Abstract
Background
The aim of this meta-analysis was to summarize the efficacy and safety of bevacizumab in the treatment of ovarian cancer.
Methods
We sought to identify randomised controlled trials (RCTs) by searching PubMed and Web of Science. Outcomes were objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse events.
Results
Four studies with 4,246 patients were included. Combination of bevacizumab and chemotherapy resulted in a statistically significant improvement in ORR (OR 2.165, 95 % CI 1.511–3.103) and in PFS (HR 0.691, 95 % CI 0.517–0.865), compared with chemotherapy alone. There was no evidence of a significant improvement in OS (HR 0.934, 95 % CI 0.826–1.041). It also had significantly increased risk of gastrointestinal events (OR 2.743, 95 % CI 1.580–4.763; P < 0.001), hypertension (OR 4.630, 95 % CI 3.737 to 5.737; P < 0.001), proteinuria (OR 4.872, 95 % CI 2.617–9.069; P < 0.001), and arterial thromboembolism (OR 1.994, 95 % CI 1.210–3.286; P = 0.007).
Conclusion
This meta-analysis suggests that the addition of bevacizumab to chemotherapy offers meaningful improvement in objective response rate and progression-free survival in ovarian cancer treatment, but does not benefit overall survival. It also significantly increased the occurrence of gastrointestinal events, hypertension, proteinuria, and arterial thromboembolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to Jemal and Bray [1], ovarian cancer is the eighth most common incident cancer in women worldwide, and the seventh most common cause of cancer death. Each year, worldwide, over 225,000 women are diagnosed with ovarian cancer and over 140,200 die, corresponding to an annual age-standardized incidence of 9.4 cases per 100,000 women, annual mortality rate of 5.1 deaths per 100,000 in developed areas. In the United States, ovarian cancer is the fifth most common cause of cancer death in women, accounting for nearly 22,280 estimated new cases and 15,500 estimated deaths annually [2]. Treatment strategies for ovarian cancer by stage include standard surgical therapy, intravenous chemotherapy with carboplatin plus a taxane, and systemic therapy [3]. New targeted biologic agents, particularly those involved with the vascular endothelial growth factor (VEGF) pathway, hold great promise for improving the outcome of ovarian cancer [4].
VEGF, a glycoprotein produced by both normal and neoplastic cells, is one of the key elements in the stimulation of angiogenesis. Numerous studies have demonstrated that VEGF over-expression plays an essential role in the growth, progression and metastatic potential of ovarian cancer [5–7]. VEGF became a fundamental target of antiangiogenic therapy for ovarian cancer.
Bevacizumab is a humanized recombinant monoclonal antibody that specifically blocks the binding of VEGF to high-affinity receptors [8]. Based on some phase III RCTs, the US Food and Drug Administration has approved bevacizumab for the treatment of metastatic HER2 negative breast cancer, metastatic renal cell carcinoma, second-line treatment of glioblastoma, first-line treatment of non-small cell lung cancer, second-line treatment of metastatic colorectal cancer, and first-line treatment of metastatic colorectal cancer [9].
Bevacizumab treatment for ovarian cancer in clinical trials appeared only in recent years. Some RCTs have reported the final results. In this article, we performed a meta-analysis to pool the results and summarize the efficacy and safety of bevacizumab in the treatment of ovarian cancer.
Methods
Database and literature search
We searched PubMed and Web of Science from their inception to 25 September 2012. The search terms included “ovarian cancer”, “bevacizumab”, and “avastin”. The search detail in PubMed was “ovarian cancer” (Title/Abstract) AND [“bevacizumab” (Title/Abstract) OR “avastin” (Title/Abstract)]. In Web of Science citation database, we selected the science citation index expanded (SCI-EXPANDED) database and conference proceedings citation index science (CPCI-S) database. The search detail used was as follows: TS = (ovarian cancer) AND (TS = bevacizumab OR TS = avastin). We also looked at posters from the annual meetings of the American society of clinical oncology (ASCO) and the European society for medical oncology (ESMO) in the past 10 years.
