Abstract
Objective
Chitotriosidase (ChT) is an activated macrophage marker. Tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) are mainly produced macrophages. The aim of the present study was to evaluate the relationship between serum ChT activity, levels of TNF-α and IL-1β in patients with mild preeclampsia and normal pregnancy.
Methods
An overall 64 cases, 32 healthy pregnant control women (control group) and 32 women with mild preeclamptic patients (study group), were enrolled in this study. At the beginning of the study, all study participants were matched for age and gestational age. Serum ChT activity was measured by fluorometer; TNF-α and IL-1β levels were measured by enzyme-linked immunosorbent assay.
Results
The mean age, gestational week, parity and gravida were similar in the two groups (p > 0.05). Serum ChT activity was significantly higher in the preeclampsia group compared to the control group (p < 0.05). Levels of TNF-α and IL-1β in patients with mild preeclampsia were similar compared to the control group (p > 0.05). In the PE group, serum ChT activity was not correlated with TNF-α and IL-1β.
Conclusion
Mild preeclampsia is found associated with higher ChT activity. This result suggests that activated macrophages play a role in the pathogenesis of preeclampsia. This suggestion needs to be confirmed in future studies with larger populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is the most common medical complication of pregnancy and characterized by the new onset of hypertension and proteinuria after the 20th week of gestation. It occurs in about 6–10% of all pregnancies and results in substantial maternal and fetal morbidity and mortality [1]. Despite extensive research to explain pathogenesis of preeclampsia, its exact pathogenesis still remains unclear. However, several pathophysiologic abnormalities such as abnormal trophoblast differentiation and invasion, placental and endothelial dysfunction, immune maladaptation and exaggerated systemic inflammatory response have been proposed to explain the development of PE [2, 3].
Decidual immune systems, mainly uterine natural killer (NK) and macrophages, are required for a normal successful pregnancy. Macrophages play a role in implantation, trophoblast invasion, uterine vascular remodeling, removal of apoptotic trophoblast cells and immunotolerance toward fetal antigens [4]. Macrophages are also located near the spiral arteries during trophoblast invasion and transformation; therefore, macrophages play a role in spiral artery remodeling [4, 5]. Also, decidual immune cells are highly activated and produce abundant amount of cytokines [4].
During the placentation process or during placentation, apoptotic bodies resulting from trophoblasts are removed by macrophages. The removal of damaged cells by natural killer and macrophages in the decidua facilitates trophoblast invasion and implantation [3, 4, 6]. However; in many studies it has been shown that PE is associated with increased apoptosis of trophoblastic cells [4]. In PE, the number and distribution of macrophages in placenta are significantly altered in comparison to normal pregnancy [4, 7, 8]. Activated macrophages secrete detectable levels of cytokine such as TNF and IL- 1, and indeed are mainly produced by macrophages [9]. In many studies, it has been shown that TNF-α and IL-1β play a role in abnormal extravillous trophoblast invasion in preeclampsia [10, 11]. In vitro studies demonstrate that macrophage-associated TNF-α induces apoptosis of extravillous trophoblasts [12]. Chitotriosidase (Chito, ChT), a member of the chitinase protein family, is mainly secreted as a 50-kDa active enzyme by activated human macrophages to the extracellular medium [13]. ChT was found to be increased in several diseases such as Gaucher’s diseases, thalassemia, atherosclerosis, bronchial asthma, malaria and neurodegenarative disorders, all displaying the common feature of elevated phagocytosis which is also a feature of PE [14]. We hypothesized that removal of increased apoptotic trophoblast cells by macrophages resulted in elevated ChT in PE activity. In the current study, our aim was to investigate the concentrations of serum IL-1β and TNF-α levels, and ChT activity in patients with PE and in normal pregnant women.
Materials and methods
The study was approved by the hospital’s ethics committee and all participants gave written informed consent. The study was carried out between February 2008 and November 2009 at the Department of Obstetrics and Gynecology of Gulhane Military Medical Faculty. The study population consisted of 32 healthy normal pregnant women (control group) and 32 women with mild PE (study group), who had been diagnosed and treated at the third trimester of pregnancy.
At the beginning of the study, all study participants were matched for age and gestational age. For the study and control groups, the women who were admitted to the study center for routine prenatal visit after the 32nd week of pregnancy were assigned. It would be better to include in the definition of severe preeclampsia an onset of disease before the 32nd week of gestation.
