Abstract
Purpose
Bevacizumab has demonstrated survival benefit in metastatic colorectal cancer (mCRC) patients when combined with chemotherapy. Several randomized clinical studies have evaluated bevacizumab in combination with chemotherapy. Meta-analysis was performed to better assess the efficacy and safety of bevacizumab with chemotherapy for mCRC.
Materials and methods
Five clinical trials randomizing a total of 3,103 mCRC patients to chemotherapy alone or to the combined treatment of chemotherapy plus bevacizumab were identified. The efficacy data included progression-free survival (PFS), overall survival (OS), and overall response rate (ORR), and the safety data contained the 60-day all-cause mortality rate, adverse events (AEs), and specific toxicity such as hypertension, thrombosis, bleeding, proteinuria, gastrointestinal perforation, diarrhea, and leucopenia.
Result
There was a significant PFS benefit (P = 0.00; hazards ratio [HR] = 0.66) and OS benefit (P = 0.00; HR = 0.77) in favor of the combined treatment. The ORR was significantly higher on the bevacizumab-containing arm (P = 0.021; relative risk [RR] = 1.5), while CR was comparable between the two arms (P = 0.09). A higher incidence of grade 3/4 AEs, grade 3/4 hypertension, grade 3/4 thromboembolic/thrombotic events, grade 3/4 bleeding, and gastrointestinal perforation was associated with the bevacizumab group. The two treatment groups were similar in terms of grade 3/4 proteinuria, grade 3/4 leukopenia, grade 3/4 diarrhea, and the 60-day all-cause mortality rate.
Conclusion
The addition of bevacizumab to chemotherapy confers a clinically meaningful and statistically significant improvement in OS, PFS, and ORR. Its side effects are predictable and manageable and do not compound the incidence or severity of toxicities from chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the fourth most common noncutaneous malignancy in the United States and the second most frequent cause of cancer-related death. In 2008, an estimated 148,810 cases of CRC will be diagnosed and 49,960 people will die from this disease [1]. In CRC, surgery and chemotherapy is the mainstay of treatment. With the discovery of the mechanisms of oncogenesis, there are two complementary approaches to chemotherapy: conventional chemotherapy, such as irinotecan, capecitabine, and oxaliplatin, and targeted therapy, such as bevacizumab, cetuximab, and panitumumab.
The first drug of choice for patients with metastatic colorectal cancer (mCRC) was the fluoropyrimidine 5-fluorouracil (5-FU) [2]. Bolus FU led to modest response rates of approximately 12% and a median survival of approximately 11 months [3]. Combining FU with leucovorin (LV), a reduced folate that increases thymidylate synthetase inhibition, improves clinical outcomes [4]. A meta-analysis indicated a response rate of 23% and a median survival of 11.5 months [4]. In the 1990s, another two agents, irinotecan and oxaliplatin, were found showing activity against advanced CRC. Several randomized trials have established the role of irinotecan and oxaliplatin as second-line agent in patients with mCRC refractory to 5-FU-based treatment [5–7]. And researches have showed that irinotecan or oxaliplatin plus FU/LV known as IFL and FOLFOX, respectively, significantly improve outcomes [8, 9]. However, some patients with mCRC are not appropriate candidates for irinotecan [9–17].
Newer approaches to mCRC therapy have focused on targeting angiogenesis inhibitors. Angiogenesis is critical to both the growth of the primary tumor and metastases [18]. And poor prognosis and an increased relapse rate are often correlated with increased blood vessel density in the primary tumor in mCRC [19]. One of the most important stimulators of angiogenesis is vascular endothelial growth factor (VEGF) [20, 21]. The potential of VEGF as an anticancer target was supported by the demonstration that a murine anti-VEGF monoclonal antibody can inhibit the growth of human tumor xenografts [22]. Subsequently, a recombinant humanized monoclonal antibody against VEGF [23], bevacizumab (Avastin), has been examined as an antiangiogenic cancer therapy. In mCRC patients, initial studies of bevacizumab showed improvements in tumor response rate and progression-free survival (PFS) when added to fluorouracil and leucovorin [24, 25]. Subsequent randomized trials showed bevacizumab to prolong median overall survival (OS; 20.3 versus 15.6 months) in combination with IFL [26]as initial treatment and FOLFOX [27] after the failure of a prior irinotecan-containing regimen (12.9 versus 10.8 months) and to improve response rates and PFS times with the addition of bevacizumab to FOLFIRI or FOLFOX in patients with untreated mCRC [28].
