Abstract
Background
This systematic review and meta-analysis analyzed randomized controlled trials (RCTs) assessing the efficacy and tolerance of incorporating bevacizumab into chemotherapy in patients with advanced ovarian cancer.
Methods
MEDLINE, Web of Science, EMBASE and the Cochrane Central Register of Controlled Trials were reviewed for RCTs evaluating add-on bevacizumab in advanced ovarian cancer. Progression-free survival (PFS), overall survival (OS), objective response rate and adverse events were obtained from RCTs comparing first- and second-line bevacizumab plus chemotherapy with chemotherapy alone for advanced ovarian cancer. Meta-analyses were performed to determine hazard ratios for time-to-event variables and odds ratios for dichotomous outcomes using random-effects or fixed-effects model based on the heterogeneity of included studies.
Results
Four RCTs, including 4246 patients, were identified and analyzed. Two trials, GOG218 and ICON7, assessing bevacizumab in first-line chemotherapy, found that bevacizumab significantly extended PFS (HR 0.82; 95 % CI 0.75–0.89) and OS (HR 0.86; 95 % CI 0.75–0.99). The other two trials, OCEANS and AURELIA, analyzing second-line bevacizumab, found that this agent extended PFS (HR 0.48; 95 % CI 0.41–0.57), but did not enhance OS (HR 0.93; 95 % CI 0.78–1.12). The most common adverse events associated with bevacizumab included hypertension, proteinuria and gastrointestinal perforation.
Conclusion
The addition of bevacizumab to chemotherapy followed by bevacizumab significantly improved PFS and OS in frontline setting and PFS in recurrent settings compared with that of chemotherapy alone in patients with advanced ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Ovarian cancer remains the eighth most common cancer and the leading cause of gynecologic cancer deaths worldwide, responsible for 225,000 new cases and 140,200 deaths per year [1]. Despite modest improvements in outcome, this disease is frequently diagnosed at advanced stages and is associated with high mortality and morbidity rates. Standard first-line treatment involves initial surgical cytoreduction followed by six cycles of adjuvant chemotherapy with carboplatin and paclitaxel [2, 3]. Despite initially high response rates to surgery and first-line chemotherapy, approximately 75 % of patients will eventually relapse because of drug resistance, with these patients having poor long-term overall survival. Survival rates in patients with ovarian cancer have remained largely unchanged over the last three decades, with a 5-year survival rate in women diagnosed with stage III or IV ovarian cancer being <30 % [4, 5]. The addition of third cytotoxic agents failed to demonstrate superior efficacy but significantly increased toxic events [6]. Therefore, the development of effective novel molecularly targeted agents with tolerable toxicity profiles is of high priority. One approach is to identify agents that target mechanisms of tumor progression, such as angiogenesis, a critical pathway in the development and progression of cancer.
Angiogenesis plays critical roles in tumor growth, invasion, and metastasis [7]. Significant progress has been made by using angiogenesis inhibitors in the treatment of cancer. Vascular endothelial growth factor (VEGF) is the best characterized angiogenic factor and is recognized as a major element in regulating angiogenesis [8]. VEGF expression has been correlated with tumor progression and poor overall survival (OS) in patients with advanced ovarian cancer [9, 10]. Preclinical studies in an ovarian cancer model have shown that direct inhibition of VEGF activity alone can reduce tumor growth, metastasis and malignant ascites formation [11–14]. In addition, anti-VEGF agents may increase the effects of chemotherapy by normalizing tumor vasculature, leading to a transient reduction in interstitial pressure and enhancing the delivery of cytotoxic drugs [15].
