Abstract
Purpose
Primary dysmenorrhea effects the life-quality of women negatively. The aim of this study was to evaluate heme oxygenase-1 (HO1) activity together with malondialdehyde (MDA) and nitric oxide (NO) levels in patients with primary dysmenorrhea.
Methods
A total of 28 nulliparous women with the diagnosis of primary dysmenorrhea and 26 healthy controls were included in this study. On the first day of menstruation, all patients underwent ultrasound examination to exclude pelvic pathology and the visual analogue scale was applied to patients. Patient’s visual analogue scale (VAS) scores, age, body mass index (BMI), menstrual cycle length (day), length of bleeding (day) were recorded. In the same day, fasting blood samples were taken from each patient for biochemical analysis.
Results
Serum MDA, NO and HO1 levels were found to be higher in women with primary dysmenorrhea compared to healthy controls (p = 0.012, p = 0.009, p < 0.001, respectively). There were no correlation among serum levels of HO1, NO and MDA, age, BMI, cycle length, pain score and menses duration in both groups. In Pearson’s correlation analysis, positive correlation was found between HO1 levels with the NO levels (r = 0.316, p < 0.05) and VAS scores (r = 0.520, p < 0.01). Also, positive correlation was found between MDA levels and VAS scores (r = 0.327, p < 0.05).
Conclusions
Serum HO1, NO and MDA levels increase in patients with primary dysmenorrhea. Antioxidant support might be helpful to reduce pain severity in primary dysmenorrhea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary dysmenorrhea is defined as a menstrual pain on the suprapubic region without any pelvic pathology including myoma, endometriosis or ovarian cyst. The pain occurs just before or on the first day and continues to peak for 3 days of the menstrual cycle with or without nausea, vomiting, diarrhea, headache or syncope [1]. The prevalence of dysmenorrhea was reported as 16 and 91% in women of reproductive age [2, 3] and this disease effects the life-quality of women negatively [4]. Although the etiology of primary dysmenorrhea has not been clearly explored,; excessive uterine production of prostaglandin and vasopressin, higher blood malondialdehyde levels and higher uterine blood flow indices were reported in patients with primary dysmenorrhea [5–8]. Many drugs have been tried to alleviate menstrual pain in women with primary dysmenorrhea, such as mefenamic acid [9], Fructus agni casti [8] and diclofenac potassium [10].
Heme oxygenase-1 (HO1), a rate-limiting enzyme, has an important role in the oxidative catabolism of heme to generate carbon monoxide, iron and biliverdin. It was shown that HO1 has cytoprotective effects in case of oxidative stress conditions [11]. Also, studies revealed that HO1 provides extensive tissue protection via anti-oxidative, anti-inflammatory, anti-apoptotic and immunoregulatory activities [12–15]. Malondialdehyde (MDA) is formed during lipid peroxidation and it is used as a marker for tissue injury [16]. Nitric oxide (NO) is an important source of free radical production in tissue damage and NO levels decrease in case of endothelial dysfunction [17]. Lee et al. [18] revealed the important role of HO1 in the formation of lipid droplets during polymicrobial sepsis and they suggested that HO1 effects lipid metabolism in response to mitochondrial dysfunction.
Increased serum MDA and NO levels were reported in subjects with primary dysmenorrhea [19]. To the best of our knowledge, HO1 levels have not been studied in primary dysmenorrhea. Thus, the aim of this study was to evaluate HO1 activity together with MDA and NO levels in patients with primary dysmenorrhea. We also investigated the possible correlations of MDA and NO with HO1 in the healthy controls and primary dysmenorrheic patients.
Materials and methods
The study protocol was approved by the Ethics Committee of Medical Faculty, Ataturk University, Erzurum, Turkey. This study was performed in the Gynecology Department of Nenehatun Hospital and patients who suffered from moderate and severe form of primary dysmenorrhea were included. Written informed consent was obtained from each participant. Women with regular menstrual cycles (25–30 days), 18–30 years old, nulliparous, non-smoking, normal ultrasound examination of uterus and adnexa, no chronic inflammatory, circulatory or surgical diseases of the abdomen–pelvis were included. The pain was within 2 or 3 years after menarche, periodical and began a few hours before the menstruation and continued for the first 3 days of the cycle [7]. Those with a body mass index (BMI) ≥30, cardiac or pulmonary diseases, endocrine and metabolic diseases, smoking, alcohol consumption, pelvic pathology (endometriosis, ovarian cyst, surgery, etc.) and using any analgesic within 24 h prior to the study were excluded from the study. The control group consisted of women without dysmenorrhea.
