Abstract
Purpose
To compare the circulating levels of cathepsins B and D in preeclamptic and normotensive pregnancies.
Methods
Seventy-two pregnant patients were enrolled in this study. Of the 72 pregnant patients, 25 were preeclamptic and 47 patients were normotensive. Serum levels of soluble cathepsins B and D were measured with an enzyme-linked immunosorbent assay (ELISA) kit.
Results
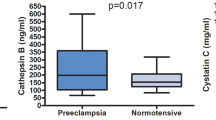
Cathepsin B levels were significantly higher in preeclamptic women than normotensive pregnant women (125.9 vs. 41.9 ng/mL; p = 0.013). The serum levels of cathepsin D were lower in preeclamptic women, but the differences were not significant (129.3 vs. 200.9 ng/mL; p = 0.077). However, cathepsin B and D levels were not correlated with severity of preeclampsia and small for gestational age. The serum levels of cathepsin D were inversely correlated with uric acid in preeclamptic patients (r = −0.527; p = 0.03).
Conclusion
The serum levels of cathepsin B levels were increased significantly in preeclamptic women. Correlation with severity of preeclampsia needs further investigation to clarify the role of cathepsin B.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is a gestational multisystem disease that affects 3–5 % of pregnancies. It remains one of the most challenging diseases in obstetrics worldwide, with increased maternal and neonatal morbidity and mortality. Perinatal consequences include stillbirth, preterm delivery, fetal growth restriction (FGR), neonatal complications, and later sequelae [1]. Although there has been a boom of research on PE, the etiology and pathophysiology are still debatable and need to be clearly established. Placental abnormalities, oxidative stress, endothelial dysfunction, inflammation, and immunity are all thought to be involved in disease progression [2].
Normal placentation requires interactions between fetal-derived trophoblast cells and maternal decidualizing uterine stroma. The invasiveness of trophoblast cells depends on the production of proteases, particularly matrix metalloproteases (MMP), plasminogen activators, and cathepsins [3, 4]. In PE, endovascular trophoblast invasion is restricted to the peripheral, decidual segments of the spiral arteries, leading to incomplete modification of maternal spiral arteries and less placental perfusion. Abnormal expression of proteinases, such as cathepsins, may contribute to this syndrome.
The cathepsin proteases are major lysosomal enzymes that are important for placental development, in mice [5] as well as humans [6, 7]. In general, these enzymes are involved in protein degradation and processing, and have been implicated in a variety of cellular processes, such as apoptosis, angiogenesis, cell proliferation, and invasion [6]. They also play essential physiological roles in antigen presentation, bone remodeling, and epidermal homeostasis, and several family members are associated with tumor development and progression [8, 9].
Cathepsin proteases include the cysteine proteases cathepsin B (CB) and cathepsin L (CL), as well as aspartyl protease cathepsin D (CD). CB is predominantly located in placental and decidual macrophages and trophoblasts, indicating that it may play a role in mediating trophoblast invasion, villous angiogenesis, and decidual apoptosis [6]. CD is the most significant aspartic protease and is widely distributed in animal and human cells. It is reportedly correlated with invasion and phagocytic activity in rodent trophoblasts [10]. Several studies on animal studies have indicated the roles of CB and CD in normal and pathologic placentation [5, 10, 11]. However, research on changes in serum concentrations of cathepsins in PE in humans is limited, although one study reported significantly elevated serum levels of CB and CL in PE [12].
In the present study, we compared the circulating levels of CB and CD in preeclamptic and normotensive pregnancies to further elucidate the etiology and pathophysiology of PE.
Materials and methods
A cross-sectional cohort study of patients with preeclampsia at Kang Dong Sacred Heart hospital was conducted from September 2011 and May 2015. We analyzed blood samples from 72 patients with normotensive pregnancies (n = 47) and PE (n = 25). Patients were recruited from the Department of Obstetrics and Gynecology, Kang Dong Sacred Heart Hospital in South Korea. The normotensive women had no signs of gestational complications or fetal distress, and all of the delivered healthy babies were of appropriate size for gestational age. One sampling was each taken in the third trimester for the normotensive and preeclamptic pregnant patients. For the preeclamptic patients, samples were taken at admission immediately following the diagnosis of disease onset and before the use of intravenous magnesium.