However, the results of these clinical trials were not completely consistent, none of which was large enough to interpret well the efficacy and safety of bevacizumab in combination with chemotherapy. And so far, there has been no meta-analysis with greater statistical power to detect real differences between the groups of patients who were and were not treated with bevacizumab.
Materials and methods
Literature search
Literature search was carried out to identify all relevant randomized controlled trials (RCTs), comparing combined chemotherapy with or without bevacizumab in mCRC. The Pubmed, Embase, and The Cochrane Library were used systematically to search for all articles published from January 2003 to August 2008 which included the following terms in their titles, abstracts, or keyword lists: bevacizumab (or Avastin), colorectal cancer.
Study selection
The reference lists of all traced articles of this topic were examined manually. Citations selected from this initial search were subsequently screened for eligibility using the follow criteria: (1) patients with mCRC; (2) combined chemotherapy with bevacizumab versus without bevacizumab and not confounding by additional agents or interventions (i.e., in the combination chemotherapy, the control and experimental arms had to differ only by bevacizumab component); (3) RCT.
Data extraction
Two reviewers abstracted data independently and reached consensus on all items. The following information were sought as follows: first author, year of publication, number of patients, number of patients eligible for response, Eastern Cooperative Oncology Group performance status, mean age, gender rate, prior adjuvant therapy, median OS, PFS, or median time to progression (TTP), overall response rate (ORR), the 60-day all-cause mortality rate, adverse events (AEs), and specific toxicity data, such as hypertension, thrombosis, bleeding, proteinuria, gastrointestinal perforation, diarrhea, and leukopenia. For trials included in this meta-analysis, if the log hazards ratio (HR) and its variance were not presented explicitly, the methods reported by Parmar et al. [29] were used to extract estimates of these statistics.
Data analysis
Meta-analysis was made of all RCTs comparing the efficacy and safety of combined chemotherapy with bevacizumab versus without bevacizumab. The outcomes used for this study were OS, defined as the time from random assignment to death from any cause, censoring patients who had not died at the date last known alive, and PFS or TTP, defined as the time from random assignment to first documented progression or all-cause mortality in the absence of previously documented tumor progression, and ORR, defined as the sum of partial and complete response rates (according to the Response Evaluation Criteria in Solid Tumors) [30], and toxicity, which was graded according to the Common Toxicity Criteria version 2 (http://ctep.cancer.gov). The overall HR for OS and PFS or TTP, the relative risks (RRs) for ORR, and the odds ratios (ORs) for AEs and the treatment-related deaths were calculated using Stata version 7.0. Data analyses were performed on the intent-to-treat population, defined as all randomly assigned patients. Pooled estimates of efficacy were calculated using a fixed-effects model [31], but the DerSimonian and Laird random effects model was used according to heterogeneity and a P value less than 0.1 was defined as heterogeneity. Bias was studied using the weighted regression tests described by Egger and colleagues [32]. In addition, sensitivity analysis was applied by omitting one study in each turn and investigated the influence of a single study on the overall meta-analysis estimate [31] when necessary.
Assessment of study quality
The methodological quality of the studies included in the meta-analysis was scored using the Jadad composite scale [33, 34]. This is a five-point scale, and one point was given when one quality criterion was met [35].
Result
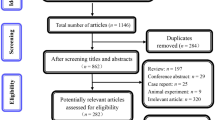
Nine RCTs were identified [36–39, 24–28]. Three publications [36–38] were excluded because of failure to address endpoints of interest, such as OS, PFS or TTP, ORR, and AEs. Another study, the interim analysis [39] in Hurwitz’s trial [26], was also omitted with the compared arms distinct not only by bevacizumab component. Thus, five trials [24–28] involving 3,103 patients with mCRC were ultimately analyzed. Two trials [27, 24] were designed as three-arm comparison and one [28] was a two-by-two factorial design, which, for purposes of meta-analysis, were all condensed to two arms (chemotherapy plus bevacizumab versus chemotherapy alone). And the only-bevacizumab arm in one trial [27] above was excluded in this meta-analysis. The key patient characteristics by trials are listed in Table 1. All of the trials included within the meta-analysis about bevacizumab appear to have been reasonably well-designed and conducted and, with the exception of one study [24], appear to have included balanced populations.