Preeclampsia is defined as increased blood pressure (≥140/90 mmHg) that occurs in a pregnant woman after 20 weeks of gestation, accompanied by proteinuria (0.3 g/24 h) [15]. Hypertension is defined as a blood pressure >140 mmHg (systolic) or >90 mmHg (diastolic) on at least two occasions and 4–6 h apart after the 20th week of gestation in women known to be normotensive previously. Proteinuria is defined as 300 mg or more protein excretion every 24 h, or protein concentration of 300 mg/L or higher in urine (≥1+ on dispstick) [3, 15]. Severe preeclampsia was diagnosed if one or more of the following were present: blood pressure of 160/110 mmHg or higher, excretion of 5 g or more of protein in a 24-h urine sample, the presence of multiorgan involvement such as oliguria, pulmonary edema, visual or cerebral disturbance and pain in the epigastric area or right upper quadrant, abnormal liver enzymes and thrombocytopenia (platelet count <100,000 per μL) [3, 15]. Women who met the preeclampsia criteria but not severe preeclampsia were classified as mild preeclampsia.
Gestational age was based on the precise date of the last menstrual period or/and ultrasound measurement of the crown-rump length in the first trimester. The exclusion criteria were as follows: severe preeclampsia, HELLP syndrome, intrauterine growth retardation (IUGR), multiple pregnancies, premature rupture of membranes, chorioamnionitis, chronic hypertension, diabetes mellitus, autoimmune disorders and inflammatory conditions. Also smoking and advanced maternal age (>35 year) and obese subjects (body mass index > 27 kg/m2) were not enrolled in this study because of its probable effects on the investigated cytokines.
None of the controls had signs of elevated blood pressure or other pregnancy complications, and all gave birth to healthy infants. None of the patients were in labor at the time of the sampling.
Blood samples and biochemical analysis
One peripheral sample of 10 cc venous blood was collected from each pregnant woman into vacutainer tubes. Plasma was isolated by centrifugation at 3,000g for 10 min at 4°C and stored at −80°C until the analyses were performed. Blood samples from PE women were drawn after the diagnosis and admission to hospital, and before glucocorticoid treatment. Blood samples from healthy pregnancy were obtained during routine antenatal care. Serum IL-1β and TNF-α concentrations were determined by a commercial enzyme-linked immunosorbent assay (CytElisa ™ Human IL-1β and CytElisa™ Human TNF-α). The ChT assay was based on the method described by Hollak et al. [13]. Briefly, ChT activity was measured by incubating 5 μL of serum with 100 μL of 22 μmol/L 4-methylumbelliferyl β-d-N,N′,N″-triacetylchitotrioside (Sigma Chemical, St. Louis, MO, USA) as substrate in Mcllvain phosphate-citrate buffer, pH 5.2, for 1 h at 37°C. The reaction was terminated by adding 120 μL of 0.5 mol/L Na2CO3–NaHCO3 buffer, pH 10.7, and the fluorescence of 4-methylumbelliferone was measured with a fluorometer with excitation set at 355 nm and emission at 460 nm (Titertek, Huntsville, AL, USA). Serum chitotriosidase activity was measured by duplication, and coefficient of variation was less than 5% in all cases.
Statistical analysis
The SPSS 15.0 statistical software package (SPSS Inc., Chicago, IL, USA) was used for all data analyses. One-sample Kolmogorov–Smirnov test was performed to confirm the assumption of normality in the two groups for outcome variables. For comparing groups according to mean demographic and clinic properties, the Student’s t test (age) or Mann–Whitney U (others variable) test was used. Differences were considered to be statistically significant if the null hypothesis could be rejected with 95% confidence; p < 0.05. Correlations between ChT activity, TNF-α and IL-1β were assessed with the Spearman’s rank test.
Results
A total of 106 subjects were found to be eligible for the study, but 42 of the participants were excluded due to advanced maternal age (>35 years) (n = 12), obesity (body mass index ≥ 27 kg/m2) (n = 8), severe preeclampsia (n = 7), refusal to participate (n = 4), chronic hypertension (n = 3) HELLP syndrome (n = 2), IUGR (n = 2), multiple pregnancies (n = 2) and diabetes mellitus (n = 2) (Fig. 1). In all, 64 patients were enrolled in the study. These 64 women were divided into two subgroups [study group (n = 32), control group (n = 32)].