On the first day of menstruation, all patients underwent ultrasound examination to exclude pelvic pathology. Because the pain isusually more severe on the first day of menstruation, the visual analogue scale (VAS) from 0 (minimum pain) to 10 (maximum pain) was applied to patients on the first day of menstruation [20]. Patient’s VAS scores, age, BMI, menstrual cycle length (day) and length of bleeding (day) were recorded. In the same day, fasting (>12 h) blood samples were taken from each patient and blood samples were centrifuged at 3000g for 10 min. Then, the obtained serum samples were kept at −80 °C until the time of analysis.
Plasma HO-1 levels were determined by Human HO-1 ELISA Kit (EKS-800, Stressgen/Assay Designs, Ann Arbor, MI, USA) according to the manufacturer’s instructions and results were expressed as ng/mL. Serum MDA levels were determined according to the method described by Ohkawa et al. [21] and values were expressed as µmol/mL. Measurement of the serum NO levels, which is the sum of nitrite and nitrate levels as the metabolite of NO, was performed according to the colorimetric Cayman method using colorimetric assay kit (Cayman chemical, Ann Arbor, MI, USA) in two-step process [22] and results were expressed as nmol/mL. All data were recorded for statistical analysis.
A power analysis for this study was calculated based on the work of Erdemli et al. [23] setting the primary outcome as HO1 levels using Russ Lenth’s Power and sample size calculation application [24]. When α error and β error was considered, respectively, as 0.05 and 0.04, with 85% power the patient number for each group was determined as a minimum of 25. The common standard deviation within a group was assumed to be 0.50.
Results were expressed as mean ± SD. Statistical analysis was performed using SPSS for Windows (version 15.0) statistical package (SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered significant. The normality of variables was tested with the Kolmogorov–Smirnov test. Because the distribution of all data is normal, unpaired t test was used to assess the differences between groups. Pearson’s correlation coefficient was used to detect correlations. Multiple regression analysis was performed.
Results
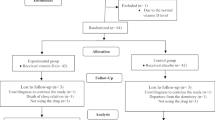
A flow chart of the patients is shown in Fig. 1. A total of 28 nulliparous women with the diagnosis of primary dysmenorrhea and 26 healthy controls were analyzed. Table 1 gives the clinical characteristics of the study population. Age, BMI, cycle length and menses duration of patients with primary dysmenorrhea were not different from those of controls. The data including the serum MDA, NO and HO1 levels of patients with primary dysmenorrhea and controls are given in Table 2. Serum MDA, NO and HO1 levels were higher in women with primary dysmenorrhea compared to healthy controls (p = 0.012, p = 0.009, p < 0.001, respectively).
In Pearson’s correlation analysis, positive correlation was found between HO1 levels with the NO levels (r = 0.316, p < 0.05) and VAS scores (r = 0.520, p < 0.01). Also, positive correlation was found between MDA levels and VAS scores (r = 0.327, p < 0.05). There was no correlation between serum levels of HO1, NO and MDA with age, BMI, cycle length or menses duration. When primary dysmenorrhea group and control group were analyzed severally, the correlation between HO1 levels and VAS scores only continued in the primary dysmenorrhea group (r = 0.516, p < 0.01) (Table 3).
A significant linear correlation was found between HO1 levels with VAS scores (p = 0.003) and NO levels (p = 0.014) in multiple regression analysis (F ratio = 4.299, p = 0.001) (Table 4).
Discussion
Although the exact pathophysiological mechanisms have not been understood completely, it has been suggested that primary dysmenorrhea is associated with increased uterine production of prostaglandin and vasopressin, increased serum malondialdehyde levels and increased uterine blood flow indices [5–8]. Whatever the reason might be, primary dysmenorrhea leads to the severe pain requiring hospital admission and it affects the life of school and work negatively [4]. Our results from this study demonstrated for the first time that serum concentrations of HO1, a rate-limiting enzyme in the oxidative catabolism of heme, are significantly higher in women with primary dysmenorrhea compared to controls. Also, serum NO and MDA levels were found to be higher in these patients compared to controls.