The mean Jadad score of the studies included was 2 (Table 2). All studies had a statement regarding randomization. Three out of five trials had described the methods of randomization. Two out of five trials reported the withdrawals and dropouts. The main study limitations pertained to the procedure for concealing the treatment allocation. There was no placebo-controlled double-blinded trial.
Efficacy
Progression-free survival
The combination of bevacizumab and chemotherapy resulted in a statistically significant improvement in PFS compared with chemotherapy alone (HR = 0.66, 95% confidence interval [95%CI] = 0.56 to 0.77, P = 0.00) (Fig. 1). There was significant heterogeneity between individual trials (P = 0.00). Sensitivity analysis showed that the heterogeneity of outcome between reported trials could be attributed mainly to the trial reported by Kabbinavar and colleagues [25] in 2005.
Median overall survival
OS data required for a meta-analysis were available from four studies [25–28]. Pooled analysis showed a significant difference (P = 0.00) in favor of the bevacizumab-containing arm (Fig. 2). With a HR equal to 0.77 and an associated 95%CI of 0.67 to 0.89, there is a 23% reduction in the hazard of death for the bevacizumab-containing group. However, heterogeneity existed between individual trials (P = 0.08). Sensitivity analysis suggested that the study reported by Kabbinavar [25] may be the main source of heterogeneity.
Response rate
All the three studies included in the meta-analysis reported ORR and CR data (seen in Table 1). The ORR was 623 (40.0%) of 1,559 in the bevacizumab plus chemotherapy group and 533 (34.5%) of 1,544 in the chemotherapy group, showing a statistically significant difference (P = 0.02) in favor of the former (Fig. 3). The RR was 1.50 with an associated 95%CI of 1.06 to 2.10, corresponding to an increase in odds of response for the addition of bevacizumab therapy. Once again, there was significant heterogeneity (P = 0.00). Sensitivity analysis identified the study reported by Kabbinavar and colleagues [25] in 2005 as the main source of heterogeneity. Moreover, 20 of 792 (2.5%) in the bevacizumab arm had complete responses compared with 11 of 807 (1.4%) in the chemotherapy-alone arm (P = 0.09) and no heterogeneity existed (P = 0.667).
Safety
The 60-day all-cause mortality rate
There was no significant difference in the 60-day all-cause mortality rate (OR = 0.82, 95%CI = 0.55 to 1.23, P = 0.34) (Table 3).
Adverse events leading to treatment discontinuation
Pooled estimates of the difference favored combined chemotherapy with bevacizumab over without bevacizumab (OR = 1.38, 95%CI = 1.14 to 1.66, P = 0.00) (Table 3).
Adverse events
Table 3 lists the AEs of particular interest, without adjustment for the median duration of therapy. The incidence of any grade 3 or 4 AEs was approximately 10 percentage points higher among patients receiving chemotherapy plus bevacizumab than receiving chemotherapy alone, with a statistically significant difference (OR = 1.79, 95%CI = 1.52 to 2.11, P = 0.00). For the specify toxicity, the statistically significant differences in pooled estimates suggest a higher incidence of grade 3/4 hypertension (OR = 4.19, 95%CI = 2.76 to 6.36, P = 0.00), grade 3/4 thromboembolic/thrombotic events (OR = 1.75, 95%CI = 1.21 to 2.53, P = 0.00), grade 3/4 bleeding (OR = 1.87, 95%CI = 1.10 to 3.12, P = 0.02), and gastrointestinal perforation (OR = 4.81, 95%CI = 1.52 to 15.3, P = 0.00) associated with the bevacizumab group. No statistically significant differences were noted in the incidence of grade 3/4 proteinuria (OR = 2.61, 95%CI = 0.87 to 7.78, P = 0.086), grade 3/4 leukopenia (OR = 1.28, 95%CI = 0.96 to 1.69, P = 0.09), and grade 3/4 diarrhea (OR = 1.26, 95%CI = 0.97 to 1.63, P = 0.08). There was no significant heterogeneity between trials (P < 0.10). Funnel plots detected no obvious publication bias (P = 0.14, Egger’s test).