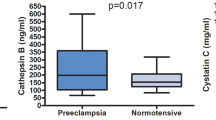
The clinical characteristics of PE and normotensive pregnant participants are shown in Table 1. There were no significant differences between the PE and normal pregnancy subjects with their age, parity, gravida, gestational period at the time of blood sampling and aspartate transaminase, alanine transaminase, hemoglobin, platelet count and lactate dehydrogenase levels (p > 0.05). As expected, women with PE had a higher systolic and diastolic blood pressure and proteinuria compared to those with normal pregnancy (p < 0.05). The birth weight of babies was lower in the PE group compared with the normotensive pregnant group (p < 0.05). Serum ChT activity was significantly higher in women with PE (median 141.1 range 86.8–202.3 nmol/ml/h) than in the control group (median 72.8 range 51.3–84.8 nmol/ml/h) (p < 0.05). There were no significant differences in maternal serum levels of TNF-α and IL-1β between the two groups (p > 0.05). Table 2 lists serum ChT activity and concentrations of TNF-α and IL-1β from the PE and normotensive participants. Distribution of ChT according to groups is shown in Fig. 2. There was no significant correlation between ChT with IL-1β and TNF-α levels (p > 0.05). In PE, also there was no significant correlation between TNFα and IL-1β (p > 0.05). In the control group, there was no significant correlation between ChT activity with IL1-1β and TNF-α (p > 0.05). But, a correlation was found between IL-1β and TNF-α in the normotensive group (p < 0.05).
Discussion
Our study showed that serum ChT activity was markedly increased in mild PE patients when compared with women with normal pregnancy. ChT activity has been assumed to be a marker of macrophage activation; our results showed that even mild PE was associated with increased macrophage activation compared to normal pregnancy. Pregnancy is a condition of controlled mild maternal systemic inflammation [16, 17]. During normal pregnancy, the decidua is colonized by cellular members of the innate immune system, mainly uterine NK and macrophages. While gestation proceeds, the NK cell numbers decrease, but the macrophage population remains high throughout pregnancy [5]. Macrophages are recruited to the closely associated extravillous cytotrophoblast shell [5]. Macrophages are also located near the spiral arteries during trophoblast invasion and transformation. In summary, decidual macrophages appear to play an important role in trophoblast invasion, vascular remodeling, implantation, placentation and finally a successful pregnancy [4]. In preeclampsia, there is increased apoptosis due to the placental hypoxia or maternal or fetal immune maladaptation [3, 18, 19]. Aberrant decidual macrophage distribution and function have been described in patients with preeclampsia [4, 7, 8]. Immunohistochemical analysis of placental bed biopsies has demonstrated an accumulation of macrophages surrounding acute atherosis lesions within the spiral arteries in preeclamptic patients [20]. The infiltrating macrophages could affect trophoblast function in various ways: induce apoptosis [21], alter differentiation or proliferation [22] and may also hamper trophoblast invasion [6].
Therefore, we investigated macrophage activity in PE, using the ChT enzyme activity. ChT is mainly secreted by active human macrophages to the extracellular medium as a 50-kDa active enzyme [13, 14]. It is a known fact that activated macrophages are the main source of the enzyme. ChT was first discovered in plasma of patients suffering from Gaucher disease. It was first found that the elevated enzyme originates from lipid-laden macrophages that accumulate in various tissues of Gaucher patients [13]. Elevation of ChT was observed in various diseases, i.e., lysosomal disorders, malaria, atherosclerosis and neurodegenerative disorders. The activating factors still remain unknown and are likely to be different in the disorders studied [13]. But, common feature of elevated ChT disorders is macrophage activation through increased phagocytosis similar to PE. Madazli et al. [23] showed that there was a significant positive correlation between ChT activity and PE. This study also showed that ChT activity was correlated with diastolic blood pressure in PE, indicating a relationship between disease severity and macrophage activation. We confirmed the significant relationship between PE and ChT activity similar to the prior study, and, due to our study group characteristics, we demonstrated that even mild PE was associated with elevated macrophage activity. Therefore, we confirm previous studies that showed macrophage activation in PE. We may speculate that mild macrophage activity is required for normal pregnancy, but increased macrophage activity may cause or at least accompany the development of PE.
In this study, we assessed ChT and TNF levels in only mild preeclamptic patients. The reason that we did not include patients with severe preeclampsia in our study was renal excretion of TNF was decreased in these patients, which might lead to false results.
Fractional excretion of TNF-α is found to be significantly reduced in women with severe preeclampsia, despite proteinuria. The decreased clearance and altered renal excretion of this cytokine may contribute to the exaggerated inflammatory response observed in preeclampsia [24].
