Abstract
Purpose
Primary dysmenorrhea, which refers to painful, spasmodic cramping in the lower abdomen just before/or during menstruation, is the most common gynecological complaint in women of reproductive age. Non-steroidal anti-inflammatory drugs have been prescribed as the first-line therapy for pain relief from dysmenorrhea. We aimed to investigate the efficacy of the daily recommended dose (150 mg) of diclofenac potassium, administered at set intervals across the first 24 h of menstruation, in treating severe menstrual pain in 24 women with severe primary dysmenorrhea.
Methods
In a randomized, placebo-controlled, double-blind cross-over study, women rated their menstrual pain intensity on a 100-mm visual analog scale across set time intervals over a 24-h period.
Results
Menstrual pain intensity was significantly reduced after taking the first capsule of diclofenac, and remained consistently lower (P < 0.0001), compared with initial pain intensity, in the morning (before treatment), throughout the day, evening, and into the next morning. Also, women rated their pain intensity as significantly lower (P < 0.001) at each time point across the 24-h time interval of the cycle when receiving diclofenac compared with the cycle when they received placebo. No woman required rescue medication when taking diclofenac potassium compared with six women taking rescue medications during the placebo trial. When taking only placebo, women rated their menstrual pain intensity as persistently severe across the first 24 h of menstruation.
Conclusion
These results show that the recommended daily dose of diclofenac potassium, in three 50 mg doses across the day and evening, offers effective menstrual pain relief across 24 h, compared with placebo, in women with severe primary dysmenorrhea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary dysmenorrhea, defined as painful, spasmodic cramping in the lower abdomen, just before and/or during menstruation, in the absence of any discernable macroscopic pelvic pathology [1], is the most common gynecological condition among women of reproductive age [2, 3]. The onset of primary dysmenorrhea usually occurs in adolescence, at or shortly after (6–24 months) menarche [4]. Primary dysmenorrheic pain is most severe during the first or second day of menstruation and typically lasts for 8–72 h [3]. The pain may radiate to the back and thighs and is frequently accompanied by systemic symptoms including nausea, vomiting, diarrhea, fatigue, and insomnia [4, 5].
Prevalence estimates of this gynecological inflammatory disorder vary between 45 and 95 % of menstruating women [6, 7] and is very severe in approximately 10–25 % of women of reproductive age [1, 4, 8, 9]. The painful menstrual cramps may be considerably disabling; dysmenorrheic pain has been documented to be as intense as renal colic pain [10] and, as such, has been associated with decreased physical activity [11–13], recurrent short-term school or work absenteeism [2], interference with women’s social and professional lives [14], and poor subjective [15, 16] and objective sleep quality [17, 18].
Primary dysmenorrhea is believed to result from excessive Prostaglandin (PG) F2α release [1, 5] which causes vasoconstriction of uterine blood vessels (uterine ischemia) and increased uterine smooth muscle contraction [4]. The contraction of the ischemic uterus is the likely cause of dysmenorrheic pain [19]. Furthermore, PG F2α lowers the threshold for pain perception by sensitizing nerve endings to pain [4, 5, 20]. Non-steroidal anti-inflammatory drugs (NSAIDs) have been prescribed as the first-line therapy for pain relief from dysmenorrhea [5, 13, 20–22]. Pharmacologically, NSAIDs inhibit the iso-enzymes of the cyclooxygenase (COX) family which catalyze the synthesis of PGs from arachidonic acid. Cyclooxygenase exists primarily in two isoforms: COX-1 and COX-2, each with different prevalence and effects in various tissues [21, 23]. Cyclooxygenase-1 is involved in a variety of regulatory and homeostatic functions such as cytoprotection of the gastric mucosa, [21, 23] whereas COX-2 is primarily upregulated in numerous pathophysiological states by pro-inflammatory agents [21, 23].
Historically, traditional NSAIDs, the selective COX-1 inhibitors, that inhibit COX-1 more than COX-2, such as naproxen and ibuprofen, and the non-selective COX inhibitors that inhibit both COX-1 and COX-2, such as diclofenac and piroxicam, [21, 23] were prescribed to treat dysmenorrhea [24, 25]. These traditional NSAIDs have analgesic efficacy superior to placebo for treating dysmenorrheic pain [14, 26–34]. However, concerns over the gastrointestinal (GI) safety associated with the inhibition of COX-1 [5, 21, 35] led to the production of the “newer” generation NSAIDs (e.g. celecoxib, rofecoxib, and lumiracoxib) that are more selective and more specific in their inhibition of COX-2, while sparing COX-1 [21]. These “newer” NSAIDs were, until recently, prescribed to treat a variety of inflammatory conditions, including dysmenorrhea [5, 20, 36]. However, many of these COX-2 specific inhibitors have been withdrawn due to cardiovascular safety concerns [6, 22, 35].