Discussion
The meta-analysis included a total of 3,103 intent-to-treat patients, with 1,559 patients in the chemotherapy-plus-bevacizumab arm and 1,544 patients in the chemotherapy-alone arm. It represents the largest randomized cohort of CRC patients treated with an addition of bevacizumab to chemotherapy for advanced disease.
This study showed that the addition of bevacizumab to chemotherapy resulted in both a clinically statistically significant reduction in the risk of dying or progression/death compared with the chemotherapy-alone group. Individually, all trials contained in this study reported that bevacizumab improves PFS when added to chemotherapy for patients with mCRC. While unlike the two trials [26, 27] included in the study, the observed trend in an improvement in OS did not reach statistical significance in the two studies reported by Kabbinavar [25] and Saltz [28], respectively. Long-term survival is clearly the ultimate goal of treatment. But in trial reported by Kabbinavar [25], many patients switch regimens (46% of the FU/LV/placebo group and 39% of the FU/LV/bevacizumab group received irinotecan, oxaliplatin, or both) when they progress and, therefore, survival differences are diluted. Such differences in postprogression therapy have also confounded the interpretation of OS in other randomized clinical trials in first-line mCRC, most notably the Intergroup Trial N9741 [40]. Generally, treatment effects seem larger for disease progression than for survival. Nonetheless, some caution should be advised when PFS is used as a surrogate marker of survival [41]. In addition, the study protocols in the included five trials [24–28] all allowed bevacizumab for treatment until disease progression (PD), and the duration of PFS was longer in the bevacizumab arm. In four of five trials, where the duration of treatment in the bevacizumab arms was longer than in the control arms, three [24, 26, 27] with longer survival time in the bevacizumab arms, one [25] not, but with confounded postprogression therapy. The trial reported by Saltz [28], which compared further-line treatment regimens between the study arms, failed to treat with bevacizumab until PD and did not reach statistical significance in the OS benefit seen for bevacizumab. These findings suggest that the duration of bevacizumab therapy is likely to be important and that treatment until PD may be necessary to maximize the clinical benefit derived from bevacizumab therapy. Meanwhile, the meta-analysis showed a statistically significant difference in favor of chemotherapy plus bevacizumab, with an objective response of 40% compared with 34.5% for chemotherapy alone.
Although this study shows chemotherapy plus bevacizumab to have greater therapeutic efficacy to chemotherapy alone in advanced CRC patients, the risk of such treatment should not be minimized but, instead, considered in balance with the benefit of increased survival.
The meta-analysis suggests that the combination of bevacizumab plus chemotherapy resulted in an approximately 10% overall increase in grade 3 and 4 toxicity, which was thought to be caused by the longer durations of treatment in the bevacizumab arms [24]. There were no significant differences in the 60-day all-cause mortality rates. But a higher proportion of patients discontinued study treatment because of AEs in the bevacizumab-containing arms compared with the chemotherapy-alone arms. Of four trials that reported the outcome, three showed no significant differences between two treatment groups, while one [28] did and indicated that most of these treatment discontinuations were attributable to chemotherapy-related events, such as neurotoxicity [42], gastrointestinal events [43], general disorders, and hematologic events [9], rather than events felt to be potentially related to bevacizumab. The side effect profile of bevacizumab in mCRC differs from that of cytotoxic chemotherapy agents in that bevacizumab has a greater risk of grade 3 or 4 hypertension and grade 3 or 4 thrombotic events, a slight increase in grade 3 or 4 bleeding and gastrointestinal perforation. The outcomes appeared to be comparable between two treatment groups about grade 3 or 4 proteinuria. And the incidence and severity of AEs that are known to occur with 5-FU/LV, such as diarrhea and leucopenia, were no greater with the addition of bevacizumab. Hypertension may be