Abstract
Purpose
The primary objective of the retrospective study was to collect speech intelligibility data on children and adolescents implanted with the vibrating ossicular prosthesis (VORP) 503.
Methods
This was a retrospective, multicentre study on 55 children and adolescents from 6 German clinics aged between 5 and 17 years suffering from mixed or conductive hearing loss implanted with a VORP 503. Pre- and postoperative bone-conduction pure tone thresholds were measured at 0.5, 1, 2 and 4 kHz, and word recognition scores in the unaided and VORP 503-aided conditions using monosyllabic speech intelligibility tests measured at 65-dB sound pressure level (SPL) were determined.
Results
Mean pre- and postoperative bone-conduction thresholds remained unchanged, showing the preservation of inner ear hearing. Speech intelligibility assessed in quiet at 65-dB SPL improved on average from 24.5% (SD ± 25.4) unaided to 86.4% (SD ± 13.4) aided. The average improvement of 61.9% (SD ± 25.3) was clinically and statistically significant. A total of three complications were found in the medical records of 55 subjects. The responsible investigators judged these events as procedure related.
Conclusion
The treatment of children suffering from conductive or mixed hearing loss with the VORP 503 implant demonstrates excellent aided benefit in terms of speech understanding and only minor complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Untreated hearing loss in young children results in speech development disorders and reduced quality of life and can also negatively impact academic performance. Surgical hearing restoration remains a challenge in children with conductive or mixed hearing loss due to complex disorders of the outer and middle ear, preventing use of conventional hearing aids. In these patients, active middle ear implants (AMEIs) can be a sufficient treatment option [1].
The Vibrant Soundbridge (VSB) is a direct drive, partially implantable middle-ear hearing system intended to provide a certain level of useful sound perception in individuals with hearing loss. The device consists of two major components: the implant, called the Vibrating Ossicular Prosthesis (VORP), and the external attachment, called the audio processor. The VORP is composed of a receiver coil, a magnet, a demodulator, a conductor link, and a floating mass transducer (FMT). The FMT reinforces the natural movement of the ossicular chain as it moves in the same direction. The VORP 503, introduced in 2014, is a further development of the well-established VORP 502 technology. In contrast to the VORP 502 the VORP 503 includes self-drilling fixation screws, a shorter, reinforced conductor link and a magnet that is MRI compatible at 1.5 T. Unlike the VORP 502, the VORP 503 has no pre-assembled attachment clip. This eliminates the need to remove the pre-assembled clip when connecting the FMT to one of the available Vibroplasty Couplers [2]. The VORP is the only CE-marked AMEI suitable for children between the ages of 5 and 17 years suffering from either sensorineural (SNHL), conductive (CHL) or mixed hearing loss (MHL) who cannot achieve success or adequate benefit from traditional therapy. The audiological results and the risk profile in children is comparable to those in adults [3,4,5]. A prospective study on 19 paediatric subjects confirmed the short-term safety and performance of the VORP 502 [6]. The present retrospective study serves as evidence that the VORP 503 is safe and effective in children and adolescents and performs in a similar way as the predecessor device.
Materials and methods
Subjects and materials
The data of this retrospective, multicentre, longitudinal, open-label case series study were collected between the end of August 2018 and middle of October 2018. Included subjects were implanted with a Vibrant Soundbridge VORP 503 between the 4th of November 2014 and the 9th of April 2018. The data sets were included for the retrospective analysis only if unaided and AMEI-aided speech intelligibility measures were available. Six German hospitals collected patient data after approval was obtained from the respective ethics committees (Technical University Dresden, EK171052018; Albert-Ludwigs-University Freiburg, 154/18 (MPG§23b); Medical Association Rheinland Pfalz, 2018-13241; Ludwig-Maximilian-University-Munich, 18-479; University Lübeck, 18-099; University Medical Centre Tübingen, 235/2018BO2).
55 subjects (32 female and 23 male) with mixed or conductive hearing loss met the study’s inclusion criteria. The mean age of the recruited patients was 9 ± 4 years (range 5–17 years). All patients met the indication criteria provided by the data sheet of the implant. The FMT of the AMEI was connected either to the short process (SP) of the incus (n = 25), the long process (LP) of the incus (n = 1), the stapes (n = 17), the round window (RW) (n = 10), the oval window (n = 1) or to a third window (n = 1). The FMT was either connected directly or using couplers (Table 1).