Study selection
The following inclusion criteria had to be fulfilled: (1) types of studies: only randomised controlled trials (RCTs) were included. (2) Types of participants: adult women with histologically proven ovarian cancer were included. (3) Types of interventions: conventional chemotherapy plus Bevacizumab versus conventional chemotherapy plus placebo, and conventional chemotherapy plus Bevacizumab versus conventional chemotherapy alone. (4) Types of outcome: objective response rate (ORR) defined by according to the response evaluation criteria in solid tumors (RECIST) [10]; progression-free survival (PFS) defined as the time from randomization to disease progression or death from any cause; overall survival (OS) defined as the time from random assignment to death from any cause; adverse events, included gastrointestinal events (grade ≥ 2), hypertension (grade ≥ 2), proteinuria (grade ≥ 3), venous thromboembolism and arterial thromboembolism.
When the same trials were reported in different papers or meetings, only the most recent reports were included. We excluded case reports, case series, one arm phase I trials, retrospective case–control studies, and phase II non-randomized trials.
Quality assessment
We assessed the quality of included studies using the Cochrane Collaboration’s tool for assessing risk of bias [11]. The tool included randomized sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Items in the risk of bias assessment were judged “adequate” (+), “unclear” (?), or having the “potential for bias” (−) for each study. Two reviewers independently assessed the quality. Disagreements were resolved by discussion.
Data extraction
We collected information about methodological characteristics (study design, randomization method, allocation concealment, blinding, follow-up, baseline comparability, and other), study characteristics (study year, country, age, treatment schedule), and outcomes (ORR, median PFS and hazard ratios with 95 % CI, median OS and hazard ratios with 95 % CI, and the incidence of all kinds of adverse events). Data were extracted independently by two reviewers. Any differences of opinion were resolved by discussion.
Statistical analysis
Statistical heterogeneity was explored by χ2 and inconsistency (I 2) statistics; an I 2 value of 50 %or more represented substantial heterogeneity [12]. If there was no heterogeneity, a fixed effects model was used for meta-analysis; otherwise, a random effect model based on the Der Simonian and Laird estimator was used [13]. Summary hazard ratios (HR) for time-to-event data or summary odds ratio (OR) for dichotomous data were calculated by taking a weighted average of individual study results. Two-sided P < 0.050 was considered statistically significant. Potential publication bias was tested by Begg’s test and Egger’s test. Subgroup meta-analyses were performed by patient inclusion criteria (first-line therapy patients or recurrent ovarian cancer patients). The risk of bias figure was drawn with review manager (RevMan) software (version 5.0.21; Update Software Ltd, Oxford, Oxon, UK), and the pooling of data was performed with Stata software, version 12.0 (Stata Corp, College Station, Texas).
Results
Eligible studies and studies quality
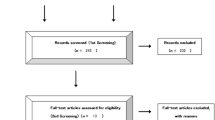
We identified four studies [14–17] that met our inclusion criteria for meta-analysis. The detailed steps of our literature search are shown in Fig. 1. A total of 4,246 patients were used in the pooled analyses. Of the four studies, sample sizes ranged from 361 to 1873. They were all phase III RCTs. One of the studies was a three-arm trial; the remaining three studies were two-arm trials. Three studies have been published; another study has been reported in the 2012 ASCO annual meeting. Table 1 shows the main characteristics of the four included studies, and Table 2 shows the outcome results of the studies. Two published studies showed low risk of bias in randomized sequence generation, blinding, incomplete outcome data, selective reporting, and other bias; while allocation concealment was unclear in all these published studies. It was unclear in all six risk of bias items in the study reported in the meeting. Figure 2 illustrates a general risk of bias across the included studies.
Objective response rate
A total of four trials [14–17] reported this outcome representing 4,246 patients. The ORR ranged from 11.8 to 74.0 %. There was significant heterogeneity between the studies (I 2 = 83.8 %, P < 0.001). The pooled OR was 2.165 (95 % CI 1.511–3.103) by random-effects (Fig. 3). It shows the combination of bevacizumab and chemotherapy resulted in a statistically significant improvement in ORR compared with chemotherapy alone. The Begg’s test (z = 0.24, P = 0.806) and the Egger’s test (t = 1.03, P = 0.378) suggested there was no significant publication bias. The subgroup analysis based on patient inclusion criteria showed that the addition of bevacizumab to standard chemotherapy for ovarian cancer led to a statistically significant improvement in ORR, both as a first-line therapy (OR = 1.895, 95 % CI 1.172–3.064), and in patients with recurrent ovarian cancer (OR = 2.767, 95 % CI 1.999–3.830).