a class effect of angiogenesis inhibitors, as it has been observed with sunitinib [44], sorafenib [45], and the investigational agents VEGF Trap [46], PTK787/ZK222584 [47], AMG706 [48], and AZD2171 [49]. But the mechanism of hypertension is less clear, possibly with the alterations of nitric oxide signaling. The incidence of hypertension in individuals receiving bevacizumab plus chemotherapy in the meta-analysis is consistent with the Avastin (bevacizumab) package insert (Genentech 2004), where severe hypertension occurred in 7–10%, compared to 2% in those not treated with bevacizumab. Meanwhile, higher rates of hypertension have also been observed in other advanced cancer patients treated with the bevacizumab [50–52]. Despite the high incidence, hypertension is generally grade 3 or lower and is manageable with standard antihypertensive medication [24–28]. In two [25, 26] of the five trials contained in the meta-analysis, no grade 4 hypertension events were reported. The relationship between bevacizumab and proteinuria is poorly understood. Several phase III studies [39, 26, 50] reported that the rates of grade 1 or 2 proteinuria were increased in bevacizumab-treated patients, while there was no statistically difference in the incidence of grade 3 or 4 proteinuria in the bevacizumab group in the study. For safety, recommendations [47, 53] for monitoring and treating patients with hypertension and proteinuria that are suggested by the manufacturer of bevacizumab and the US FDA should be carefully followed. In addition, a high background rate of thromboembolic disease was itself associated with patients suffering from mCRC, most likely because of the malignancy, the procoagulant nature of surgery and cytotoxic chemotherapy, and other factors (such as prolonged bed rest) [54]. But this study shows a higher rate of grade 3 or 4 thromboembolic events that happened in the bevacizumab group. An exploratory analysis of pooled data from five RCTs [55] composed of 1,745 patients found that the rate of arterial thromboembolic event (ATEs) was more than twice as high with the addition of bevacizumab to chemotherapy. Furthermore, a study [56] identified age ≥65 years and a history of ATEs as independent risk factors for ATEs, with the exception of exposure to bevacizumab. However, the investigations did not conclude that these risk factors should be an absolute contraindication to bevacizumab therapy. Even these high-risk patients suboptimal for first-line irinotecan-containing therapy [14–17, 57] appeared to benefit in terms of OS and PFS to a similar degree as the study population as a whole [55]. And the risk of an ATE related to bevacizumab and its concomitant risk of mortality, while increased, is small in comparison with cancer-related mortality. Therefore, both patients and their physicians should carefully weigh the potential risks and benefits of treatment with bevacizumab. A pooled analysis mentioned previously [55] suggested that the use of low-dose aspirin (≤325 mg/day) was correlated with a lower incidence of ATEs in high-risk patients treated with bevacizumab. But a higher rate of grade 3 or 4 bleeding with the addition of bevacizumab to chemotherapy was not correlated with the anticoagulation therapy after the development of thromboembolism during treatment with bevacizumab [28, 58]. This meta-analysis also shows that the chemotherapy-plus-bevacizumab group was associated with a higher incidence of gastrointestinal perforation. The event occurred in a small number of patients, but appeared to be potentially life-threatening. Patients with mCRC who are treated with bevacizumab should be closely monitored for signs and symptoms of gastrointestinal perforation, including abdominal pain that is associated with constipation or vomiting.
In summary, the addition of bevacizumab to chemotherapy confers a clinically meaningful and statistically significant improvement in OS, PFS, and ORR. Its side effects are predictable and manageable and do not compound the incidence or severity of toxicities from chemotherapy. With the recommendations for patient selection and monitoring mentioned above, bevacizumab is a valuable addition to the current chemotherapy regimens used in mCRC, producing a significant survival benefit, especially for the high-risk patient with irinotecan- or oxaliplatin-refractory while without additional toxicity.