As such, health care providers and women with dysmenorrhea have to now resort back to the “older” generation, non-selective COX inhibitors/NSAIDs to treat menstrual pain, with varying degrees of success. Therefore, there is still a need to identify which one of these non-selective COX inhibitors/NSAIDs is best able to treat menstrual pain quickly and safely. One such NSAID that is proving to be beneficial in treating dysmenorrhea is diclofenac, a non-selective NSAID with a fourfold selectivity of COX-2 over COX-1 [21]. Its poor selectivity of COX-1, as well as its pharmacological properties which allow diclofenac to exert an extended duration of action, despite its rapid systemic elimination, means that it has a low side-effect profile [21, 23, 37, 38], rendering it a safe option for the treatment of the repetitive, acute pain experienced monthly by women with dysmenorrhea. Several studies have shown that diclofenac is an effective treatment for dysmenorrhea in the short term, from 2 to 8 h after treatment administration [14, 29, 39]. Studies have varied in terms of daily doses being taken (from 1 to 6 times daily) [14, 29, 34, 39, 40] with most studies allowing participants to choose their own dosing schedule based on the need for pain relief [14, 29, 34, 40]. To our knowledge, only one study, which was conducted by our research group, has monitored dysmenorrheic pain severity over a period of more than 8 h with diclofenac potassium versus placebo [40]. In this study, pain intensity was measured before and 2 h after treatment (diclofenac, rofecoxib, meloxicam or placebo) over a 2- to-3-day period. However, dosages were not consistent among women, as each woman was allowed to self-medicate with up to two pills daily [40].
In this study we aimed to investigate further the efficacy of the daily recommended dose of diclofenac potassium (150 mg), administered as 50 mg capsules, at three intervals across the first day of menstruation, compared with placebo, in treating severe primary dysmenorrhea in a randomized, double-blind, crossover study. We assessed dysmenorrheic pain severity before and after each capsule, as well as the following morning to determine the progression of pain across 24 h with and without effective treatment.
Methods
Screening phase
Thirty-three women from a university student population volunteered to participate in the study and gave written informed consent before participation. Ethical clearance was obtained from the University of the Witwatersrand’s Committee for Research on Human Subjects, which adheres to the principles of the Declaration of Helsinki (Clearance No. M050537).
The volunteers were first interviewed and screened to ensure that they were generally healthy women with severe primary dysmenorrhea. A 100-mm visual analog scale (VAS) anchored from “no pain at all” to “the worst pain I have ever felt” was used to determine the severity of each volunteer’s retrospective dysmenorrheic pain (over the past 6 months). The VAS has been shown to be a reliable and valid measure of experimental and clinical pain, which is sensitive to the effects of treatment of clinical pain and to small changes in pain intensity [41–45].
Women were asked to make a mark on the 100-mm VAS to indicate the intensity of their pain, and VAS scores were obtained by measuring the distance, in mm, between the beginning anchor point (“no pain at all”) to the mark filled in by the subjects. The VAS assessment of dysmenorrheic pain intensity is highly correlated with the pain rating index and the present pain index derived from the McGill pain questionnaire [40]. Women who rated their menstrual pain, for the past 6 months, above 60 mm on the VAS were considered to have severe dysmenorrheic pain [46]. Women also completed a customized questionnaire about their menstrual cycles and history of menstrual pain, based on Andersch and Milsom [8]. They were asked about the impact of dysmenorrheic pain on daily activity, the presence of associated symptoms, and their analgesic requirements. Volunteers who had a history of pathological conditions associated with uterine pain, indicating secondary dysmenorrhea and not primary dysmenorrhea, were excluded from the study. In addition, volunteers were immediately excluded from the study if they presented with irregular menstrual cycles (defined as lasting <21 or >35 days and/or with more than 4 days variation between cycles) [47], insufficient dysmenorrheic pain (<60 mm on the VAS), or sensitivity to NSAIDs. For participation in the study, the women were required to have a history of primary dysmenorrhea, starting shortly after menarche [19], and were also required to be nulliparous individuals who were not taking chronic medication (including oral contraceptives) for at least 6 months before the study. In addition, the 30-item version of the General Health Questionnaire was used for psychological screening, and only women who scored <6 indicating normal psychological status were included in the study [48]. After completion of the screening procedures, 24 eligible women with a history of primary dysmenorrhea agreed to participate in the study.