Methods
Identification of trials
MEDLINE (1950 through April 2015), Web of Science (1950 through April 2015), EMBASE (1966 through April 2015), and Chinese VIP (1989 through April 2015) databases, as well as The Cochrane Central Register of Controlled Trials, were searched for relevant trials, with no limitation of language. The search strategy used terms related to “ovarian cancer” and “bevacizumab” in all fields. Additionally, all relevant review articles, references of included studies and the abstracts presented at the annual meetings of the American Society of Clinical Oncology and the European Society for Medical Oncology up to April 2015 were manually searched. Registers of clinical trials (http://clinicaltrials.gov/; http://www.who.int/ictrp/en/) were also searched, and the study authors, investigators and manufacturers of bevacizumab were contacted when relevant data were not clear.
Outcomes
The combination of chemotherapy and bevacizumab was considered the experimental arm and chemotherapy alone as the standard comparator. OS and PFS were the primary outcomes analyzed. PFS was defined as time from randomization until tumor progression, death or date of last contact. OS was defined as the time from randomization to death or date of last contact. Secondary outcomes were objective response rate (ORR), toxicity and quality-of-life. Adverse events were graded and recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) [16].
Trial selection criteria
All prospective randomized controlled trials (RCTs), published or unpublished, comparing bevacizumab plus chemotherapy with chemotherapy alone for patients with advanced ovarian cancer were eligible, both in first- and second-line treatment. Studies involving the use of other targeted agents or immunotherapy were excluded. If duplications existed, only the most recent or most complete report of clinical trials was included and the updated data were used for our analysis. Two reviewers (Li, Zhou) examined the list of references and individually selected the studies.
Data extraction
Two reviewers (Li, Zhou) independently extracted data from publications and trial reports. All data were checked for consistency with the trial protocol, statistical report and publications. Authors were contacted to obtain any missing information. Any disagreement was resolved by discussion and consensus according to Kappa index [17]. A third reviewer (Chen) was consulted in case of divergence. Details recorded included number of patients, performance status, treatment, outcomes, and length of follow-up.
Statistical analysis
Meta-analyses for nonheterogeneous trials were performed using RevMan 5.3 software (Cochrane collaboration’s information management system). Fixed-effect models were used to calculate pooled hazard ratios (HRs) for time-to-event outcomes, and odds ratios (ORs) for dichotomous variables, with two-sided 95 % confidence intervals (CI) and P values. Forest plots were depicted for each summary effect. The diamond at the bottom of the plot summarizes the best estimate of pooled valid outcomes (the width representing its corresponding 95 % CI). The effect of treatment in each study was expressed as a ratio of the bevacizumab-containing arm over the chemotherapy alone arm. A HR value of less than one and an OR value of more than one indicated a benefit for the inclusion of bevacizumab. P < 0.05 was regarded as statistically significant. Statistical heterogeneity was evaluated through Chi-square test [18], and expressed in I 2 index. Values over 50 % indicate large heterogeneity [19]. If heterogeneity was detected, the random-effects model was used after a possible explanation was investigated. Eligible studies were classified into subcategories depending on the treatment lines. In first-line treatment, both two trials (GOG218 and ICON7) showed much greater survival benefit in patients with high risk for progression (FIGO stage IV disease or FIGO stage III disease and >1.0 cm of residual disease after debulking surgery), we also made a subgroup analysis in patients of this population.
Methodological quality of included studies
To assess trial quality, the following items were extracted: sample size calculation, the sequence of randomization, blinding, use of placebo, allocation concealment, source of funding and intention-to-treat (ITT).
Results
Research results
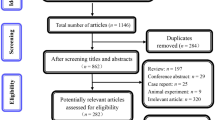
Our search yielded 389 references after excluding duplicates. After excluding review articles, case reports, meta-analyses, phase I studies, single-arm studies, and retrospective studies, 16 references were retrieved, and their full texts were assessed for eligibility. Four phase III RCTs with a total of 4246 patients were finally identified as eligible and incorporated into this review: the ICON7 [20], GOG218 [21], OCEANS [22] and AURELIA [23] trials. The selection procedure is further summarized in Fig. 1, as recommended by the PRISMA statement [24].