Progression-free survival
A total of four trials [14–17] reported this outcome representing 4,246 patients. The median of PFS ranged from 3.4 to 19.8 months. There was significant heterogeneity between the studies (I 2 = 92.1 %, P < 0.001). The pooled HR was 0.691 (95 % CI 0.517–0.865) by random-effects (Fig. 4). It shows the combination of bevacizumab and chemotherapy resulted in a statistically significant improvement in PFS compared with chemotherapy alone. The Begg’s test (z = 1.22, P = 0.221) and the Egger’s test (t = 4.93, P = 0.016) suggested there was no significant publication bias. The subgroup analysis based on patient inclusion criteria showed that the addition of bevacizumab to standard chemotherapy for ovarian cancer led to a statistically significant improvement in PFS, both as a first-line therapy (HR = 0.828, 95 % CI 0.710–0.946), and in patients with recurrent ovarian cancer (HR = 0.482, 95 % CI 0.405–0.559).
Overall survival
A total of three trials [14–16] reported this outcome representing 3,885 patients. The median of OS ranged from 33.3 to 39.7 months. There was no significant heterogeneity between the studies (I 2 = 0.0 %, P = 0.553). The pooled HR was 0.934 (95 % CI 0.826–1.041), using a fixed-effect model (Fig. 5). It shows the combination of bevacizumab and chemotherapy resulted in no significant improvement in OS compared with chemotherapy alone. The Begg’s test (z = 0.34, P = 0.734) and the Egger’s test (t = 1.50, P = 0.273) suggested there was no significant publication bias. The subgroup analysis based on patient inclusion criteria showed that the addition of bevacizumab to standard chemotherapy for ovarian cancer was not associated with a significant improvement in OS, either as a first-line therapy (HR = 0.916, 95 % CI 0.799–1.033), or in patients with recurrent ovarian cancer (HR = 1.027, 95 % CI 0.757–1.296).
Adverse events
Compared with controls, ovarian cancer patients treated with bevacizumab had a 2.743 times (95 % CI 1.580–4.763; P < 0.001) increased risk of grade ≥ 2 gastrointestinal events; 4.630 times (95 % CI 3.737–5.737; P < 0.001) increased risk of grade ≥2 hypertension; 4.872 times (95 % CI 2.617–9.069; P < 0.001) increased risk of grade ≥3 proteinuria; and 1.994 times (95 % CI 1.210–3.286; P = 0.007) increased risk of arterial thromboembolism. There was no evidence of a significant increased risk of venous thromboembolism (OR 1.205; 95 % CI 0.934–1.554; P = 0.151). The plots of meta-analysis for adverse events are listed in the Appendix.
Discussion
Tumor angiogenesis is critical for transition of a cancer from the avascular phase to the vascular phase. In the absence of neovascularization, most solid tumors stop growing when they are 2–3 mm in size and enter a dormant stage [18]. Work in animal models has shown that treatment with a VEGF-specific monoclonal antibody was associated with a decrease in the density of vessels, and inhibition of growth, for multiple tumor types, although there was no effect on the growth rate of the tumor cells in vitro [8, 19]. The US FDA has approved bevacizumab for the treatment of breast cancer, renal cell carcinoma, glioblastoma, non-small cell lung cancer, and colorectal cancer. In this meta-analysis, we pooled the results from four phase III RCTs. The results showed that the addition of bevacizumab to chemotherapy offers meaningful improvement in ORR and PFS in ovarian cancer treatment, but no significant improvement in OS. The treatment efficacy of bevacizumab in ovarian cancer is similar to that seen with other cancers. In breast cancer treatment, combination of bevacizumab and chemotherapy resulted in a statistically significant improvement in PFS (HR = 0.70, 95 % CI 0.60–0.82) and ORR (RR = 1.26, 95 % CI 1.17–1.37) compared with chemotherapy alone, there was no significant difference in OS [20]. In renal cell carcinoma treatment, bevacizumab can significantly prolong the PFS compared with the placebo group (HR 2.55; P < 0.001) [21]. In non-small cell lung cancer treatment, the addition of bevacizumab to chemotherapy resulted in a significantly longer OS (HR 0.89; 95 % CI 0.79–0.99), longer PFS (HR 0.73; 95 % CI 0.66–0.82) and higher response rates (OR 2.34; 95 % CI 1.89–2.89) [22]. In colorectal cancer treatment, there was a significant PFS benefit (HR = 0.66; P < 0.01) and OS benefit (HR = 0.77; P < 0.01) in favor of the bevacizumab combined treatment; the ORR was significantly higher on the bevacizumab-containing arm (RR = 1.5; P = 0.021) [23].