Procedures
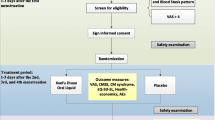
The women were required to follow a randomized, double-blinded, placebo-controlled, crossover medicated procedure on the day they experienced their most intense dysmenorrheic pain. All women experienced their most severe dysmenorrheic pain on day 1, the first day of menstruation. Study medications were diclofenac potassium (50 mg, Cataflam D, Novartis, South Africa) or placebo (cane sugar); disguised in identical gelatine capsules. The study was conducted over two menstrual periods, with each medication being taken in a separate menstrual period. Women were randomly assigned to a treatment sequence based on a Latin square design. Fourteen women had placebo first and the remaining ten women had diclofenac potassium first. Each woman was provided with a diary in which to document menstrual pain severity, times of medication administration, adverse events, and details of any rescue medications taken. The protocol for treatment and pain severity evaluation is outlined in Fig. 1. When the women first felt sufficient dysmenorrheic pain to require pain relief, they were instructed to record the time, as well as their pain intensity on a VAS anchored from “no pain at all” to “the worst pain I have ever felt”, before self-medicating with a prescribed capsule taken with a glass of water. The onset of dysmenorrheic pain for all the women was in the morning hours; therefore, all the women took their first prescribed capsule in the morning, shortly after waking (between 8:00 and 10:00 a.m.). Participants were instructed to rate their menstrual pain severity on a second VAS 2 h after taking medication; an indication of any pain relief that the medication may have provided. Five hours after taking the first capsule, regardless of whether pain relief was required, the women completed a third VAS before taking the second prescribed capsule. Two hours after taking the second prescribed capsule, the women completed a fourth VAS; again serving as an indication of any pain relief provided by the medication. In the evenings, before going to sleep, the women completed a fifth VAS to indicate the intensity of their dysmenorrheic pain and took the third prescribed capsule. All women went to bed between 21:00 and 23:00 p.m. Thus the time interval between capsules 1 and 2 was always 5 h, while the time interval between capsules 2 and 3 varied between 6 and 10 h. Last, upon waking the following morning, the women rated the intensity of their current dysmenorrheic pain on another VAS.
In the event that any of the women required further pain relief from their dysmenorrheic pain during the day they were allowed to use their own rescue medication (that which they normally would take). However, the women were encouraged to try and not take rescue medication unless it was absolutely necessary. Such information was recorded and is reported in Table 2. However, regardless of whether the women took rescue medication or not, they were still expected to complete the prescribed capsule administration as described in the research procedure above. When the women required further pain relief from their dysmenorrheic pain during the night, however, they were given another (fourth) prescribed capsule (50 mg diclofenac potassium or placebo) and were encouraged to take the prescribed capsule before resorting to their own rescue medication (see Table 2).
Statistical analyses
All data were analyzed using STATISTICA (StatSoft, Tulsa, version 5, 1996). A two-tailed probability of P < 0.05 was considered to be statistically significant. All VAS measurements (in mm), used to describe dysmenorrheic pain, were normalized before statistical analyses using the arcsine transformation; as advised when a large number of values fall within the extremes of the scale [49]. However, all text and graphs report the VAS data as back transformed values (in mm). All VAS data are thus expressed as mean ± standard deviation (SD).
Each woman’s 24-h progression of dysmenorrheic pain intensity (VAS) scores were plotted on separate graphs and best-fit curves were fitted (table curve 2D, version 3 for Win 32, Jandel Scientific Software, AISN Software Inc., San Rafael, CA, USA). Area under the VAS-time curve (AUC, mm h−1) was calculated and a paired t test was used to compare the AUC between treatments (diclofenac versus placebo, n = 24).
A two-way repeated-measures analysis of variance (two-way RM-ANOVA) with time and treatment administered as main effects was used to compare the intensity of dysmenorrheic pain, as measured by the VAS (mm), between the treatments (diclofenac versus placebo, n = 24), across the various times of assessment over the 24-h period. Where appropriate, the Student–Newman–Keuls (SNK) post hoc test was used to assess the origin of any significant differences detected by the ANOVA model.
The McNemar’s test was used to determine whether the number of women who took rescue medication during diclofenac treatment versus placebo was statistically significant.
A secondary analysis using the same statistical procedures described above was performed on the group of women who did not use rescue medication on any occasion during the 24-h placebo treatment (n = 18) to evaluate the true placebo effect on dysmenorrheic pain.
Results
The characteristics of the 24 women who participated in the study are shown in Table 1.
All of the women followed and completed the study medication procedure; however, 6 of the 24 women opted to take rescue medication when placebo failed to provide pain relief. The rescue medications taken, as well as the dose and time at which they were taken, are displayed in Table 2.