Characteristics of included studies
A total of 3621 patients were finally enrolled in this meta-analysis. We only recruited the bevacizumab-throughout group in GOG218 study to reduced heterogeneity because throughout therapy was applied to ICON7. All four trials included survival data, adequate follow-up, no other chemotherapy, no radiation therapy, and had information on various prognostic variables, including age, performance status, stage, grade, and residual disease. The ICON7 and GOG218 trials evaluated the addition of bevacizumab as first-line therapy; AURELIA trial evaluated platinum-resistant recurrent ovarian cancer, and OCEANS evaluated platinum-sensitive recurrent OC. Three trials (ICON7, OCEANS and AURELIA) were two-armed studies; the GOG218 trial was three-armed, with patients receiving chemotherapy alone or bevacizumab plus chemotherapy, with the latter group either receiving or not receiving bevacizumab maintenance therapy. Patients in the ICON7 study received 7.5 mg/kg bevacizumab, whereas patients in the other three trials received 15 mg/kg bevacizumab. Bevacizumab maintenance was limited to 12 months in ICON7 and 15 months in GOG218; in the two second-line trials (OCEANS and AURELIA), bevacizumab was continued until disease progression (Tables 1, 2).
The primary endpoint in all four trials was PFS, with disease progression defined according to the response evaluation criteria in solid tumors (RECIST) [25], based on radiologic, clinical, and/or symptomatic indicators of progression. Progression based on elevated CA-125 alone, according to Gynecologic Cancer InterGroup criteria [26], was permitted only in GOG218. ORR was not evaluated in GOG218.
Quality of included studies
Of the four RCTs, GOG218 and AURELIA were open-label trials; ICON7 and OCEANS were double-blinded, placebo-controlled trials with appropriate randomization. Sample size calculation was mentioned in all trials. All were designed to test the superiority of bevacizumab efficacy. None mentioned allocation concealment. Crossover was permitted in all studies. All analyzed ITT populations and were multicenter, randomized trials. The funding source had no role in the design, collection, analysis, interpretation, or writing of the study. The quality of included studies is summarized in Table 3.
Results of meta-analysis
PFS
Compared with chemotherapy alone, chemotherapy with concurrent and maintenance bevacizumab improved PFS in both first- (HR 0.82; 95 % CI 0.75–0.89) and second-line (HR 0.48; 95 % CI 0.41–0.57) settings. Patients at high risk for progression (FIGO stage III >1 cm residual/stage IV) during first-line treatment showed a greater effect size of PFS (HR 0.68; 95 % CI 0.55–0.84) (Fig. 2a).
a Forest plots of hazard ratios (HRs) for progression-free survival (PFS) of patients with advanced ovarian cancer treated with bevacizumab plus standard chemotherapy, compared with chemotherapy alone, as first- and second-line treatment regimens. Chi-squared tests showed no significant heterogeneity among the trials. CI confidence interval. b Forest plots of hazard ratios (HRs) for overall survival (OS) of patients with advanced ovarian cancer treated with bevacizumab plus standard chemotherapy, compared with chemotherapy alone, as first- and second-line treatment regimens. Chi-squared tests showed no significant heterogeneity among the trials. c Forest plots of Odds ratios (ORs) for objective response rate (ORR) of bevacizumab plus standard chemotherapy, compared with chemotherapy alone, as first- and second-line treatment regimens in patients with advanced ovarian cancer. Chi-squared tests showed no significant heterogeneity among the trials. CI confidence interval
OS
Meta-analysis of the randomized trials showed that first-line bevacizumab was associated with a significant survival benefit (HR 0.86; 95 % CI 0.75–0.99). However, it had no effect in recurrent settings (HR 0.93; 95 % CI 0.78–1.12) (Fig. 2b).
