Abstract
The mechanistic target of rapamycin (mTOR) is involved in the regulation of cellular growth, proliferation, lipid synthesis, and protein translation. The mTOR pathway involves two complexes: the mechanistic target of rapamycin complex 1 (mTORC1) and the mechanistic target of rapamycin complex 2 (mTORC2). Both mTOR complexes have been implicated in the development and progression of various skin diseases including melanoma, psoriasis, and acne vulgaris. Here, we review the role of both mTORC1 and mTORC2 as well as their upstream modulators, phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt), and their downstream targets in various dermatologic diseases. Phytochemicals, plant-derived naturally occurring compounds, have been shown to regulate the mTOR pathway and may serve as novel therapeutic agents in dermatological disease. Here, we review phytochemicals in the context of the mTOR pathway and their potential use in cutaneous disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanistic target of rapamycin, also known as the mammalian target of rapamycin, (mTOR) responds to numerous environmental signals and plays a role in regulating multiple cellular functions including proliferation, lipogenesis, and gluconeogenesis [42]. Multiple diseases have altered mTOR pathway regulation, such as cancer, obesity, diabetes, and psoriasis [4, 14, 18, 23, 24, 30, 38, 42, 75]. Some phytochemicals, which are plant-derived naturally occurring chemicals, act as mTOR pathway inhibitors and may serve as a source of novel topical and oral therapies for dermatological disease. Here, we review the mTOR pathway, their relevance in dermatological disease, and discuss several phytochemicals that have been evaluated for their role in mTOR pathway inhibition.
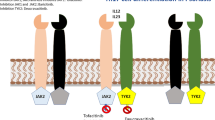
Mechanistic target of rapamycin (mTOR)-signaling pathway
A serine/threonine protein kinase, mTOR responds to cell membrane ligand binding signaling pathways and interfaces with other proteins to form two different complexes, mTORC1 and mTORC2 [42]. The various proteins forming mTORC1 and mTORC2 and their signaling pathways are depicted in Fig. 1. Three highly researched upstream modulators of mTOR are the phosphatidylinositol 3-kinases (PI3Ks), protein kinase B (Akt) that is regulated by PI3K, and tumor necrosis factor alpha (TNFα). Increased expression of PI3K, Akt, and TNFα increases mTORC1 activity [2, 3, 58, 65, 84, 89]. PI3K is currently the only known upstream modulator of mTORC2 and its expression leads to increased mTORC2 activity [19]. PI3K is capable of signaling mTORC1 through activating Akt, which then prevents the proper formation of the tuberous sclerosis complex (TSC). Similarly, TNFα is capable of activating mTORC1 through inhibiting TSC [43]. Once active, mTORC1 phosphorylates the translational regulators eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1) [9, 10]. Likewise, mTORC2 activation leads to increased Akt and serum and glucocorticoid-induced protein kinase 1 (SGK1) phosphorylation [47, 72]. The importance of and details concerning these upstream and downstream targets to dermatologic diseases will be addressed.
mTORC1 regulation and activity
The phosphatidylinositol 3-kinases (PI3Ks) phosphorylate and activate protein kinase B (Akt), a serine/threonine kinase [2]. PI3K can activate 3-phosphoinositide-dependent protein kinase-1 (PDK1) to phosphorylate Akt at Thr308 as well [3]. Upon activation, Akt phosphorylates the tuberous sclerosis complex 2 (TSC2) preventing it from forming a complex with TSC1 [65]. TSC1/2 negatively modulates mTOR activity by converting the Ras homolog enriched in brain (Rheb) to its inactive GDP form [32, 80]. Akt can also activate mTORC1 through disassociating proline-rich Akt substrate 40 kDa (PRAS40) from regulatory-associated protein of mammalian target of rapamycin (raptor) [84] and/or by directly phosphorylating mTORC1 at Ser2448 [58]. Therefore, Akt has multiple modes through which it activates mTORC1. There are also other methods reviewed in Laplante and Sabatini [42] that regulate mTORC1 through modulation of TSC1/2 such as the Wnt pathway, adenosine monophosphate-activated protein kinase (AMPK), and the amino acids leucine and arginine that are not discussed here.
Once active, mTORC1 phosphorylates the translational regulators eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1) [9, 10]. Phosphorylated eIF4E is necessary to form the eIF4F complex that is essential for translation initiation. 4E-BP1 binds to eIF4E to inhibit it and phosphorylation of 4E-BP1 causes it to dissociate. Phosphorylation of S6K1 leads to its activation and an increase in protein translation and cell proliferation. mTORC1 is also involved in the regulation of lipid synthesis by downstream activation of sterol regulatory element-binding protein, a transcription factor that upregulates lipogenesis [41, 76].
mTORC2 regulation and activity
In contrast to mTORC1, mTORC2 is less responsive to rapamycin treatment [70]. Growth factors induce mTORC2 activity via PI3K but other upstream modulators of mTORC2 remain unknown [19]. It has been proposed that PI3K promotes mTORC2 binding to ribosomes, which directly activates mTORC2 [92]. mTORC2 activates Akt through phosphorylation at Ser473 [72], but prolonged treatment with rapamycin of 24–72 h inhibited mTORC2 assembly through dissociation of the rictor–mTOR complex [71]. However, rapamycin treatment had varying efficacy on decreasing Akt phosphorylation depending on cell type [71]. mTORC2 is capable of regulating cellular growth, proliferation, and apoptosis from its control over Akt. mTORC2 also phosphorylates serum and glucocorticoid-induced protein kinase 1 (SGK1) at Ser422, which plays a role in stimulating sodium ion transport and regulating cellular growth [47]. Rapamycin treatment decreased S6K1 activity regulated by mTORC1, but not SGK1 phosphorylation [25]. Furthermore, mTORC2 can affect the actin cytoskeleton and impact cell shape through its regulation of specific kinases [35].