Methods
Due to the retrospective character of the study, no additional interventions or contact to the patients were undertaken. The respective principal investigator transferred pseudonymized user data without personally identifiable information which adhere to the selection criteria defined in the protocol. The whole data set was statistically analysed and is shown in the present publication.
The last preoperative bone-conduction (BC) thresholds at frequencies 0.5, 1, 2 and 4 kHz (= PTA4) were compared to the last postoperative measurement. Speech intelligibility was tested by word recognition scores (WRS). The WRS, measured by the Freiburger Monosyllable Test (27 subjects), the Mainzer children speech test (6 subjects) or the Göttinger children speech test (22 subjects) at 65-dB sound pressure level (SPL) in quiet, compared the last available unaided to the last available AMEI-aided scores. The mean follow-up after surgery was 10.7 (SD = 8.5) months (range 3–41 months).
The safety of the surgical procedure and the AMEI was examined by recording the number and the relative frequency (%) of complications per subject.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (IBM, Armonk, New York). Graphs were created with GraphPad Prism 6 (GraphPad Software, Inc.). Interferential statistics was applied to test for significant differences in speech intelligibility between the last evaluated unaided and aided speech test outcomes. Kolmogorov–Smirnov test showed that data were not normally distributed, therefore, the Wilcoxon signed-rank test was used for data comparison. Changes in speech intelligibility were calculated for the whole population and for each individual speech intelligibility test. An improvement in speech intelligibility of ≥ 20% from the unaided to the aided condition was considered clinically relevant. The Wilcoxon signed-rank test was also applied to compare unaided pre- and postoperative PTA4 BC thresholds. Since none of the subjects suffered from purely sensorineural hearing loss, PTA4 AC thresholds were not calculated. Deteriorations of up to 10 dB in BC PTA4 threshold from the preoperative to postoperative test results per subject were not considered clinically significant.
p values of less than 0.05 were considered to indicate statistical significance.
Results
Bone-conduction thresholds
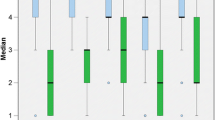
Pre- and postoperative BC PTA4 thresholds were available for 52 out of 55 analysed unilaterally implanted subjects. The mean preoperative BC PTA4 threshold was 15.3 dB HL (standard deviation [SD] = 8.5, range 0–43.5 dB HL), which remained stable (15.2 dB HL, SD = 6.9) without significant difference (p < 0.391) postoperatively (see Fig. 1). With regard to individual BC PTA4 thresholds, one subject experienced a deterioration of 11.3 dB HL. The same subject had a clinically significant WRS improvement of 50%. Two other subjects experienced a BC PTA4 threshold improvement of more than 20 dB HL after VSB implantation.
Mean PTA4 (pure tone average at frequencies 0.5, 1, 2 and 4 kHz) bone-conduction (BC) thresholds for 52 subjects comparing the preoperative with the postoperative AMEI-aided conditions. Boxes extend from the 25th to the 75th percentiles; median horizontal lines, + mean, N subjects, circles individual values
Speech intelligibility as assessed by the word recognition score
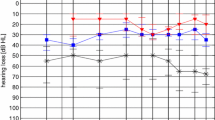
Speech intelligibility was determined for all 55 subjects using age-adapted tests. The average WRS for the total study group was 24.5% (SD = 25.4) in the unaided and 86.4% (SD = 13.4) in the AMEI-aided situations. The average improvement of 61.9% (SD = 24.5) was clinically, and according to the results of a Wilcoxon signed-rank test, also statistically significant (p < 0.001).
Speech intelligibility was assessed with the Freiburger Monosyllabic test in older subjects (n = 27) with an average age at implantation of 11 years (SD = 4.2). The WRS improved by 54.3% (SD = 22.1) from 29.1% (SD = 25.3) unaided to 83.3% (SD = 14) aided (p < 0.001). Twenty-two study subjects with a mean age of 7 years (SD = 3) underwent the Göttinger children's speech test. The WRS improved on average by 75% (SD = 23.3), from 13.2% (SD = 23) unaided to 88.2% (SD = 13.7) aided (p < 0.001).
The six youngest patients with an average age of 6 years (SD = 2) were evaluated using the Mainzer children's speech test. Their mean WRS improved from 45% unaided (SD = 16.4) to 93.3% (SD = 5.2) aided. The average improvement of 48.3% (SD = 19.4) was statistically significant (p < 0.0313). Figure 2 presents the graphical and Table 2 the numerical data sets.