ORR
The summary effect sizes of response rate that were evaluated in three studies, were significantly higher in the bevacizumab arm with an OR of 2.20 (95 % CI 1.79–2.70) in the upfront setting and an OR of 2.91 (95 % CI 2.20–3.84) in recurrent setting, indicating that response rates were greater with than without bevacizumab regardless of line of treatment (Fig. 2c).
Toxicity
Our meta-analysis found that bevacizumab was associated with additional toxicities. The most common adverse events associated with bevacizumab were hypertension and proteinuria. The summary incidence of Grade ≥3 hypertension was 19.3 %, with an OR of 16.1 (95 % CI 9.88–26.25). Grade ≥3 proteinuria was significantly more common in the bevacizumab arm (OR, 5.14; 95 % CI 2.34–11.27), with an incidence of 2.19 %. Of note, the incidence of grade ≥2 gastrointestinal perforation (GIP) was significantly higher with than without bevacizumab (OR, 2.94; 95 % CI 1.45–5.95; 1.83 % vs. 0.65 %, P = 0.03). Other adverse events, such as arterial thromboembolism, wound healing disruption and bleeding, were also significantly more common in bevacizumab treated women. Bevacizumab was also associated with slightly, but not significantly, higher rates of Grade ≥3 neutropenia or febrile neutropenia and central nervous system complications such as reversible posterior leukoencephalopathy syndrome. Importantly, no new safety signals were identified in our review (Figure 3).
Forest plots of odds ratios (ORs) for toxicity of bevacizumab plus standard chemotherapy, compared with chemotherapy alone, as first- and second-line treatment regimens in patients with advanced ovarian cancer. Chi-squared tests showed no significant heterogeneity among the trials. CI confidence interval
Discussion
This pooled analysis showed that the addition of bevacizumab to upfront chemotherapy, followed by maintenance therapy with bevacizumab, resulted in statistically significant benefits in both PFS and ORR, independently of treatment lines. The benefits were consistent and seemed applicable to all patients with ovarian cancer. However, no significant OS benefit was observed in second-line settings. Patients at high risk for progression seemed to be more suitable for bevacizumab therapy.
The dose and duration of bevacizumab differed among the four included trials. In three trials (GOG218, OCEANS and AURELIA), patients received 15 mg/kg bevacizumab, a regimen based on those approved in patients with non-small-cell lung cancer and metastatic breast cancer [27, 28]. In contrast, patients in the ICON7 trial received 7.5 mg/kg bevacizumab, based on one of the licensed doses for metastatic colorectal cancer. Two trials (GOG218 and ICON7), involving 3401 patients, were available for comparison of first-line bevacizumab plus chemotherapy with chemotherapy alone. In the GOG218 trial, there was no statistically significant improvement in OS (HR 0.88; 95 % CI 0.75–1.04) despite a substantial improvement in PFS (HR 0.77; 95 % CI 0.68–0.87). In contrast, ICON7 showed that bevacizumab had a significant effect on OS, especially in patients with high-risk disease similar to the GOG218 population (HR 0.68; 95 % CI 0.55–0.84). This discrepancy may be because of the crossover effect in the GOG218 trial, in that a large proportion of patients (28 %) received post-progression therapy with anti-VEGF agents, whereas in ICON7, only 2.5 % of the patients in the control group received post-progression antiangiogenic agents. In addition, the effect size for PFS was larger in GOG218 than in ICON7, which may reflect the higher dose of bevacizumab (15 mg/kg vs. 7.5 mg/kg), the inclusion of more seriously ill patients and the longer duration of maintenance bevacizumab (15 months vs. 12 months) in ICON7. Moreover, compared with the summary outcomes in the upfront trials, pooled analysis showed greater benefits favoring the use of bevacizumab, as shown by PFS (HR 0.48; 95 % CI 0.41–0.57) and ORR (OR, 2.91; 95 % CI 2.20–3.84), in patients with recurrent disease, suggesting the sensitivity to bevacizumab in this population and the longer treatment duration, with bevacizumab continued until progression. Additional investigations are needed to optimize bevacizumab dose, timing and treatment duration.