The progression of dysmenorrheic pain intensity, expressed as the average VAS scores, throughout the day when the women were taking diclofenac potassium compared with placebo (including the six women who took rescue medications) is displayed in Fig. 2. A two-way RM-ANOVA revealed significant treatment [F(5,115) = 31.27, P < 0.0001], time [F(1,23) = 32.60, P < 0.0001] and treatment-time interaction [F(5,115) = 8.47, P < 0.0001] effects. As demonstrated by the starting points of the two curves in Fig. 2 (Time “0”), the women’s intensity of dysmenorrheic pain before any treatment was not significantly different between the two trials (diclofenac versus placebo; P = 0.90). The progression of pain intensity throughout the day, as shown in Fig. 2, followed two distinct patterns, depending on the treatment received (diclofenac or placebo). As confirmed by post hoc analyses, the pain intensity of the women taking diclofenac potassium was significantly reduced after taking capsule 1 and remained at a significantly lower intensity at all the time points compared with the initial (Time “0”) pain intensity (SNK: P < 0.0001 between Time “0” and all subsequent time-points; Fig. 2). In addition, women rated their pain intensity as significantly lower when taking diclofenac potassium compared with placebo at each time point after commencing treatment (after Time “0”) (P < 0.0001; Fig. 2). When the women were taking placebo, pain intensity was significantly lower at 7 h (after capsule 2; SNK: P = 0.001) and in the morning (SNK: P = 0.0007) compared with Time “0”.
The progression of dysmenorrheic pain intensity over time, as measured by the visual analog scale (back transformed VAS mean and SD) when the 24 women with primary dysmenorrhea were given either diclofenac potassium or placebo (including the 6 women who took rescue medications as shown in Table 2). Pain intensity was assessed at six different time points across 24 h; before taking capsule 1 (time = 0), 2 h after taking capsule 1 (time = +2), before taking capsule 2 (time = +5), 2 h after taking capsule 2 (time = +7), in the evening before going to bed (before taking capsule 3), and in the morning upon waking. Asterisk represents a significant difference in the VAS score from the initial VAS score (before treatment at time = 0):*P < 0.05, **P < 0.01, ***P < 0.0001. Dagger represents a significant difference in the VAS score between diclofenac potassium and placebo at each time point: ††† P < 0.0001
Furthermore, the area under the VAS-time curve (mm h−1) was significantly greater when the women were taking placebo compared with when they were taking diclofenac potassium [t(23) = 8.48; P < 0.0001], indicating that the women rated the intensity of their dysmenorrheic pain as more severe throughout the 24-h assessment period when they were taking placebo (including those women who used rescue medications) compared with when they were taking diclofenac potassium.
Results were similar when the six women who took rescue medication were excluded from the analysis. The two-way RM-ANOVA between diclofenac and placebo alone (n = 18), showed significant treatment [F(1,34) = 29.5, P < 0.0001], time [F(5,170) = 30.5, P < 0.0001), and interaction [F(5,170) = 11.4, P < 0.0001) effects. SNK post hoc analyses showed that following treatment with the first capsule, the pain ratings after the administration of diclofenac potassium were significantly lower than after placebo administration (P < 0.0001) at all time points. The pure placebo response for dysmenorrheic pain over the first 24 h of menstruation is shown in Fig. 3. Pain severity was lower from 5 h after taking the first capsule onwards, compared with that before taking the first capsule although pain ratings remained, on average, at or above a severe level (60 mm) for the 24 h period.
The progression of dysmenorrheic pain intensity over time, as measured by the visual analog scale (back transformed VAS mean and SD) when 18 women with primary dysmenorrhea were treated with placebo and resisted taking any rescue medications. Pain intensity was assessed at six different time points across 24 h; before taking capsule 1 (time = 0), 2 h after taking capsule 1 (time = +2), before taking capsule 2 (time = +5), 2 h after taking capsule 2 (time = +7), in the evening before going to bed (before taking capsule 3), and in the morning upon waking. Asterisk represents a significant difference in the VAS score from the initial VAS score (before treatment at time = 0):*P < 0.05, **P < 0.01
No rescue medications were taken when the women’s dysmenorrheic pain was treated with diclofenac potassium, and no woman took the fourth capsule of diclofenac potassium during the night. In addition, there were no reported adverse events when the women were taking diclofenac potassium, placebo or rescue medications for their menstrual pain.
Discussion
We have shown that diclofenac potassium, administered in three 50 mg doses across a day, effectively attenuated menstrual pain throughout the day and night, and into the following morning compared with placebo in women experiencing severe dysmenorrhea. None of the women required rescue medication when they treated their dysmenorrheic pain with diclofenac potassium, whereas six of them (25 %) used rescue medication when taking placebo. The 150-mg recommended daily dose of diclofenac potassium is, therefore, effective in treating dysmenorrheic pain.