References
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
de Gramont A, Bosset J-F, Milan C, Rougier P, Bouché O, Etienne P-L, Marvan F, Louvet C, Guillot T, François E, Bedenne L (1997) Randomized trial comparing monthly low-dose leucovorin and Xuorouracil bolus with bimonthly high-dose leucovorin and Xuorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15:808–815
Colorectal Meta-analysis Collaboration (2000) Palliative chemotherapy for advanced or mCRC. Cochrane Database Syst Rev (2):CD001545
Anonymous (1992) Advanced colorectal cancer meta-analysis project: modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 10:896–903
Cunningham D, Pyrhönen S, James RD, Punt CJA, Hickish TF, Heikkila R, Johannesen TB, Starkhammer H, Topham CA, Awad L, Jacques C, Herait P (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after Xuorouracil failure for patients with mCRC. Lancet 352:1413–1418
Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C (1998) Randomised trial of irinotecan versus Xuorouracil by continuous infusion after Xuorouracil failure in patients with mCRC. Lancet 352:1407–1412
de Gramont A, Vignoud J, Tournigand C, Louvet C, André T, Varette C, Raymond E, Moreau S, Le Bail N, Krulik M (1997) Oxaliplatin with high-dose leucovorin and 5-Xuorouracil 48-hour continuous infusion in pretreated mCRC. Eur J Cancer 33:214–219
Saltz LB, Cox JV, Blanke C et al (2000) Irinotecan Study Group: irinotecan plus fluorouracil and leucovorin for mCRC. N Engl J Med 343:905–914
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Rougier P, Bugat R, Douillard JY et al (1997) Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol 15:251–260
Freyer G, Rougier P, Bugat R et al (2000) Prognostic factors for tumour response, progressionfree survival and toxicity in mCRC patients given irinotecan (CPT-11) as second-line chemotherapy after 5FU failure: CPT-11 F205, F220, F221 and V222 study groups. Br J Cancer 83:431–437
Knight RD, Miller LL, Pirotta N et al (2000) First-line irinotecan (C), fluorouracil (F), leucovorin (L) especially improves survival (OS) in mCRC (MCRC) patients (PT) with favorable prognostic indicators. Proc Am Soc Clin Oncol 19:255a, (abstract 991)
Food and Drug Administration Center for Drug Evaluation and Research [FDA CDER] (2000) Camptosar (irinotecan, CPT-11): first-line therapy of mCRC. Presented at the 65th Meeting of the Oncologic Drugs Advisory Committee, March 16. Available at http://www.fda.gov/ohrms/dockets/ac/00/slides/3592sle.ppt
Bleiberg H, Cvitkovic E (1996) Characterisation and clinical management of CPT-11 (irinotecan)-induced adverse events: the European perspective. Eur J Cancer 32A(Suppl 3):S18–S23
Food and Drug Administration Center for Drug Evaluation and Research [FDA CDER] (1996) CPT-11 for the treatment of patients with mCRC that has progressed following initial 5-FU based chemotherapy. Presented at the 50th Meeting of the Oncologic Drugs Advisory Committee, Bethesda, MD, June 13
Rougier P, Bugat R (1996) CPT-11 in the treatment of colorectal cancer: clinical efficacy and safety profile. Semin Oncol 23(1 Suppl 3):34–41
Rothenberg ML, Cox JV, DeVore RF et al (1999) A multicenter, phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal carcinoma. Cancer 85:786–795
Algire GH, Chalkley HW, Legallais FY et al (1945) Vascular reactions of normal and malignant tissue in vivo, I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst 6:73–85
Reinmuth N, Parikh AA, Ahmad SA, Liu W, Stoeltzing O, Fan F et al (2003) Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc Res Tech 60:199–207
Ferrara N (1999) Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77:527–543
McMahon G (2000) VEGF receptor signaling in tumor angiogenesis. Oncologist 5:3–10
Kim KJ, Li B, Winer J et al (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844
Presta LG, Chen H, O’Connor SJ et al (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57:4593–4599
Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G et al (2003) Phase II, randomized trial comparing Bevacizumab plus fluorouracil (FU)/Leucovorin (LV) with FU/LV alone in patients with MCRC. J Clin Oncol 21:60–65
Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR et al (2005) Addition of Bevacizumab to Bolus Fluorouracil and Leucovorin in first-line MCRC: results of a randomized phase II trial. J Clin Oncol 23:3697–3705
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al (2004) Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for MCRC. N Engl J Med 350:2335–2342
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR et al (2007) Bevacizumab in combination with Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for previously treated mCRC: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in MCRC: a randomized phase III study. J Clin Oncol 26:2013–2019