Preeclampsia is associated with the imbalance between proinflammatory (Th1) (such as TNF-α-IL-1β) and anti-inflammatory (Th2) cytokines [3, 17, 18]. TNF and IL-1β are mainly produced by activated macrophages [9]. Local TNF-α production could be critically involved in the physiological balance of trophoblast turnover (apoptosis), invasion, renewal and spiral artery remodeling [9, 25]. It has been shown that excess apoptosis results in increased proinflammatory cytokines as TNF-α secretion by macrophages in PE. A study showed that activated macrophages around the spiral arterioles secrete high levels of TNF-α that lead to the apoptotic death of the invading extravillous trophoblasts [12]. It was also shown that activated macrophages have the potential to inhibit trophoblast migration [26]. Studies have shown that increased decidual TNF-α levels increase decidual cell expression of a number of monocyte/macrophage chemoattractant factors, which lead to the recruitment and activation of macrophages in the placental bed [27, 28]. TNF-α may not be sufficient for apoptosis of extravillous trophoblast, but both elevated macrophages in decidua and TNF-α are required for apoptosis [16]. In a recent observation, it has been found that TNF neutralizing antibody reduced the lipopolysaccharide—activated macrophages mediated trophoblast invasion inhibition [29]. There is a conflict on TNF-α level in PE in the literature. Although several studies have reported elevated circulating TNF-α in patients with PE [30–33], others failed to detect a correlation between TNF-α and the later onset of PE [34, 35]. Therefore, we investigated both macrophage activity and TNF-α and IL-1β cytokines in PE in relation to macrophages activity. Preeclamptic patients had higher concentrations of TNF-α levels, but the difference was not significant. It is a known fact that the normal pregnancy process is in favor of TH2 immunity; recent studies have shown that TH1 cytokines are also required for pregnancy [36]. These findings are in line with our results. We also found a correlation between TNF-α and IL-1β, suggesting that a certain amount of cytokines is necessary for a successful pregnancy. Although several studies support the theory of shift toward Th1 immunity in PE, many reports demonstrate a lack of reproducibility. There are conflicting results about cytokine levels between normal and preeclamptic pregnancy. These discrepancies and conflicts may be due to different experimental approaches/techniques such as ELISA or PCR, blood collection problems, the nature of cytokines such as short half-life, blood sampling after glucocorticoid treatment, different stage of gestation, different study designs or inclusion criteria and different PE groups [37, 38]. Our results regarding TNF-α may arise from these factors. One study demonstrated that IFN-gamma, TNF-alpha and LPS were associated with the up-regulation of chito gene expression in human macrophages [39]. We could not find a significant relationship between ChT activity and TNF-α. Lockwood et al. [10] showed that TNF-α and IL-1β play a role in abnormal extravillous trophoblast invasion in preeclampsia. In this study, we found that there was no significant change in the levels of IL1β in the serum of women with PE compared with normal pregnancy. There is a controversy about IL-1β in PE, while some authors reported increased blood serum levels compared with normal pregnant women [33], some were not able to detect a significant difference between the serum IL-1β levels of normal pregnant and preeclamptic women, similar to our study [34, 35]. In summary, TNF-α and IL-1β were not found to be increased in preeclamptic pregnancies. However, our study population was too small to allow definitive conclusions. Also, we included patients with milder disease (as reflected by lower blood pressure and later onset of disease) to our study. Since cytokines and immune systems act in networks, it seems unlikely that a single cytokine or a single immune system element could explain the total of a normal trophoblastic development.
The results of the present study indicate that women suffering from even mild PE possess significantly increased ChT activity, thus suggesting that increased macrophage activity may play a major role in the etiopathogenesis of PE.