Pharmacologically, NSAIDs are prescribed as the agent of choice to treat dysmenorrhea [5, 13, 20–22] as they are able to effectively inhibit the COX-2 enzyme responsible for the production of PGs implicated in the etiology of dysmenorrhea. Since the withdrawal of the once-thought safer “newer” generation COX-2 selective and COX-2 specific NSAIDs from the market [6, 22, 35], the traditional “older” generation non-selective COX-inhibitors/NSAIDs, such as diclofenac and ibuprofen, are now being prescribed more frequently to safely treat dysmenorrhea. We have shown that the “older” generation non-selective COX inhibitor, diclofenac, is effective in treating dysmenorrheic pain.
Our study results are in agreement with those of several other studies that have shown that diclofenac (sodium and potassium) is effective in alleviating dysmenorrheic pain. Compared with placebo, 25 mg of diclofenac sodium, administered three times daily, was found to significantly reduce menstrual pain in 35 women with dysmenorrhea [34]. However, in this study pain assessment was only done once after menstruation and women were asked to compare current pain intensity with that of the previous menstruation, requiring retrospective recall [34]. Others have reported diclofenac sodium (50 mg up to three times daily, when needed) to be more effective and more tolerable in the treatment of dysmenorrhea, compared with a smooth muscle relaxant, at least during a 2-h assessment following first treatment [14]. Similarly, diclofenac dispersible (equivalent to 50 mg diclofenac sodium) taken up to four times daily was found to provide better menstrual pain relief than placebo over a 6-h period [29]. In addition, a single dose of aceclofenac (100 mg), a glycolic acid ester of diclofenac [50], used either alone [28] or in combination with a smooth muscle relaxant [51], has been reported as a safe and well-tolerated analgesic for primary dysmenorrhea.
Similarly, diclofenac potassium has been found to be highly effective in relieving menstrual pain in 11 women with primary dysmenorrhea [40]. In this study, a maximum of two capsules of diclofenac potassium (50 mg) was prescribed daily and pain assessment done immediately before and 2 h after taking the capsule [40]. Other studies have investigated the efficacy of diclofenac in the treatment of dysmenorrhea; however, they have been excluded from “The Cochrane Collaboration” [52], either due to lacking of randomization or lack of blinding and are thus not discussed here.
Substantiating the efficacy of diclofenac potassium, via effective pain relief, we previously have reported that diclofenac potassium restores the dysmenorrheic pain-induced reduction in physical activities [39] and dysmenorrheic pain-induced reduction in sleep quality [18]. Our data extend these findings to show that diclofenac potassium, when taken at three time-points across the day, provides effective treatment of severe dysmenorrheic pain across the day and through the night, such that pain intensity is lower across 24 h, compared with placebo. We chose to instruct participants to take three capsules of medication across the day regardless of their level of dysmenorrheic pain at the time. We, therefore, cannot comment whether a lower dose over a 24-h period might also be effective. Direct comparisons, however, with other studies are also difficult to make due to the varied doses (from 1 to 6 times daily) [14, 29, 34, 39, 40], the freedom of participants to choose their own dosing schedule based on the need for pain relief [14, 29, 34, 40], and the fact that most studies only monitored pain for 2–8 h after treatment administration [14, 29, 39].
It has been previously recommended that treatment of primary dysmenorrhea with NSAIDs, in adolescents at least, could be initiated 1–2 days before the onset of menstruation [20]. We have shown that taking the recommended daily dose of diclofenac potassium in three capsules across the first day of menstruation is an effective method to alleviate menstrual pain for at least 24 h. The protocol employed by this study may be a better option given that the duration of treatment with NSAIDs for primary dysmenorrhea is recommended to be no longer than 3 days, to avoid possible adverse effects [22].
As described above, most research on the use of diclofenac for the treatment of dysmenorrhea has used diclofenac sodium. Although pharmacokinetic studies show similar bioavailability between diclofenac sodium and potassium, the mean time to reach maximal plasma levels is shorter with diclofenac potassium (±30 min) [53] compared with diclofenac sodium (±1.5 to 2 h) [37]. Such properties may account for a faster onset of action with diclofenac potassium; for example diclofenac potassium significantly reduced postoperative dental pain intensity scores starting at 15 min, compared with diclofenac sodium at 2 h [54]. However, no studies have directly compared the time efficacy of diclofenac potassium and diclofenac sodium in alleviating primary dysmenorrheic pain specifically.