Parmar MKB, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F (2000) Methods for meta-analysis in medical research. Wiley, Chichester
Egger M, Smith GD, Altman DG (2001) Systematic reviews in health care: meta-analysis in context, 2nd edn. BMJ Books, London
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Kjaergard LL, Villumsen J, Gluud C (2001) Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135:982–989
Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M et al (1998) Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352:609–613
Blackwell K, Hurwitz H, Liebérman G, Novotny W, Snyder S, Dewhirst M (2004) Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 101:77–82
Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS et al (2006) Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24:217–227
Zheng H, Chen JZ, Liao WJ, Luo RC (2006) Efficacy of Avastin in combination with irinotecan for metastatic colorectal cancer. Nan Fang Yi Ke Da Xue Xue Bao 26:689–691
Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E et al (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508
Goldberg RM, Sargent DJ, Morton RF et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated mCRC. J Clin Oncol 22:23–30
Johnson RK, Ringland C, Stokes JB et al (2006) Response rate or time to progression as predictors of survival in trials of mCRC or non-small-cell lung cancer: a meta-analysis. Lancet Oncol 7:741–746
Land SR, Kopec JA, Cecchini RS et al (2007) Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 25:2205–2211
Rougier P, Van Cutsem E, Bajetta E et al (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with mCRC. Lancet 352:1407–1412
Sutent (sunitinib malate) prescribing information. Pfizer, New York, NY. Available at https://www.pfizeroncology.com/product-info/sutent-description.jsp?setShowOn=../product-info/product-centers.jsp&setShowHighlightOn=../product-info/sutent-indication.jsp
Nexavar (sorafenib) prescribing information. Onyx Pharmaceuticals, Emeryville, CA. Available at http://www.nexavar.co.uk/pi.htm
Dupont J, Schwartz L, Koutcher J et al (2004) Phase I and pharmacokinetic study of VEGF Trap administered subcutaneously to patients with advanced solid malignancies. J Clin Oncol 22:197, (abstract 3009)
Gordon MS, Cunningham D (2005) Managing patients treated with bevacizumab combination therapy. Oncology 69(Suppl 3):25–33
Rosen L, Kurzrock R, Jackson E et al (2005) Safety and pharmacokinetics of AMG 706 in patients with advanced solid tumors. J Clin Oncol 23(Suppl):195s, (abstract 3013)
Morris C, Jurgensmeier J, Robertson J et al (2006) AZD2171, an oral, highly potent, and reversible inhibitor of VEGFR, signaling with potential for the treatment of advanced colorectal cancer. Presented at 2006 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology, San Francisco, CA, January 28–30, Abstract 242
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Miller KD, Wang M, Gralow J et al (2005) A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer: a trial coordinated by the Eastern Cooperative Oncology Group (E2100). Breast Cancer Res Treat 94(Suppl 1):S6, (abstract 3)
Sandler AB, Gray R, Brahmer J et al (2005) Randomized phase II/III trial of paclitaxel (P) plus carboplatin (C) with or without bevacizumab (NSC #704865) in patients with advanced non-squamous non-small cell lung cancer (NSCLC): an Eastern Cooperative Oncology Group (ECOG) Trial-E4599. J Clin Oncol 23(Suppl):2s, (abstract LBA4)
Avastin (bevacizumab) prescribing information. Genentech, South San Francisco, CA. Available at http://www.cptech.org/ip/health/avastin.html
Ma L, Francia G, Viloria-Petit A et al (2005) In vitro procoagulant activity induced in endothelial cells by chemotherapy and antiangiogenic drug combinations: modulation by lower-dose chemotherapy. Cancer Res 65:5365–5373
Skillings JR, Johnson DH, Miller K et al (2005) Arterial thromboembolic events in a pooled analysis of 5 randomized, controlled trials of bevacizumab with chemotherapy. J Clin Oncol 23(Suppl):196s, (abstract 3019)
Scappaticci FA, Skillings JR, Holden SN, Gerber H, Miller K, Kabbinavar F (2007) Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99:1232–1239
Rougier P, Bugat R, Douillard JY et al (1997) Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol 15:251–260
Hambleton J, Novotny W, Hurwitz H et al (2004) Bevacizumab does not increase bleeding in patients with mCRC receiving concurrent anticoagulation. J Clin Oncol 22:252, (abstract 3528)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Y., Tan, A., Gao, F. et al. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis 24, 677–685 (2009). https://doi.org/10.1007/s00384-009-0655-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-009-0655-9