The PFS curves were near each other in the two upfront trials (GOG218 and ICON7), in which bevacizumab continuation was limited to 12 and 15 months, respectively. That convergence was not observed in the recurrent trials (OCEANS and AURELIA), where bevacizumab was continued until disease progression. Moreover, in GOG218, patients were randomized into three arms, including patients treated with bevacizumab plus chemotherapy, followed by bevacizumab maintenance; bevacizumab plus chemotherapy; and chemotherapy alone. In that trial, patients treated with chemotherapy plus concurrent bevacizumab did not show a significant improvement in PFS compared with that of chemotherapy alone (HR 0.908; 95 % CI 0.795–1.040; P = 0.16). These findings suggested that the magnitude of benefit of bevacizumab may correlate with treatment duration, and that administration of bevacizumab until progression may represent the optimal treatment schedule. However, none of these studies was designed to evaluate the usefulness of extended bevacizumab monotherapy beyond chemotherapy. Future studies should be designed to test the effects of bevacizumab maintenance treatment after completion of cytotoxic therapy.
To date, OS has been considered the most clinically relevant endpoint in oncology trials because of its relevance and objectivity. PFS, however, is the most commonly used surrogate endpoint, with a lower likelihood of confounding because of treatment after progression. The prognostic effect of PFS on OS remains inconclusive for patients with advanced ovarian cancer receiving biologically targeted agents. The 2010 Gynecologic Cancer InterGroup (GCIG) consensus has therefore recommended that both PFS and OS be important end points in determining the value of any new therapeutic strategy. PFS is more often the preferred primary end point for trials because of the crossover effect after progression [29]. Indeed, none of the trials included in this meta-analysis found significantly increased OS with bevacizumab, despite improving PFS, in patients with advanced ovarian cancer. This lack of benefit may be because of the high percentage of patients in the chemotherapy arm receiving crossover therapy after progression and relatively small population in a single study, making the interpretation of PFS problematic [30].
Bevacizumab was associated with higher rates of drug-related toxic effects without harming patients’ quality-of-life. The main significant side effects related to bevacizumab treatment were hypertension and proteinuria, both frequently encountered in other disease settings in which bevacizumab is used. Evaluation of serious adverse events in ovarian cancer patients showed that bevacizumab treatment resulted in a significantly increased risk of GIP compared with patients treated with chemotherapy alone (OR 2.94; 95 % CI 1.45–5.59). The rate of GIP in patients treated with bevacizumab plus chemotherapy was 1.83 %, much lower than in earlier trials in platinum-resistant patients (11.4 %) [31], but higher than in a meta-analysis of patients with other diseases treated with bevacizumab (0.9 %; 95 % CI 0.7–1.2), with an OR of 2.14 (95 % CI 1.19–3.85) compared with control treatments [32].
Two similar meta-analyses have been performed looking at the same four randomized controlled trials. However, the present study have updated the final data that was published derived from these four trials [33, 34]. Firstly, meta-analysis performed by Ye et al. [33] did not observed significant improvement of OS in BEV + chemotherapy group, for they did not use the updated HRs of OS. In our study, we pooled the updated data of GOG218 and ICON7, with improved OS (HR 0.87, CI 0.77–0.99, and P = 0.026). This result was consistent with the ones published in 2013 by Zhou et al. [34]. The significantly improved OS in bevacizumab plus chemotherapy group was observed in Zhou et al. and our study by pooling the two similar study in which neither of them did not observed OS benefit. This contrary indicates that a meta-analysis with larger sample size by pooling similar studies was required to detect the significant difference. Secondly, OS was evaluated in three of these trials since AURELIA did not have this outcome at the time of publication. We pooled the AURELIA in our meta-analysis.