It was reported that elevated prostaglandin (PG) and vasopressin levels reduce uterine artery blood flow due to the vasoconstriction and myometrial contractions in patients with primary dysmenorrhea and, ischemia occurs. Ischemia was held responsible for pain formation in patients with dysmenorrhea [5, 6]. As it is known, oxidative stress occurs in case of ischemia [16–18]. Indeed, Yeh et al. [25] and Turhan et al. [7] reported higher plasma MDA levels, an oxidative stress marker in patients with dysmenorrhea compared to those in healthy controls. They suggested that lipid peroxidation and oxidative stress play a significant role in the etiopathogenesis of primary dysmenorrhea. Dikensoy et al. [19] also reported increased plasma levels of MDA, NO and adrenomedullin in subjects with primary dysmenorrhea compared to the controls. Similar to above studies [7, 19, 25], we found elevated HO1, NO and MDA levels in patients with primary dysmenorrhea compared to the control group.
Nitric oxide is a major paracrine mediator and it takes roles in various biological processes such as vascular functions and inflammation. It was shown that endometrium-derived NO may be important in the pathogenesis of dysmenorrhea and NO donors may be beneficial in the treatment of dysmenorrhoea [26]. In this current study, we found elevated serum NO levels in patients with primary dysmenorrhea compared to the control group. Similar to our study results, Dikensoy et al. [19] and Sun et al. [27] reported increased plasma NO levels in subjects with primary dysmenorrhea compared to the controls. Also, Akdemir et al. [28] reported higher serum ADMA, an endogenous competitive inhibitor of NO synthesis, levels in women with dysmenorrhea compared to healthy controls. They suggested that NO donors may relieve pelvic pain by reversing endothelial dysfunction.
Heme oxygenase is a rate-limiting enzyme and it takes part in heme catabolism towards biliverdin, carbon monoxide and free iron. Studies in the literature showed that this enzyme has got anti-oxidative, anti-inflammatory, anti-apoptotic and immunoregulatory activities [12–15]. Erdemli et al. [23] reported higher HO1 levels in patients with preeclampsia when compared to normotensive pregnant women. They concluded that HO1 may be important in the pathogenesis of preeclampsia. On the other hand, Xin et al. [29] investigated anti-oxidative stress effect of HO1 in patients with gestational diabetes mellitus. They reported increased expression of HO1 to protect against oxidative stress induced by high glucose levels. Similar to these studies, we found elevated serum HO1 levels in patients with primary dysmenorrhea compared to the control group. Also, we found a positive correlation between HO1 levels with the NO levels and VAS scores. We suggested that HO1 levels were increased by compensatory mechanisms to protect the balance between oxidant and antioxidant system in patients with dysmenorrhea.
To our knowledge, there isno study in the literature investigating serum HO1 levels in dysmenorrhea. We first demonstrated higher serum HO1 levels in patients with primary dysmenorrhea. The limitation of this present study was the relatively small patient population. Our results need to be confirmed in larger prospective studies.
In conclusion, serum HO1, NO and MDA levels increase in primary dysmenorrhea. Antioxidant support might be helpful to reduce pain severity in primary dysmenorrhea. This study provides new insight into the pathogenesis of primary dysmenorrhea and it may help in the development of new therapeutic strategies. Further investigations are necessary to detect the association between antioxidant activities and primary dysmenorrhea.