Word recognition scores as measured by the Freiburger, Göttinger or Mainzer test presented at 65-dB SPL; the unaided is compared to the AMEI-aided WRS. Boxes extend from the 25th to the 75th percentiles; median horizontal lines, + mean, N subjects, circles individual values; ALL refers to the total population in the study
Complications
Three device- or procedure-related complications were observed. One subject had an AMEI-aided WRS of only 50%. Further examinations revealed that the SP-Coupler was displaced. The subject underwent a successful revision surgery. A second patient suffering from Partial Trisomy 21 was a partial AMEI non-user. Another Trisomy 21 child experienced a device protrusion through the tympanic membrane due to a local infection and Eustachian tube dysfunction. This patient underwent a successful revision surgery as well.
Discussion
The primary objective of the present retrospective study was to collect VORP 503 user data to investigate improvement in speech intelligibility in quiet in children and adolescents. The results of three different German word recognition tests suitable for children of different ages were considered for speech intelligibility assessment. Regardless of the test administered, the children achieved a mean AMEI-aided speech intelligibility rate of 86.4% (SD ± 13.8). This result confirms data from recent publications [4, 5, 7, 8] reporting on the safety and performance of the VORP 502 implanted in children.
Procedure risks and device safety were evaluated by comparing pre- and postoperative BC PTA4 thresholds. In addition, complications found in the respective medical records were analysed. There was no significant difference between average pre- and postoperative BC PTA4 thresholds. Individual BC threshold changes had no negative consequence on the WRS outcome. Similar results for a total of 93 children were reported in different studies [4, 5, 8,9,10].
Three complications were recorded in the present study. The complications were defined as procedure related, and two of them required revision surgery. The revision surgery rate for the present retrospective study was 3.6%. A similar revision rate of 3.2% due to insufficient coupling was reported by Frenzel et al. [6].
The anatomical development of the middle-ear structures are, with the exception of the tympanum, complete at birth [11]. There is, however, a difference between adults and paediatric patients in terms of the prevalence and origin of specific ear pathologies. Typically, SNHL is congenital in children and occurs in 2–4 neonates per 1000 in developed countries [12]. These children are normally provided with conventional hearing aids or—in patients with more severe hearing loss—with a cochlear implant. This circumstance is reflected by the present study as no subject suffering from SNHL was included for analysis.
The prevalence of MHL in adults is not well defined and occurrence rates are not reported in the literature. In Australian preschool children, MHL is the third leading cause of hearing impairment [13]. However, almost all of those children have impaired middle ear effusion with a normal middle ear anatomy which mainly can be treated conventionally. Only rare cases with severe chronic inflammations require alternative treatment. In the present study, 12 VSB users suffered from MHL. Children with a pure CHL make up the largest paediatric patient population that fall within the indication criteria for the Vibrant Soundbridge. The present study clearly supports this fact as 43 out of the 55 analysed subjects had CHL. Of these subjects, those suffering from malformations formed the largest group (n = 41). Patients with malformations of the middle ear including atresia are unable to use conventional hearing aids. Bone-conduction devices are an alternative with some audiologic disadvantages, especially regarding directional hearing. Middle ear implants can solve these problems, as they bypass the pathologic external auditory canal and defect middle ear structures [14]. The VSB is the only approved middle ear implant for children aged 5 and above. Due to its single-point attachment technique and variety of different Vibroplasty couplers available, the FMT can be placed on any suitable vibratory structure in the middle ear. In the present study, the FMT was successfully attached to the short process of the incus (n = 25), the stapes (n = 17) or the long process of the incus (n = 1). If the ossicular chain is missing, the FMT can even be attached to the round window membrane (n = 10) or adapted to the stapes foot plate (n = 1). In one patient suffering from a cholesteatoma, the FMT was successfully placed in the third window. All coupling variations are summarized in Table 1.
There is a general consensus that congenital and especially bilateral early childhood hearing loss should be treated as soon as possible. Also children with unilateral hearing loss have significant deficits in speech-in-noise discrimination as well as directional hearing which is related to poor academic performance [15, 16]. Early treatment with a conductive hearing device (on a headband) soon after birth positively affects the maturation of the auditory pathway. However dissatisfaction with comfort, sound quality, and aesthetics often limits the use of these devices [17]. The AMEI allows a comfortable hearing rehabilitation in preschool children. This is supported by the fact that the median age at VSB implantation is 6 years in the present study population.