References
Sibai BM (2003) Diagnosis and management of gestational hypertension-preeclampsia. Obstet Gynecol 102:181–192
Sibai BM (2008) Hypertensive disorders of pregnancy: the United States perspective. Curr Opin Obstet Gynecol 20:102–106
Sibai BM, Dekker G, Kupferminc M (2005) Preeclampsia. Lancet 365:785–799
Nagamatsu Takeshi, Schust DannyJ (2010) The immunomodulatory roles of macrophages at the maternal–fetal interface. Reprod Sci 17:209–218
Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL (2009) Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 174(5):1959–1971
Renaud SJ, Postovit LM, Macdonald-Good fellow SK, McDonald GT, Caldwell JD, Graham CH (2005) Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod 73:237–243
Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, Kaufmann P, Rath W (1999) The distribution of macrophages in spiral arteries of the placental bed in preeclampsia differs from that in healthy patients. Placenta 20:229–233
Redline RW (2001) Macrophages in the basal plate of preeclamptic placentae. Placenta 22:229–233
Haider S, Knöfler M (2009) Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta 30:111–123
Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buckwalder LF et al (2008) Matrix mettaloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod 78:1064–1072
Meisser A, Chardonnens D, Campana A, Bischof P (1999) Effects of tumor necrosis factor-alpha, interleukin1-alpha, macrophage colony stimulating factor and transforming growth factor beta on trophoblastic matrix metalloproteinases. Mol Hum Reprod 5:252–260
Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B (2001) Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest 81:1143–1152
Hollak CE, van Weely S, van Oers MH, Aerts JM (1994) Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest 93:1288
Malaguarnera L (2006) Chitotriosidase: the yin and yang. Cell Mol Life Sci 63:3018–3029
National High Blood Pressure Education Program Working Group (2000) Report on high blood pressure in pregnancy. National Institutes of Health Publication No. 00–3029. National Institutes of Health, Washington
Rusterholz C, Hahn S, Holzgreve W (2007) Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol 29:151–162
Wegmann TG, Lin H, Guilbert L, Mosmann TR (1993) Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today 14:353–356
Redman CW, Sargent IL (2003) Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta 24:2I–27
Straszewski S, Kamsteeg M (2002) THe role of the Fas/Fas ligand system in female reproductive organs: survival and apoptosis. Biochem Pharmacol 64:1305–1315
Katabuchi H, Yih S, Ohba T et al (2003) Characterization of macrophages in the decidual atherotic spiral artery with special reference to the cytology of foam cells. Med Electron Microsc 36(4):253–262
Crocker IP, Cooper S, Ong SC, Baker PN (2003) Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol 162:637–643
Knofler M, Mosl B, Bauer S, Griesinger G, Husslein P (2000) TNF-alpha/TNFRI in primary and immortalized first trimester cytotrophoblasts. Placenta 21:525–553
Madazli R, Kucur M, Gezer A, Isman F, Bulut B (2008) Chitotriosidase and YKL-40 in normal and pre-eclamptic pregnancies. Int J Gynaecol Obstet 100:239–243
Cackovic M, Buhimschi CS, Zhao G, Funai EF, Norwitz ER, Kuczynski E, Lockwood CJ, Buhimschi IA (2008) Fractional excretion of tumor necrosis factor-alpha in women with severe preeclampsia. Obstet Gynecol 112:93–100
Knofler M, Mosl B, Bauer S, Griesinger G, Husslein P (2000) TNF-alpha/TNFR1in primary and immortalized first trimester cytotrophoblasts. Placenta 21:525–535
Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M (2004) Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab 89:812–822
Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F et al (2006) Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol 168:445–452
Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T et al (2006) Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol 72:60–73
Renaud SJ, Macdonald-Goodfellow SK, Graham CH (2007) Coordinated regulation of human trophoblast invasiveness by macrophages and interleukin 10. Biol Reprod 76:448–454
Jonsson Y, Ruber M, Matthiesen L, Berg G, Nieminen K, Sharma S, Ernerudh J, Ekerfelt C (2006) Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol 70:83–91
Dong M, He J, Wang Z, Xie X, Wang H (2005) Placental imbalance of Th1–and Th2 type cytokines in preeclampsia. Acta Obstet Gynecol Scand 84:788–793
Bernardi F, Guolo F, Bortolin T, Petronilho F, Dal-Pizzol F (2008) Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J Obstet Gynaecol Res 34(6):948–995
Luppi P, Deloia JA (2006) Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol 118:268–275
Montagnana M, Lippi G, Albiero A, Salvagno GL, Tranchi M, Guidi GC (2008) Serum pro-inflamatory cytokines in physiological and pre-eclamptic pregnancies. Gynecol Endocrinol 24:113–116
Serin IS, Ozcelik B, Basbug M, Kilic H, Okur D, Erez R (2002) Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol 100:143–145
Chaouat G (2007) The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol 29:95–113
Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW (2007) Inflammatory changes in preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv 62:191–2011
Peracoli JC, Rudge MV, Peracoli MT (2007) Tumor necrosis factor-alpha in gestation and puerperium of women with gestational hypertension and preeclampsia. Am J Reprod Immunol 57:177–185
Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L (2005) Effect of interferon-gamma, interleukin 10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clin Chem Lab Med 43:499–502
Conflict of interest
There is no conflict of interest of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alanbay, I., Coksuer, H., Ercan, C.M. et al. Chitotriosidase, interleukin-1 beta and tumor necrosis factor alpha levels in mild preeclampsia. Arch Gynecol Obstet 285, 1505–1511 (2012). https://doi.org/10.1007/s00404-011-2157-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-011-2157-6