References
De Sanctis V, Soliman A, Bernasconi S, Bianchin L, Bona G, Bozzola M, Buzi F, De Sanctis C, Tonini G, Rigon F, Perissinotto E (2015) Primary dysmenorrhea in adolescents: prevalence, impact and recent knowledge. Pediatr Endocrinol Rev 13:512–520
Esen I, Oğuz B, Serin HM (2016) Menstrual characteristics of pubertal adolescent girls: a questionnaire based study in Turkey. J Clin Res Pediatr Endocrinol 8:192–196
Ju H, Jones M, Mishra G (2014) The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 36:104–113
Habibi N, Huang MS, Gan WY, Zulida R, Safavi SM (2015) Prevalence of primary dysmenorrhea and factors associated with its intensity among undergraduate students: a cross-sectional study. Pain Manag Nurs 16:855–861
Liedman R, Hansson SR, Howe D, Igidbashian S, Russell RJ, Akerlund M (2008) Endometrial expression of vasopressin, oxytocin and their receptors in patients with primary dysmenorrhoea and healthy volunteers at ovulation. Eur J Obstet Gynecol Reprod Biol 137:189–192
Yang L, Cao Z, Yu B, Chai C (2015) An in vivo mouse model of primary dysmenorrhea. Exp Anim 64:295–303
Turhan N, Celik H, Duvan Cİ, Onaran Y, Aydın M, Armutcu F (2012) Investigation of oxidative balance in patients with dysmenorrhea by multiple serum markers. J Turk Ger Gynecol Assoc 13:233–236
Aksoy AN, Gözükara I, Kabil Kucur S (2014) Evaluation of the efficacy of Fructus agni casti in women with severe primary dysmenorrhea: a prospective comparative Doppler study. J Obstet Gynaecol Res 40:779–784
Shirvani MA, Motahari-Tabari N, Alipour A (2015) The effect of mefenamic acid and ginger on pain relief in primary dysmenorrhea: a randomized clinical trial. Arch Gynecol Obstet 291:1277–1281
Iacovides S, Baker FC, Avidon I (2014) The 24-h progression of menstrual pain in women with primary dysmenorrhea when given diclofenac potassium: a randomized, double-blinded, placebo-controlled crossover study. Arch Gynecol Obstet 289:993–1002
Otterbein LE, Mantell LL, Choi AM (1999) Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol 276:688–694
Ryter SW, Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28:289–309
Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG (2003) Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev 55:551–571
Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, Miyamoto Y, Maeda H (2003) Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer 88:902–909
Soares MP, Marguti I, Cunha A, Larsen R (2009) Immunoregulatory effects of HO-1: how does it work? Curr Opin Pharmacol 9:482–489
Gaweł S, Wardas M, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 57:453–455
Waltz P, Escobar D, Botero AM, Zuckerbraun BS (2015) Nitrate/nitrite as critical mediators to limit oxidative injury and inflammation. Antioxid Redox Signal 23:328–339
Lee SJ, Zhang J, Choi AM, Kim HP (2013) Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid Med Cell Longev 2013: 327167.
Dikensoy E, Balat O, Pençe S, Balat A, Cekmen M, Yurekli M (2008) Malondialdehyde, nitric oxide and adrenomedullin levels in patients with primary dysmenorrhea. J Obstet Gynaecol Res 34:1049–1053
Crichton N (2000) Information point: Visual Analogue Scale (VAS). J Clin Nurse 10:706
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Lundberg JO, Weitzberg E (2005) NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol 25:915–922
Erdemli HK, Yıldırımlar P, Alper TY, Kocabaş R, Salis O, Bedir A (2014) Increased serum heme oxygenase-1 levels as a diagnostic marker of oxidative stress in preeclampsia. Hypertens Pregnancy 33:488–497
Lenth RV (2006) Java applets for power and sample size (computer software). http://www.stat.uiowa.edu/~rlenth/Power
Yeh ML, Chen HH, So EC, Liu CF (2004) A study of serum malondialdehyde and interleukin-6 levels in young women with dysmenorrhea in Taiwan. Life Sci 75:669–673
Facchinetti F, Sgarbi L, Piccinini F, Volpe A (2002) A comparison of glyceryl trinitrate with diclofenac for the treatment of primary dysmenorrhea: an open, randomized, cross-over trial. Gynecol Endocrinol 16:39–43
Sun MF, Huang HC, Lin SC, Chang LP, Liu CF (2005) Evaluation of nitric oxide and homocysteine levels in primary dysmenorrheal women in Taiwan. Life Sci 76:2005–2009
Akdemir N, Cinemre H, Bilir C, Akin O, Akdemir R (2010) Increased serum asymmetric dimethylarginine levels in primary dysmenorrhea. Gynecol Obstet Invest 69:153–156
Xin G, Du J, Wang YT, Liang TT (2014) Effect of oxidative stress on heme oxygenase-1 expression in patients with gestational diabetes mellitus. Exp Ther Med 7:478–482
Author contributions
ANA: project development, data collection, manuscript writing and editing. EL: project development, data collection and management, manuscript editing. ALO: project development, data analysis, manuscript editing. EPTY: project development, data collection, manuscript writing and editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Aksoy, A.N., Laloglu, E., Ozkaya, A.L. et al. Serum heme oxygenase-1 levels in patients with primary dysmenorrhea. Arch Gynecol Obstet 295, 929–934 (2017). https://doi.org/10.1007/s00404-017-4312-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4312-1