PE was defined as gestational hypertension [systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg] arising after 20 weeks of gestation on at least two occasions (4 h to 1 week apart) with proteinuria (≥300 mg in a 24-h urine collection or one dipstick measurement ≥1+) [13]. The preeclamptic patients were divided into subgroups according to clinical manifestations [i.e., the presence and severity of small-for-gestational-age (SGA) infants]. Severe PE was defined as the presence of one or more of the following criteria: blood pressure >160/110 mmHg, proteinuria ≥2 g/day, thrombocytopenia, elevated liver enzymes, low platelet (HELLP) syndrome, and FGR. SGA was defined as an infant whose birth weight was below the tenth percentile for gestational age. All of the patients were non-smokers. We excluded patients with multiple gestations, chronic hypertension, diabetes, nephritic syndrome, thyroid diseases, other metabolic disorders, fetal anomalies, or isolated SGA.
Immediately after collecting 2 mL of blood from each patient, the serum was centrifuged at 2500 rpm for 10 min at room temperature and stored at −70 °C until analysis, with only one freeze/thaw cycle for allocation of sample aliquots. Serum levels of CB and CD were determined using enzyme-linked immunosorbent assay kits (ELISA; CB: ABCAM, Cambridge, UK; CD: Abnova, Taipei, Taiwan) according to the manufacturer’s protocol. Samples from each patient were measured in parallel and in duplicate to avoid interassay variance. According to the manufacturer’s specifications, the assay range was 156–10,000 pg/mL, and the average sensitivity for CB was 5 pg/mL. For CD, the assay range was 156–10,000 pg/mL, and the average sensitivity was 10 pg/mL. All of the subjects provided informed consent prior to sample collection. This study was approved by the Institutional Review Board at the Kang Dong Sacred Heart Hospital, Hallym University.
Statistical analysis
We calculated 28 patients with preeclampsia appropriate with power of 80 %, ratio of unexposed to exposed of 2 and 5–10 % of prevalence of preeclampsia among the unexposed. Statistical analysis was performed with the SPSS for Windows software package (ver. 21.0; SPSS Inc., Chicago, IL, USA). All of the measured values are presented as medians and interquartile range. Comparisons were performed using the non-parametric Mann–Whitney U test, and the correlations between parameters were analyzed using Spearman’s rank correlation coefficient, with p values less than 0.05 indicating statistical significance. A receiver-operating characteristic (ROC) curve was used to determine the relationship between the sensitivity (true positive rate) and the false positive rate, and to select the best cutoff concentrations of serum CB and CD for the prediction of PE using MedCalc software (ver. 14.8.1; MedCalc, Mariakerke, Belgium).
Results
A total of 83 patients were recruited. During the run-in period, there was no dropout among preeclampsia, but 11 patients in normal pregnancy group dropped out, because they were lost to follow up. Tables 1 and 2 summarize the clinical and laboratory characteristics of the 72 enrolled patients. There were no statistically significant differences in age, nulliparity, or weight gain between preeclamptic and normotensive patients. However, the subjects with PE had higher mean blood pressure values and prepregnancy BMI, and lower birth weight and gestational age at delivery, compared with the normotensive pregnancy group (p < 0.05). In total, 10 and 13 of the preeclamptic subjects were characterized as having severe PE and SGA infants, respectively. The serum CB and CD concentrations are shown in Figs. 1 and 2. CB levels were significantly higher in PE than in normal pregnancy (p = 0.013). CD levels were lower in PE than in normal pregnancy, but the difference was not significant (p = 0.077).
Correlation between serum concentrations of CB and CD and other clinical results
Maternal age, maternal blood pressure, and prepregnancy BMI did not correlate with CB and CD either in preeclampsia and normal pregnancy group (p > 0.05). We compared CB and CD levels between patients with mild and severe PE, and in PE patients with and without SGA. The median CB and CD concentrations were not significantly different according to the severity of PE and SGA (Fig. 3). We evaluated the correlations between both enzymes and other markers of PE severity. The CD concentration showed an inverse correlation with uric acid level (r = −0.527; p = 0.03) (Fig. 4). There were no significant correlations between CB and 24-h urine protein, C-reactive protein, or uric acid.
Predictive value of CB
A final predictive model was constructed and used to select the cutoff values to classify serum CB levels as elevated or not elevated (area under the curve = 0.736; SE = 0.08, p = 0.013; Fig. 5). A cutoff value of 123.5 ng/mL appeared to represent a reasonable compromise between the sensitivity of 88 % and specificity of 53.3 %.
Discussion
This study designed to assess the association between cathepsins and preeclampsia demonstrated that CB levels in serum were significantly higher in preeclamptic patients, whereas CD levels were lower in that group. Also, serum concentration of CB levels more than 123.5 ng/mL can be useful for the clinical management of preeclampsia. However, there were no correlations between severity of PE and CB and CD levels. Also, CB and CD levels were not correlated with SGA status.