Most previous investigations of efficacy of agents in treating primary dysmenorrhea have been conducted on less-selected, and in some cases, larger cohorts, than ours. Our study group was a non-clinical sample of young women recruited from a University population. As such, our results may not be generalizable to all adolescents and women with primary dysmenorrhea. A limitation of our study was the small sample size. However, each woman acted as her own control which allowed for a more robust within-subject analysis to be performed. Despite the reduced statistical power sensitivity associated with a small sample size, we were still able to detect robust significant findings in the VAS pain ratings in the ANOVA model. The women in our study reported no adverse effects during or after administration of diclofenac potassium. However, our study was not intended nor powered to detect adverse events. It would take a far greater cohort to resolve differences in adverse effects associated with COX inhibitors taken for 2 days every month, by otherwise-healthy young women. Although not specifically in dysmenorrheic patients, the safety and tolerability of diclofenac has been reported in an extensive review of worldwide clinical trials involving over 100,000 patients who were treated with diclofenac [55]. Given that GI issues are generally of less concern in acute use of NSAIDs compared with chronic use [22, 56], and given diclofenac potassium’s poor selectivity of COX-1 [21], it is a safe GI option for the treatment of the acute pain experienced monthly by women with dysmenorrhea.
Our study is in agreement with most studies in that we found an NSAID to be superior to placebo in alleviating dysmenorrheic pain. Almost all randomized control trials investigating NSAIDs for the treatment of dysmenorrhea have reported a superiority of the active treatment compared with placebo [22, 26–33, 40, 52]. In agreement with other studies [14, 39, 40], the women who received diclofenac potassium did not require rescue medication during the day or night. In comparison, six of the women chose to self-medicate with their preferred rescue medication when they were given placebo for their menstrual pain (Table 2). The removal of these six women from the statistical analysis revealed that diclofenac potassium offered superior pain relief to placebo at each time point across the 24-h assessment period and also showed the daily progression of dysmenorrheic pain when women were taking placebo alone (Fig. 3).
Little is known about the daily progression of dysmenorrheic pain. All 24 women in our study reported a morning onset of severe menstrual pain, which coincided with the beginning of menstruation. The onset of menstruation of the women in our study coincides with data showing that the majority of women (±54 %) start menstruating in morning, while ±32 % starting in the afternoon and early evening, and only about ±14 % starting during the night [57]. In terms of the onset of pain, however, our study’s findings are in contrast with a report showing increased primary dysmenorrheic pain during the evening, coinciding with reduced uterine blood flow during the evening [58]. Since the main factors attributed to the etiology of primary dysmenorrheic pain, namely prostaglandins, vasopressin, and uterine blood flow, and contractility, have all been found to display diurnal variation [59, 60], it is plausible that dysmenorrheic pain may also have a diurnal rhythm. However, our analysis of dysmenorrheic pain severity in women treated only with placebo revealed only a small decrease in pain severity 5 h after the first capsule, which then remained at a constantly severe level (60 mm, on average) for the remainder of the 24-h assessment period. More studies, on larger samples, however, should be conducted to further investigate the daily variation in dysmenorrheic pain.
Primary dysmenorrhea in women of child-bearing age is extremely common, and the pain is often poorly managed and debilitating [10]. We have shown that the recommended daily dose of a readily available NSAID, diclofenac potassium, in 50 mg doses across the day and evening offers effective menstrual pain relief that lasts throughout the day, evening, and into the next morning, compared with placebo.