One important aspect to mention is that several patients implanted with the VSB would also qualify for a bone-conduction implant. In some cases, the anatomical situation would even prevent a VSB implantation. In contrast to VSB surgery, the implantation of bone-conduction devices may be easier, however, binaural hearing and corresponding localization ability could be impaired to some degree [18, 19]. One bone-conduction device stimulates both cochleae whereas the VSB selectively stimulates the affected ear. It is assumed that a selective and early stimulation of the cochlea allows the child to develop true binaural hearing. Therefore, an AMEI like the VORP 503 applied within the first years of life may lead to a better hearing rehabilitation compared to a BC device [20]. Additionally, the BC decrease with time because of the malformation and/or genetic profile should be considered in the preoperative setting. However, because of the wide range of middle ear anatomy and preoperative audiological performance in congenital hearing loss, comparative studies are difficult to design, and placebo-controlled studies are not ethically feasible. Therefore, both options should be considered and adapted in every individual case.
Considering that MRI has become a common diagnostic tool used in many therapeutic areas, every child will most likely undergo more than one MRI scan throughout his or her lifetime. Especially in children the use of X-ray diagnostic tools like CT should be avoided to prevent long-term sequelae. Thus, the VORP 503 bears additional advantage being the only CE-marked middle-ear implant that is MRI safe at 1.5 T.
All patients should be thoroughly counselled about existing treatment options [21]; however, the present retrospective study strengthens the position of the AMEI as a suitable device for children and adolescents suffering from conductive and mixed hearing loss when conventional hearing aids are not suitable. Although retrospective studies are generally inferior to prospective studies, they do reflect real-world situations. To our knowledge, the present study includes the most comprehensive data set regarding VORP 503 users aged between 5 and 17 years. The subjects in this study were followed for an average of 9 months after surgery (range 3–41 months).
In conclusion, the results support VSB implantation in children suffering from conductive or mixed hearing loss, to be very beneficial in terms of speech understanding. Only minor complications occurred and were successfully treated. No new risks were identified. The types and severity of complications experienced were similar to adult subjects. Although long-term data on the expected lifetime of the device are still missing, we conclude that the VSB is a safe and effective treatment for paediatric patients.
References
Kesser BW, Krook K, Gray LC (2013) Impact of unilateral conductive hearing loss due to aural atresia on academic performance in children. Laryngoscope 123(9):2270–2275. https://doi.org/10.1002/lary.24055
Labassi S, Beliaeff M, Pean V, Van de Heyning P (2017) The Vibrant Soundbridge((R)) middle ear implant: a historical overview. Cochlear Implants Int 18(6):314–323. https://doi.org/10.1080/14670100.2017.1358913
Zernotti ME, Arauz SL, Di Gregorio MF, Arauz SA, Tabernero P, Romero MC (2013) Vibrant Soundbridge in congenital osseous atresia: multicenter study of 12 patients with osseous atresia. Acta Otolaryngol 133(6):569–573. https://doi.org/10.3109/00016489.2012.762117
Roman S, Denoyelle F, Farinetti A, Garabedian EN, Triglia JM (2012) Middle ear implant in conductive and mixed congenital hearing loss in children. Int J Pediatr Otorhinolaryngol 76(12):1775–1778. https://doi.org/10.1016/j.ijporl.2012.08.022
Claros P, Pujol Mdel C (2013) Active middle ear implants: Vibroplasty in children and adolescents with acquired or congenital middle ear disorders. Acta Otolaryngol 133(6):612–619. https://doi.org/10.3109/00016489.2013.765969
Frenzel H, Sprinzl G, Streitberger C, Stark T, Wollenberg B, Wolf-Magele A, Giarbini N, Strenger T, Muller J, Hempel JM (2015) The Vibrant Soundbridge in children and adolescents: preliminary European multicenter results. Otol Neurotol 36(7):1216–1222. https://doi.org/10.1097/MAO.0000000000000796
Hempel JM, Braun T, Berghaus A (2013) Funktionelle und ästhetische rehabilitation der Mikrotie bei Kindern und Jugendlichen. HNO 61(8):655–661. https://doi.org/10.1007/s00106-013-2694-3