CB was the first member of the lysosomal cysteine protease family to be described, and it possesses both endopeptidase and exopeptidase activities [14]. It degrades or modifies proteins, such as albumin, collagen, fibrinogen, fibronectin, and IgG [15, 16]. The function of CB in placentation is important, as it is involved in trophoblast differentiation, morphogenesis of the placental villous tree, and angiogenesis in the early villus; it exerts these actions by expressing factors, such as vascular endothelial growth factor (VEGF) [17–19]. Veranou et al. [6] reported higher concentrations of cathepsins B and K, but lower concentrations of cathepsins L and F, in preeclamptic placentas compared with normal placentas. Therefore, it is likely that deregulation of this enzyme may cause abnormal placentation such as PE.
The systemic inflammatory response is evoked by the diminished perfusion and hypoxic environment caused by abnormal trophoblastic invasion in PE. A previous study showed that the secretion of inflammatory cytokines in preeclamptic women is markedly increased [20], which may explain the elevated CB concentration seen in the inflammatory response of PE. Studies on cathepsins have demonstrated that cathepsin S is released from macrophages, where CB mRNA is also expressed, and from microglial cells induced by cytokines that are increased during inflammatory processes [21]. Larsson et al. [22] also demonstrated significantly elevated serum levels of CB during the inflammatory response. Another hypothesis posits that CB is abundantly secreted into the maternal blood from the syncytium because of ischemia/hypoxia of the placenta, which represents one of the main features of PE. Cathepsins are active under low-pH and hypoxic conditions.
We previously demonstrated that serum CD levels were significantly lower in preeclamptic patients than in the normotensive subjects (129.3 vs. 202.1 ng/mL, p = 0.033) but similar to those in non-pregnant patients (118.1 ng/mL) [23]. We also found that compared with the non-pregnant subjects, there was significant elevation of CD level in the normotensive pregnant subjects (p < 0.001). However, the median CD concentration was not significantly different according to the severity of PE and small for gestational age. With this result, we hypothesized that it may not be produced or activated sufficiently in preeclamptic patients, thus leading to pathological, clinical manifestations of PE. In the present study with more number of cases, we found a similar trend toward decreased levels of CD in preeclamptic compared with normotensive pregnancy, but the difference was not significant, and there was no correlation with severity of disease. The method used in this study to detect serum levels of CD may not appear to accurately predict disease activity. However, one intriguing finding was that CD levels were inversely correlated with uric acid levels. Higher levels of uric acid are associated with a poor perinatal outcome in PE; [24] therefore, lower levels of CD may explain the severity of PE.
Kim et al. [25] used Western blotting to show CD overexpression in placenta from preeclamptic patients. The majority of hormones, proteins, and enzymes are synthesized in the syncytium. CD may be abundantly expressed in placenta, such that circulating levels are lower, as was the case in our study. A previous study on high-temperature requirement A1 (HtrA1), a serine protease secreted from the placenta, showed that its concentration in maternal plasma is inversely correlated with its placental expression [26].
The limitations of our study are the following. First, being a cross-sectional study, it is difficult to determine whether elevated cathepsin B is a cause or consequence of preeclampsia. Second, all the measurements were held on after the onset of disease. We need to measure cathepsins B and D in the first and second trimesters of pregnancy, before the onset of disease, to elucidate its diagnostic value. Third, the sample size was too small to restrict the power for proving relationships between the severity of preeclampsia and the proteases. Nevertheless, our study has several strengths as well. This study is the first study conducted to investigate serum cathepsins B and D at the same time in preeclampsia. Abnormal expression of these proteases may be important clue to solve the pathophysiology of preeclampsia in the future.
In conclusion, we demonstrated the CB is significantly increased in the serum of women affected by PE, in contrast to serum concentrations of CD that decreased in this condition. Differential expression or aberrant release of cathepsin proteases from trophoblasts or other types of cells may indicate abnormal placentation, thus further exacerbating the pathologic condition of PE. Therefore, genetic and histopathologic methods should be used to determine why serum concentrations of these enzymes are deregulated in this disease state and further investigation and prospective clinical trials are needed to elucidate the exact role of cathepsins B and D in preeclampsia.