References
Dawood MY (1987) Dysmenorrhea and prostaglandins. In: Gold JJ, Josimovich JB (eds) Gynecologic endocrinology, vol 4. Plenum Publishing Corporation, New York, pp 405–421
Coco AS (1999) Primary dysmenorrhea. Am Fam Physician 60(2):489–496
Proctor M, Farquhar C (2002) Dysmenorrhoea. Clin Evid 7:1639–1653
Hofmeyr GJ (1996) Dysmenorrhoea. In: Bassin J (ed) Topics in obstetrics and gynaecology. Julmar Communications, Johannesburg, pp 269–274
Ruoff G, Lema M (2003) Strategies in pain management: new and potential indications for COX-2 specific inhibitors. J Pain Symptom Manage 25(2 Suppl):S21–S31
Proctor M, Farquhar C (2006) Diagnosis and management of dysmenorrhoea. BMJ (Clin Res Ed) 332(7550):1134–1138
Unsal A, Ayranci U, Tozun M, Arslan G, Calik E (2010) Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Upsala J Med Sci 115(2):138–145
Andersch B, Milsom I (1982) An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol 144(6):655–660
Sundell G, Milsom I, Andersch B (1990) Factors influencing the prevalence and severity of dysmenorrhoea in young women. Br J Obstet Gynaecol 97(7):588–594
Ayan M, Sogut E, Tas U, Erdemir F, Sahin M, Suren M, Kaya Z, Demirturk F (2012) Pain levels associated with renal colic and primary dysmenorrhea: a prospective controlled study with objective and subjective outcomes. Arch Gynecol Obstet 286:403–409
Chen CH, Lin YH, Heitkemper MM, Wu KM (2006) The self-care strategies of girls with primary dysmenorrhea: a focus group study in Taiwan. Health Care Women Int 27(5):418–427
Chantler I, Mitchell D, Fuller A (2009) Actigraphy quantifies reduced voluntary physical activity in women with primary dysmenorrhea. J Pain 10(1):38–46
Dawood MY (1995) Dysmenorrhea. Endometrium 6(2):363–377
Facchinetti F, Sgarbi L, Piccinini F, Volpe A (2002) A comparison of glyceryl trinitrate with diclofenac for the treatment of primary dysmenorrhea: an open, randomized, cross-over trial. Gynecol Endocrinol 16(1):39–43
Ohde S, Tokuda Y, Takahashi O, Yanai H, Hinohara S, Fukui T (2008) Dysmenorrhea among Japanese women. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 100:13–17
El-Gilany AH, Badawi K, El-Fedawy S (2005) Epidemiology of dysmenorrhoea among adolescent students in Mansoura, Egypt. East Mediterr Health J 11(1–2):155–163
Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D (1999) High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol 277(6 Pt 1):E1013–E1021
Iacovides S, Avidon I, Bentley A, Baker FC (2009) Diclofenac potassium restores objective and subjective measures of sleep quality in women with primary dysmenorrhea. Sleep 32(8):1019–1026
Dawood MY (1985) Dysmenorrhea. J Reprod Med 30(3):154–167
Harel Z (2004) Cyclooxygenase-2 specific inhibitors in the treatment of dysmenorrhea. J Pediatr Adolesc Gynecol 17(2):75–79
Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR (1999) Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA 96:7563–7568
Zahradnik HP, Hanjalic-Beck A, Groth K (2010) Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception 81(3):185–196
Frolich JC (1997) A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol Sci 18(1):30–34
Dawood MY (2006) Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol 108(2):428–441
Marjoribanks J, Proctor ML, Farquhar C (2003) Nonsteroidal anti-inflammatory drugs for primary dysmenorrhoea. Cochrane Database Syst Rev (Online) (4):CD001751
Budoff PW (1982) Zomepirac sodium in the treatment of primary dysmenorrhea syndrome. N Engl J Med 307(12):714–719
Hanson FW, Izu A, Henzl MR (1978) Naproxen sodium in dysmenorrhea. Its influence in allowing continuation of work/school activities. Obstet Gynecol 52(5):583–587
Letzel H, Megard Y, Lamarca R, Raber A, Fortea J (2006) The efficacy and safety of aceclofenac versus placebo and naproxen in women with primary dysmenorrhoea. Eur J Obstet Gynecol Reprod Biol 129(2):162–168
Marchini M, Tozzi L, Bakshi R, Pistai R, Fedele L (1995) Comparative efficacy of diclofenac dispersible 50 mg and ibuprofen 400 mg in patients with primary dysmenorrhea. A randomized, double-blind, within-patient, placebo-controlled study. Int J Clin Pharmacol Ther 33(9):491–497
Mehlisch DR (1988) Ketoprofen, ibuprofen, and placebo in the treatment of primary dysmenorrhea: a double-blind crossover comparison. J Clin Pharmacol 28(12 Suppl):S29–S33
Milsom I, Minic M, Dawood MY, Akin MD, Spann J, Niland NF, Squire RA (2002) Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen, and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clin Ther 24(9):1384–1400
Zhang WY, Li Wan Po A (1998) Efficacy of minor analgesics in primary dysmenorrhoea: a systematic review. Br J Obstet Gynaecol 105(7):780–789
Mehlisch DR (1990) Double-blind crossover comparison of ketoprofen, naproxen, and placebo in patients with primary dysmenorrhea. Clin Ther 12(5):398–409