Mandala M, Colletti L, Colletti V (2011) Treatment of the atretic ear with round window Vibrant Soundbridge implantation in infants and children: electrocochleography and audiologic outcomes. Otol Neurotol 32(8):1250–1255. https://doi.org/10.1097/MAO.0b013e31822e9513
Celerier C, Thierry B, Coudert C, Blanchard M, Loundon N, Garabedian EN, Denoyelle F (2017) Results of VSB implantation at the short process of the incus in children with ear atresia. Int J Pediatr Otorhinolaryngol 93:83–87. https://doi.org/10.1016/j.ijporl.2016.12.038
Zhao S, Gong S, Han D, Zhang H, Ma X, Li Y, Chen X, Ren R, Li Y (2016) Round window application of an active middle ear implant (AMEI) system in congenital oval window atresia. Acta Otolaryngol 136(1):23–33. https://doi.org/10.3109/00016489.2014.1003091
Powles-Glover N, Maconochie M (2018) Prenatal and postnatal development of the mammalian ear. Birth Defects Res 110(3):228–245. https://doi.org/10.1002/bdr2.1167
Smith RJ, Bale JF Jr, White KR (2005) Sensorineural hearing loss in children. Lancet 365(9462):879–890. https://doi.org/10.1016/S0140-6736(05)71047-3
Choi SM, Robyn AU, Kei J, Wilson WJ (2017) Rates of hearing loss in primary school children in Australia: a systematic review. Speech Lang Hear 20(3):154–162. https://doi.org/10.1080/2050571X.2016.1259199
Suzuki J, Kodera K, Nagai K, Yabe T (1994) Long-term clinical results of the partially implantable piezoelectric middle ear implant. Ear Nose Throat J 73(2):104–107
Rohlfs AK, Friedhoff J, Bohnert A, Breitfuss A, Hess M, Muller F, Strauch A, Rohrs M, Wiesner T (2017) Unilateral hearing loss in children: a retrospective study and a review of the current literature. Eur J Pediatr 176(4):475–486. https://doi.org/10.1007/s00431-016-2827-2
Bess FH, Tharpe AM, Gibler AM (1986) Auditory performance of children with unilateral sensorineural hearing loss. Ear Hear 7(1):20–26
Frenzel H, Schonweiler R, Hanke F, Steffen A, Wollenberg B (2012) The Lubeck flowchart for functional and aesthetic rehabilitation of aural atresia and microtia. Otol Neurotol 33(8):1363–1367. https://doi.org/10.1097/MAO.0b013e3182659adf
Leinung M, Zaretsky E, Lange BP, Hoffmann V, Stover T, Hey C (2017) Vibrant Soundbridge((R)) in preschool children with unilateral aural atresia: acceptance and benefit. Eur Arch Otorhinolaryngol 274(1):159–165. https://doi.org/10.1007/s00405-016-4265-1
Dazert S, Thomas JP, Volkenstein S (2015) Surgical and technical modalities for hearing restoration in ear malformations. Facial Plast Surg 31(6):581–586. https://doi.org/10.1055/s-0035-1569062
Frenzel H (2018) Hearing rehabilitation in congenital middle ear malformation. Adv Otorhinolaryngol 81:32–42. https://doi.org/10.1159/000485525
Beutner D, Delb W, Frenzel H, Hoppe U, Huttenbrink KB, Mlynski R, Limberger A, Schonweiler R, Schwab B, Todt I, Walger M, Wesarg T, Zahnert T, Zeh R, Adano, Dghno, Dga, Deutsche Cochlea Implantat Gesellschaft D, Dgpp (2018) Guideline "Implantable hearing aids"-short version: German S2k guideline of the Working Group of German-Speaking Audiologists, Neurootologists and Otologists (ADANO), of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO) in collaboration with the German Society of Audiology (DGA), the German Society of Phoniatrics and Pediatric Audiology (DGPP), and patient representatives. HNO 66(Suppl 2):71–76. https://doi.org/10.1007/s00106-018-0533-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MED-EL, Innsbruck, Austria initiated and organized this study and gave support for statistical analysis and manuscript preparation. The authors declare that they have no conflict of interest.
Ethical approval
Since the present work was a retrospective study, all procedures involving human participants were performed before data collection. According to the law and the national ethical guidelines, ethical approvals were obtained.
Informed consent
The present retrospective data collection was not subject to prior consent from the device user, as personally identifiable information was irreversibly anonymized by the respective study site.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lailach, S., Zahnert, T., Maurer, J. et al. The vibrating ossicular prosthesis in children and adolescents: a retrospective study. Eur Arch Otorhinolaryngol 277, 55–60 (2020). https://doi.org/10.1007/s00405-019-05667-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05667-3