Studies have attempted to increase disease control in patients with advanced ovarian cancer by modifying the dose, schedule or route of administration of chemotherapy. Intraperitoneal chemotherapy has demonstrated a survival advantage over intravenous therapy, but only for women with small amount of residual disease after surgery; this route of administration has not been uniformly adopted because of concerns about toxicity, administration and quality-of-life [35–38]. Furthermore, based recent international trials showed that neoadjuvant chemotherapy yielded equivalent OS (29 vs. 30 months) and lower rates of postoperative complications compared with conventional, suggesting that neoadjuvant chemotherapy is an option for patients with extensive stage IIIC/IV disease who were not candidates for surgery [39, 40]. Phase III trials have shown that dose-dense weekly paclitaxel administration and paclitaxel consolidation therapy significantly improved PFS and OS [41–43]. Paclitaxel consolidation therapy has been reported more cost-effective than bevacizumab after upfront chemotherapy [44]. Therefore, two confirmatory trials, GOG 0262 (NCT01167712) and GOG 0212 (NCT00108745), are ongoing to optimize the two treatment regimens. These strategies should be considered when selected treatment for patients with advanced ovarian cancer. Future trials should to assess the efficacy of adding bevacizumab to these alterative treatment strategies.
Recent phase II trials have shown that other antiangiogenic agents (e.g., cediranib, pazopanib, and cabozantanib) and targeted agents such as angiopoietin inhibitors (e.g., AMG 386) have modest efficacy in the treatment of recurrent ovarian cancer [45–48]. Recently, survival benefits have been found in phase II trial for evaluating the efficacy of olaparib (poly ADP ribose polymerase inhibitors) in patients with advanced ovarian cancer who carry hereditary BRCA mutations and have previously received three or more lines of therapy, and FDA has granted accelerated approval to the drugs [49]. Further large-scale trials are under way to better define the role of these agents in the treatment of ovarian cancer. Bevacizumab is the first of the new molecularly targeted agents to show clear efficacy in the management of patients with primary and recurrent ovarian cancer. Concerns about the costs of these drugs; their increased toxicity, especially the high incidence of GIP; and the requirement for prolonged treatment indicate the need to identify risk factors for severe side effects and biomarkers for response. Phase IV trials are under way to assess potential clinical and biologic prognostic factors associated with benefits from this therapy.
Our study has several strengths. The data collected from four large, multicenter phase III trials was sufficiently powered to test the efficacy and safety of first- and second-line treatment with bevacizumab. All the studies included in this review were well designed and of high quality.
This study, however, also has several limitations. First, only four trials were found to meet eligibility criteria. As yet unpublished trials with negative results may have been performed, despite the extensive search of all relevant references. Furthermore, trials in this meta-analysis had different patient populations, lines of treatment, doses of bevacizumab, concurrent chemotherapies, follow-up durations and lengths of bevacizumab maintenance. Last, this meta-analysis pooled the data rather than assessing individual trials.
Although the ultimate goal of cancer therapy is to cure patients of their disease, treatments of diseases like advanced ovarian cancer may be effective but are rarely curative. Thus, the goals of treatment should include prolongation of survival with acceptable side effects profile and good quality-of-life. In addition, cost-effectiveness should be considered when making treatment decisions, inasmuch as bevacizumab is very expensive, especially as maintenance therapy [50, 51]. Physicians should assess the balance between the median 4 month improvement in PFS and increased toxicity, reduced quality-of-life and high cost when considering bevacizumab as first- or second-line treatment in patients with advanced ovarian cancer.
Conclusions
Our meta-analysis of randomized trials suggested that bevacizumab, in combination with chemotherapy, significantly increased PFS and ORR in both the first- and second-line settings, with acceptable toxicity and preserved quality-of-life. OS, however, was slightly improved only in first-line settings. The addition of bevacizumab to chemotherapy provides a new option for women for advanced ovarian cancer. The potential for side effects and cost should be taken into account when deciding on treatment. Future efforts should focus on identifying optimal treatment regimens and biomarkers of predicting response to bevacizumab, thus helping select patients most likely to benefit from combined therapy.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200.