We also did subgroup analysis based on patient inclusion criteria (first-line therapy patients or recurrent patients). Two studies (GOG-0218 [14] and ICON7 [15]) enrolled patients for bevacizumab first-line therapy; another two studies (OCEANS [16] and AURELIA [17]) enrolled patients with recurrent ovarian cancer. For first-line therapy, some phase I/II trials have already shown evidence of efficacy for combined bevacizumab. A single-arm phase II trial of treatment with paclitaxel, carboplatin and bevacizumab (PCB), in 62 patients with stage ≥IC epithelial mullerian tumors, showed a radiographic response of 75 % (among 28 women with measurable disease), with CA-125 responses in 76 % of patients; the progression-free survival rate at 36 months was 58 % [24]. Micha et al. [25] reported, in 22 PCB treated advanced-stage ovarian cancer patients, the total response rate of 80 %, and the disease progression rate was 5 %. In our subgroup meta-analysis of bevacizumab versus standard chemotherapy as a first-line therapy, we found a significant improvement in ORR (OR = 1.942, 95 % CI 1.275–2.956), as well as PFS (HR = 0.828, 95 % CI 0.710–0.946). The GOG-0218 study also compared bevacizumab maintenance with no bevacizumab maintenance in first-line therapy. The results showed relative to control treatment, the HR for progression or death was 0.908 (95 % CI 0.795–1.040; P = 0.16) with bevacizumab initiation and 0.717 (95 % CI 0.625–0.824; P < 0.001) with bevacizumab throughout [14]. Our meta-analysis showed no evidence of an improvement in OS associated with the addition of bevacizumab to standard therapy. However, in the ICON7 study, although there was no evidence of a difference in OS between the two treatment arms overall (HR 0.85, 95 % CI 0.69–1.04), the OS was significantly improved in the subgroup of patients at high risk for progression, with the median OS 28.8 months in the standard therapy group and 36.6 months in the bevacizumab group (HR 0.64; 95 % CI 0.48–0.85; P = 0.002) [15].
For recurrent ovarian cancer, some phase I/II trials also have already shown evidence of efficacy for combined bevacizumab. Garcia AA reported a single-arm trial of bevacizumab and cyclophosphamide, in 70 patients with recurrent ovarian cancer, in which, the probability of being alive and progression-free at 6 months was 56 % (±6 % SE); partial response rate was 24 %; median time to progression and survival were 7.2 and 16.9 months, respectively [26]. Chura et al. [27] reported a similar single-arm trial of bevacizumab and cyclophosphamide, in 15 patients with heavily pretreated recurrent ovarian cancer, 13.3 % of patients had a complete response after 4 months of therapy; 40.0 % patients had a partial response; the median duration of this response was 3.9 months (2.3–10.4). 20 % patients had stable disease of 4.0–5.5 months’ duration, and 26.7 % patients had progressive disease. In our subgroup meta-analysis of bevacizumab versus standard chemotherapy for recurrent ovarian cancer, we found a significant improvement in ORR (OR = 2.767, 95 % CI 1.999–3.830), as well as PFS (HR = 0.482, 95 % CI 0.405–0.559). The two included studies enrolled platinum-sensitive patients (OCEANS [16]) and platinum-resistant patients (AURELIA [17]). Both studies showed an improvement in ORR and PFS, suggesting that bevacizumab may be used in both platinum-resistant and platinum-sensitive patients.
Bevacizumab has shown evidence of efficacy in ovarian cancer treatment as an addition to chemotherapy in first-line therapy or in recurrent disease. But we also should pay attention to the adverse events associated with bevacizumab. In our meta-analysis, we found ovarian cancer patients treated with bevacizumab had an increased risk of grade ≥2 gastrointestinal events, grade ≥2 hypertension, grade ≥3 proteinuria, and arterial thromboembolism. Patients should be monitored for these events throughout the course of bevacizumab therapy. Several other meta-analyses of bevacizumab therapy in cancer patients have found similar evidence of associated adverse events [28–31]. Shord recommended blood pressure should be routinely monitored, and hypertension should be medically managed with antihypertensive drugs as deemed appropriate during bevacizumab therapy; proteinuria should be monitored for every 3 to 4 weeks, and bevacizumab should be discontinued with persistent proteinuria of >2+; and thromboembolism events should be managed in accordance with guidelines established by the American College of Chest Physicians [32].