References
Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ (2011) Maternal preeclampsia and neonatal outcomes. J Pregnancy 2011:214365
Sibai B, Dekker G, Kupferminc M (2005) Pre-eclampsia. Lancet 365:785–799
Rabinovici J, Goldsmith PC, Roberts VJ, Vaughan J, Vale W, Jaffe RB (1991) Localization and secretion of inhibin/activin subunits in the human and subhuman primate fetal gonads. J Clin Endocrinol Metab 73:1141–1149
Feinberg RF, Kao LC, Haimowitz JE et al (1989) Plasminogen activator inhibitor types 1 and 2 in human trophoblasts. PAI-1 is an immunocytochemical marker of invading trophoblasts. Lab Invest 61:20–26
Amarante-Paffaro AM, Hoshida MS, Yokota S et al (2011) Localization of cathepsins D and B at the maternal-fetal interface and the invasiveness of the trophoblast during the postimplantation period in the mouse. Cells Tissues Organs 193:417–425
Varanou A, Withington SL, Lakasing L, Williamson C, Burton GJ, Hemberger M (2006) The importance of cysteine cathepsin proteases for placental development. J Mol Med (Berl) 84:305–317
Moses EK, Freed KA, Higgins JR, Brennecke SP (1999) Alternative forms of a novel aspartyl protease gene are differentially expressed in human gestational tissues. Mol Hum Reprod 5:983–989
Turk V, Turk B, Turk D (2001) Lysosomal cysteine proteases: facts and opportunities. EMBO J 20:4629–4633
Joyce JA, Baruch A, Chehade K et al (2004) Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 5:443–453
Afonso S, Romagnano L, Babiarz B (1999) Expression of cathepsin proteinases by mouse trophoblast in vivo and in vitro. Dev Dyn 216:374–384
Afonso S, Romagnano L, Babiarz B (1997) The expression and function of cystatin C and cathepsin B and cathepsin L during mouse embryo implantation and placentation. Development 124:3415–3425
Dong M, Wang H, Huang H (2007) Alterations of serum cathepsins B and L in pre-eclampsia. Clin Chim Acta 377:285–287
Schroeder BM (2002) ACOG practice bulletin on diagnosing and managing preeclampsia and eclampsia. American College of Obstetricians and Gynecologists. Am Fam Phys 66:330–331
Frlan R, Gobec S (2006) Inhibitors of cathepsin B. Curr Med Chem 13:2309–2327
Coulibaly S, Schwihla H, Abrahamson M et al (1999) Modulation of invasive properties of murine squamous carcinoma cells by heterologous expression of cathepsin B and cystatin C. Int J Cancer 83:526–531
Gole B, Duran Alonso MB, Dolenc V, Lah T (2009) Post-translational regulation of cathepsin B, but not of other cysteine cathepsins, contributes to increased glioblastoma cell invasiveness in vitro. Pathol Oncol Res 15:711–723
Khan S, Katabuchi H, Araki M, Nishimura R, Okamura H (2000) Human villous macrophage-conditioned media enhance human trophoblast growth and differentiation in vitro. Biol Reprod 62:1075–1083
Anteby EY, Natanson-Yaron S, Greenfield C et al (2005) Human placental Hofbauer cells express Sprouty proteins: a possible modulating mechanism of villous branching. Placenta 26:476–483
Demir R, Kayisli UA, Seval Y et al (2004) Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta 25:560–572
Goulis DG, Walker IA, de Swiet M, Redman CW, Williamson C (2004) Preeclampsia with abnormal liver function tests is associated with cholestasis in a subgroup of cases. Hypertens Pregnancy 23:19–27
Liuzzo JP, Petanceska SS, Moscatelli D, Devi LA (1999) Inflammatory mediators regulate cathepsin S in macrophages and microglia: a role in attenuating heparan sulfate interactions. Mol Med 5:320–333
Akerfeldt T, Larsson A (2011) Inflammatory response is associated with increased cathepsin B and decreased cathepsin S concentrations in the circulation. Scand J Clin Lab Invest 71:203–207
Kim HY, Lee M, Kang HW, Moon C (2013) Cathepsin D levels are reduced in patients with preeclampsia in Korean population. Clin Biochem 46:1808–1811
Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA (2012) Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG 119:484–492
Kim YN, Kim HK, Warda M et al (2007) Toward a better understanding of preeclampsia: comparative proteomic analysis of preeclamptic placentas. Proteom Clin Appl 1:1625–1636
Marzioni D, Lorenzi T, Altobelli E et al (2012) Alterations of maternal plasma HTRA1 level in preeclampsia complicated by IUGR. Placenta 33:1036–1038
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Kang Dong Sacred Heart Hospital Research Fund (2014-02).
Conflict of interest
Author HY Kim declares that she has no conflict of interest. Author BW Kim declares that he has no conflict of interest. Author YJ Kim declares that she has no conflict of interest. The authors alone are responsible for the content and writing of the article. Authors do not have a financial relationship with the organization that sponsored the research. Authors have had full control of all primary data and agree to allow the Journal to review their data.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kim, H.Y., Kim, B.W. & Kim, Y.J. Elevated serum cathepsin B concentration in pregnant women is associated with preeclampsia. Arch Gynecol Obstet 294, 1145–1150 (2016). https://doi.org/10.1007/s00404-016-4129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4129-3