects may be divided into three different categories: (1) modification of hearing thresholds mostly in the high frequency range secondary to a functional effect (i.e., surgery), (2) modification of hearing thresholds secondary to an anatomical effect (i.e., lysis of the incus and fibrosis of the middle ear) and (3) modification of hearing thresholds secondary to the mass loading of the ossicular chain.
The audiological follow-up data for the group of 39 implanted patients suggest a significant change in hearing thresholds over time at 500 Hz (Δ=2.79 dB, P=0.008) and 4,000 Hz (Δ=3.34 dB, P=0.002), while the thresholds at 1,000 Hz and 2,000 Hz were shown to be clinically stable over time. Although the observed difference in hearing thresholds was rather limited, further examination of the inter-aural threshold data was examined for the frequencies 500 and 4,000 Hz to determine the significance of the change in hearing thresholds in the implanted ear over time using the contralateral ear as the control for each patient. With respect to changes in hearing thresholds noted for the frequency 4,000 Hz, no clinically significant difference was noted between the ears. As a consequence, we may conclude that at least part of the observed variation in pure-tone air conduction thresholds over time may be due to the natural history of the deafness presented by the patient.
Concerning the 500 Hz, even if the mean difference of the hearing threshold over the two time intervals was small (Δ=2.79 dB), one wonders if this change could be due to the presence of the device in the middle ear. Mass loading of the ossicular chain may induce a shift in hearing thresholds by modification of the ossicular coupling and/or the middle ear transfer function. However, functional studies on mass-loaded ossicular chains are few. As shown in clinical studies, loading the ossicular chain with a light mass (1.5 mg) can result in observable auditory threshold modifications in some ears, particularly for the higher frequencies (2.0 to 3.0 kHz) [4]. Increasing the stapes mass would be expected to lower the normal resonant frequency of the human stapes/footplate complex, which is thought to be near 4.0 kHz [4]. However the mass of the FMT (25 mg) is placed at the incus long process-stapes complex level. Nishihara et al. [7] showed that an addition of mass on the incus long process and stapes produced a decrease in stapes displacement for the high frequencies. Wilson et al. [10] studied the effect of mass loading upon the response of the ear to high frequencies by recording auditory-evoked potentials. The authors concluded that a decrease of response occurred as the load upon the stapes increased.
To study a larger band of frequencies, Gan et al. [3] used laser interferometry on fresh or fresh-frozen cadaveric temporal bones. They placed masses (two masses were tested: 37.5 mg and 25.5 mg) on the incudostapedial joint and demonstrated that the greater the mass of the implant, the less displacement was measured at the stapes footplate, although linearity of the middle ear function did not change. These authors demonstrated also that all frequencies may decrease (from 250 Hz with a maximum at frequencies above 1 kHz). Concerning our data series, the pure-tone air-conduction hearing thresholds for the implanted ear for two frequencies (500 Hz and 4,000 Hz) showed a significant decrease post-surgery (2.79 and 3.34 dB HL, respectively). However, within this patient population, the progression in hearing threshold shift for 4,000 Hz was not observed to be significantly different than that observed in the contralateral ear.
Conclusion
Since the law of gravity applies also for the ossicular chain, any additional load will increase the inertia of the ossicles. As a consequence, the residual hearing and frequency response may alter following implantation of a middle ear prosthesis. The present study of 39 patients implanted with a VSB device with a mid- to long term follow-up has found a significant modification of hearing thresholds for the frequencies 0.5 and 4 kHz. However, the shift of threshold was rather limited (2.79 and 3.34 dB, respectively), and this variation was not statistically different from the evolution of the opposite non-operated ear. We now need to evaluate implanted patients with a follow-up of several years to confirm the good tolerance of the FMT in the middle ear.
References
Ball G, Huber A, Goode RL (1997) Scanning laser doppler vibrometry of the middle ear ossicles. Ear Nose Throat J 76:213–218
Gan RZ, Wood MW, Ball GR, Dietz TG, Dormer KJ (1997) Implantable hearing device performance measured by laser doppler interferometry. Ear Nose Throat J 76:297–309
Gan RZ, Dyer RK, Wood MW, Dormer KJ (2001) Mass loading on the ossicles and middle ear function. Ann Otol Rhinol Laryngol 110:478–485
Goode R, Nakamura K, Gyo K, Aritomo H (1989) Comments on “Acoustic transfer characteristics in the human middle ears studied by a SQUID magnetometer method.” J Acoust Soc Am 86:2446–2449
Fisch U, Cremers CW, Lenarz T, Weber B, Babighian G, Uziel AS, Proops DW, O’Connor AF, Charachon R, Helms J, Fraysse B (2001) Clinical experience with the Vibrant Soundbridge implant device. Otol Neurotol 22:962–972
Fraysse B, Lavieille JP, Schmerber S, Enee V, Truy E, Vincent C, Vaneecloo FM, Sterkers O (2001) A multicenter study of the Vibrant Soundbridge middle ear implant: early clinical results and experience. Otol Neurotol 22:952–961
Nishihara S, Aritomo H, Goode R (1993) Effects of changes in mass on middle ear function. Otolaryngol Head Neck Surg 109:899–910
Snik A, Cremers C (2000) The effect of the Floating Mass Transducer in the middle ear on hearing sensitivity. Am J Otol 21:42–48
Sterkers O, Boucarra D, Labassi S, Bebear JP, Dubreuil C, Frachet B, Fraysse B, Lavieille JP, Magnan J, Martin C, Truy E, Uziel A, Vaneecloo FM (2003) A middle ear implant, the Symphonix Vibrant Soundbridge: retrospective study of the first 125 patients implanted in France. Otol Neurotol 24:427–436
Wilson EP, Deddens AE, Lesser TH, Fredrickson JM (1990) Implantable hearing aids: changes in the evoked-auditory potentials of the monkey in response to increased loading of the stapes. Am J Otolaryngol 11:149–152
Acknowledgements
We would like to thank S. Labassi and M. Beliaeff for their editorial assistance and clinical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vincent, C., Fraysse, B., Lavieille, JP. et al. A longitudinal study on postoperative hearing thresholds with the Vibrant Soundbridge device. Eur Arch Otorhinolaryngol 261, 493–496 (2004). https://doi.org/10.1007/s00405-003-0669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-003-0669-9