McGuire WP 3rd, Markman M. Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer. 2003;89(Suppl 3):S3–8.
Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92.
Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120(3):612–8.
Bookman MA. The addition of new drugs to standard therapy in the first-line treatment of ovarian cancer. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2010;21(7):211–7.
Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–16.
Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56(3):794–814.
Hefler LA, Zeillinger R, Grimm C, Sood AK, Cheng WF, Gadducci A, et al. Preoperative serum vascular endothelial growth factor as a prognostic parameter in ovarian cancer. Gynecol Oncol. 2006;103(2):512–7.
Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76(9):1221–7.
Xu L, Yoneda J, Herrera C, Wood J, Killion JJ, Fidler IJ. Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol. 2000;16(3):445–54.
Sher I, Adham SA, Petrik J, Coomber BL. Autocrine VEGF-A/KDR loop protects epithelial ovarian carcinoma cells from anoikis. Int J Cancer. 2009;124(3):553–61.
Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9(15):5721–8.
Hu L, Hofmann J, Zaloudek C, Ferrara N, Hamilton T, Jaffe RB. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am J Pathol. 2002;161(5):1917–24.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Bethesda M. Common terminology criteria for adverse events (CTCAE), v3.0 http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30. 2006. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30.
Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–9.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N Engl J Med. 2011;365(26):2473–83.
Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. Oceans: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45.
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the auRELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–16.
Rustin GJS, Bast RC, Kelloff GJ, Barrett JC, Carter SK, Nisen PD, et al. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin Cancer Res. 2004;10(11):3919–26.
Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593–9.
Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25(33):5165–71.
Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2011;21(4):750–5.
Korn EL, Freidlin B, Abrams JS. Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J Clin Oncol. 2011;29(17):2439–42.
Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(33):5180–6.
Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10(6):559–68.
Ye Q, Chen HL. Bevacizumab in the treatment of ovarian cancer: a meta-analysis from four phase III randomized controlled trials. Arch Gynecol Obstet. 2013;288(3):655–66.
Zhou M, Yu P, Qu X, Liu Y, Zhang J. Phase III trials of standard chemotherapy with or without bevacizumab for ovarian cancer: a meta-analysis. PLoS One. 2013;8(12):e81858.
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43.
Alberts DS. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5.
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(4):1001–7.
Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;11:CD005340.
Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53.
Rauh-Hain JA, Rodriguez N, Growdon WB, Goodman AK, Boruta DM 2nd, Horowitz NS, et al. Primary debulking surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann Surg Oncol. 2012;19(3):959–65.
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–8.
Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21(13):2460–5.
Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol. 2009;114(2):195–8.
Lesnock JL, Farris C, Krivak TC, Smith KJ, Markman M. Consolidation paclitaxel is more cost-effective than bevacizumab following upfront treatment of advanced epithelial ovarian cancer. Gynecol Oncol. 2011;122(3):473–8.
Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(4):372–9.
Karlan BY, Oza AM, Richardson GE, Provencher DM, Hansen VL, Buck M, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(4):362–71.
Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(33):5601–6.
Friedlander M, Hancock KC, Rischin D, Messing MJ, Stringer CA, Matthys GM, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119(1):32–7.
Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97.
Hensley ML. Big costs for little gain in ovarian cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(10):1230–2.
Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM Jr. At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(10):1247–51.
Acknowledgments
Li Jialu, Zhou Likun, and Ba Yi contributed equally to this work.
Conflict of interest
Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of interest form.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Li, L. Zhou, and Y. Ba contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, J., Zhou, L., Chen, X. et al. Addition of bevacizumab to chemotherapy in patients with ovarian cancer: a systematic review and meta-analysis of randomized trials. Clin Transl Oncol 17, 673–683 (2015). https://doi.org/10.1007/s12094-015-1293-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1293-z