There are some limitations in this meta-analysis. First, there was significant heterogeneity between the studies. Second, only four phase III RCTs were included in the study. Further, some RCTs for accessing bevacizumab in treatment of ovarian cancer are ongoing such as NCT00262847, NCT00565851, NCT00951496, NCT01462890, NCT01081262, NCT00483782, NCT01239732 and NCT00434642. We are waiting for the results of these studies. Third, the data on overall survival are not complete for some studies (e.g. ICON7 and OCEANS). Fourth, we pooled summary HR for time-to-event data by generic inverse variance method. Individual patient data meta-analysis will make the results more accurate.
In conclusion, our meta-analysis suggested that addition of bevacizumab to chemotherapy offers a meaningful improvement in objective response rate and progression-free survival in first-line therapy for ovarian cancer, as well as in treatment of recurrent disease. But addition of bevacizumab does not benefit overall survival for ovarian cancer, and will significantly increase the occurrence of adverse events.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62(1):10–29
Hennessy BT, Coleman RL, Markman M (2009) Ovarian cancer. Lancet 374(9698):1371–1382
Jelovac D, Armstrong DK (2011) Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 61(3):183–203
Kryczek I, Lange A, Mottram P et al (2005) CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res 65(2):465–472
Zhang L, Yang N, Park JW et al (2003) Tumor-derived vascular endothelial growth factor up-regulates angiopoietins-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 63(12):3403–3412
Byrne AT, Ross L, Holash J et al (2003) Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res 9(15):5721–5728
Ferrara N, Hillan KJ, Gerber HP et al (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400
FDA Approval for bevacizumab. (Updated: 11/18/2011). http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Burger RA, Brady MF, Bookman MA et al (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2483
Perren TJ, Swart AM, Pfisterer J et al (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496
Aghajanian C, Finkler N, Rutherford T et al (2012) OCEANS: a randomized, double-blind, placebo-controlled phase iii trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30(17):2039–2045
Pujade-Lauraine E, Hilpert F, Weber B, et al (2012) AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). http://meeting.ascopubs.org/cgi/content/short/30/18_suppl/LBA5002?rss=1. Accessed 29 Mar 2013
Folkman J, Cole P, Zimmerman S (1966) Tumor behavior in isolated perfused organs: in vitro growth and metastases of biopsy material in rabbit thyroid and canine intestinal segment. Ann Surg 164:491–502
Kim KJ, Li B, Winer J et al (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362(6423):841–844
Valachis A, Polyzos NP, Patsopoulos NA, Georgoulias V, Mavroudis D, Mauri D (2010) Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat 122(1):1–7
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349(5):427–434
Lima AB, Macedo LT, Sasse AD (2011) Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 6(8):e22681
Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z (2009) A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis 24(6):677–685
Penson RT, Dizon DS, Cannistra SA et al (2010) Phase II study of carboplatin, paclitaxel, and bevacizumab with maintenance bevacizumab as first-line chemotherapy for advanced mullerian tumors. J Clin Oncol 28(1):154–159
Micha JP, Goldstein BH, Rettenmaier MA, Genesen M, Graham C, Bader K, Lopez KL, Nickle M, Brown JV 3rd (2007) A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer 17(4):771–776
Garcia AA, Hirte H, Fleming G et al (2008) Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol 26(1):76–82
Chura JC, Van Iseghem K, Downs LS Jr et al (2007) Bevacizumab plus cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Gynecol Oncol 107(2):326–330
Ranpura V, Pulipati B, Chu D, Zhu X, Wu S (2010) Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 23(5):460–468
Zhu X, Wu S, Dahut WL, Parikh CR (2007) Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49(2):186–193
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S (2008) Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300(19):2277–2285
Hapani S, Chu D, Wu S (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 10(6):559–568
Shord SS, Bressler LR, Tierney LA, Cuellar S, George A (2009) Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm 66(11):999–1013
Acknowledgments
We thank anonymous reviewers and the editor for several insightful comments that significantly improved the paper. We are also grateful to anonymous reviewer for correcting syntax error in the paper.
Conflicts of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
GI events

Hypertension

Proteinuria

Arterial TE

Venous TE

Rights and permissions
About this article
Cite this article
Ye, Q., Chen, HL. Bevacizumab in the treatment of ovarian cancer: a meta-analysis from four phase III randomized controlled trials. Arch Gynecol Obstet 288, 655–666 (2013). https://doi.org/10.1007/s00404-013-2820-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2820-1