Riihiluoma P, Wuolijoki E, Pulkkinen MO (1981) Treatment of primary dysmenorrhea with diclofenac sodium. Eur J Obstet Gynecol Reprod Biol 12(3):189–194
McQuay HJ, Moore RA (2003) Side effects of COX-2 inhibitors and other NSAIDs. In: Dostrovsky JO, Carr DB, Koltzenburg KM (eds) Proceedings of the 10th world congress on pain, IASP press, Seattle, pp 499–510
Daniels S, Gitton X, Zhou W, Stricker K, Barton S (2008) Efficacy and tolerability of lumiracoxib 200 mg once daily for treatment of primary dysmenorrhea: results from two randomized controlled trials. J Women’s Health 17(3):423–437
Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS (1980) Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs 20:24–48
O’Brien WM (1986) Adverse reactions to nonsteroidal anti-inflammatory drugs. Diclofenac compared with other nonsteroidal anti-inflammatory drugs. Am J Med 80(4B):70–80
Chantler I, Mitchell D, Fuller A (2009) Diclofenac potassium attenuates dysmenorrhoeic pain and restores exercise performance in women with primary dysmenorrhoea. J Pain 10:191–200
Chantler I, Mitchell D, Fuller A (2008) The effects of three cyclo-oxygenase inhibitors with different cyclo-oxygenase-2 specificity on intensity of primary dysmenorrhoeic pain. Clin J Pain 24:39–44
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17(1):45–56
Scott J, Huskisson EC (1976) Graphic representation of pain. Pain 2(2):175–184
Revill SI, Robinson JO, Rosen M, Hogg MI (1976) The reliability of a linear analogue for evaluating pain. Anaesthesia 31(9):1191–1198
Price DD, Bush FM, Long S, Harkins SW (1994) A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 56(2):217–226
Coll AM, Ameen J (2006) Profiles of pain after day surgery: patients’ experiences of three different operation types. J Adv Nurs 53(2):178–187
Collins SL, Moore RA, McQuay HJ (1997) The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 72(1–2):95–97
Harmon D, O’Connor P, Gleasa O, Gardiner J (2000) Menstrual cycle irregularity and the incidence of nausea and vomiting after laparoscopy. Anaesthesia 55(12):1164–1167
Goldberg DP, Rickels K, Downing R, Hesbacher P (1976) A comparison of two psychiatric screening tests. Br J Psychiatry 129:61–67
Dexter F, Chestnut DH (1995) Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology 82(4):896–902
Hinz B, Rau T, Auge D, Werner U, Ramer R, Rietbrock S, Brune K (2003) Aceclofenac spares cyclooxygenase 1 as a result of limited but sustained biotransformation to diclofenac. Clin Pharmacol Ther 74(3):222–235
Pareek A, Chandurkar NB, Patil RT, Agrawal SN, Uday RB, Tambe SG (2010) Efficacy and safety of aceclofenac and drotaverine fixed-dose combination in the treatment of primary dysmenorrhoea: a double-blind, double-dummy, randomized comparative study with aceclofenac. Eur J Obstet Gynecol Reprod Biol 152(1):86–90
Marjoribanks J, Proctor M, Farquhar C, Derks RS (2010) Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev (Online) (1):CD001751
Moore N (2007) Diclofenac potassium 12.5 mg tablets for mild to moderate pain and fever: a review of its pharmacology, clinical efficacy and safety. Clin Drug Investig 27(3):163–195
Bakshi R, Jacobs LD, Lehnert S, Picha B, Reuther J (1992) A double-blind, placebo-controlled trial comparing the analgesic efficacy of two formulations of diclofenac in postoperative dental pain. Curr Therap Res 52(3):435–442
Willkens RF (1985) Worldwide clinical safety experience with diclofenac. Semin Arthritis Rheum 15(2 Suppl 1):105–110
Langford RM, Evans N (2002) Developments in specific cyclooxygenase therapy for acute pain. Acute Pain 4(1):1–4
Malek J, Gleich J, Maly V (1962) Characteristics of the daily rhythm of menstruation and labor. Ann N Y Acad Sci 98:1042–1055
Celik H, Gurates B, Parmaksiz C, Polat A, Hanay F, Kavak B, Yavuz A, Artas ZD (2009) Severity of pain and circadian changes in uterine artery blood flow in primary dysmenorrhea. Arch Gynecol Obstet 280(4):589–592
Zaidi J, Jurkovic D, Campbell S, Pittrof R, McGregor A, Tan SL (1995) Description of circadian rhythm in uterine artery blood flow during the peri-ovulatory period. Hum Reprod (Oxf, Engl) 10(7):1642–1646
Lundstrom V, Eneroth P, Swahn ML (1984) Diurnal variation of uterine contractility. Br J Obstet Gynaecol 91(2):155–159
Acknowledgments
This study was financially supported by the Faculty Research Council (FRC), University of the Witwatersrand, Faculty of Health Science.
Conflict of interest
The authors have no conflict of interest related to the work in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iacovides, S., Baker, F.C. & Avidon, I. The 24-h progression of menstrual pain in women with primary dysmenorrhea when given diclofenac potassium: a randomized, double-blinded, placebo-controlled crossover study. Arch Gynecol Obstet 289, 993–1002 (2014). https://doi.org/10.1007/s00404-013-3073-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-3073-8