Dermatological diseases associated with the mTOR pathway
Skin cancers
The mTOR pathway is associated with many diseases including the development of melanomas and skin cancers caused by UVA and UVB exposure. Exposure of human keratinocytes to UVA and UVB led to an increased activation of PI3K with a more pronounced activation by UVB and phosphorylation of Akt at Ser473 by UVB and at Thr308 by UVA. UV exposure also led to increased mTOR and S6K1 phosphorylation as well [78]. Supporting this theory, UV exposure was shown to increase S6K1 phosphorylation in mouse epidermal JB6 Cl41 cells caused by mTOR and not Akt. Pretreatment with rapamycin, decreased S6K1 phosphorylation when exposed to UVB and UVC but knockdown of Akt did not affect S6K1 phosphorylation [31]. This suggests that UVB and UVC exposure is capable of activating the PI3K/Akt/mTOR pathway downstream of Akt although mTOR activation was not directly tested. Deletion of mTOR in mice and keratinocyte cells exposed to UVB rays led to increased apoptosis and decreased S6K1 and Akt phosphorylation. mTORC2 negatively regulates UVB-induced apoptosis as shown through rictor knockdown in mice embryoblasts, while mTORC1 is responsible for phosphorylating S6K1 [12].
mTOR is a major contributor to melanomas. 76 out of 107 melanomas had moderately to severely increased S6K1 phosphorylation, while only 3/67 benign nevi showed moderately increased phosphorylation [38]. Further studies were conducted on six distinct melanoma cell lines and treatment with rapamycin was shown to inhibit proliferation in three of the cell lines. Four of six lines also showed increased S6K1 phosphorylation only 30 min after amino acid withdrawal thereby showing mTOR dysregulation in melanocytes [38]. Increased Akt phosphorylation, an upstream regulator of mTOR activity, was present in 71 % of primary melanomas and rapamycin treatment resulted in decreased proliferation in three different cell lines [54].
Using reverse phase protein microarray analysis, increased activation of PI3K/Akt and mTOR was observed in advanced and non-advanced squamous cell carcinomas compared to actinic keratoses [22]. Cyclin-dependent kinase 2 (CDK2) activation strongly correlated with Akt and mTOR phosphorylation suggesting that it may be a target for the Akt/mTOR pathway in squamous cell carcinoma [15]. While significantly elevated levels of phosphorylated mTOR were found in all 15 human squamous cell carcinoma samples, it was completely absent in 12/13 basal cell carcinoma samples [37]. This result likely explains the increased efficacy of mTOR inhibitors on squamous cell carcinomas compared to basal cell carcinomas.
An overexpression of Rheb was found to be present in several different carcinoma histotypes, and a meta-analysis of published cancer cytogenetic and transcriptome database showed an increase in the activity of the RHEB locus. Increased Rheb expression in murine epidermal keratinocytes led to mTOR activation and induction of skin tumors [48]. Mutations in the BRAF gene is a marker of malignant melanoma and show higher p90 ribosomal S6 kinase (RSK) activity [26]. The activated BRAF gene leads to the activation of mTORC1 to increase protein translation and cell proliferation in human melanoma cell lines. Furthermore, knockdown of RSK using RNAi led to decreased mTORC1 activity [68]. Activating mutations in the PIK3CA gene that promotes PI3K activity is present in a subset of merkel cell carcinomas 6/60. Furthermore, PI3K activity that leads to Akt and mTOR activation was present in all merkel cell carcinoma lines that lacked the merkel cell polyoma virus, which usually indicates a worse prognosis [57]. Taken together, the mTOR pathway is activated in both nonmelanoma and melanoma skin cancers.
Tuberous sclerosis complex
TSC is an autosomal dominant disorder that is characterized by the development of benign tumor-like lesions in multiple organ systems. As discussed above, TSC1/2 is important upstream modulator for mTORC1 and mutations in both TSC1/2 tumor suppressor genes leading to mTORC1 activation. Current mTOR inhibitors that can be used for the treatment of TSC are reviewed elsewhere [18].
Vascular lesions and neoplasms
Failure of endothelial cells to remodel or undergo programmed cell death can lead to vascular malformations, which are abnormal persistence of blood vessels. Overexpression of Akt in murine endothelial cells leads to vascular malformations [63]. Since Akt regulates mTORC1 and is activated by mTORC2, the mTOR pathway is likely to be involved in vascular malformations as well. Expression of both Akt and mTOR was increased in human hemangioma tissue samples in the proliferative phase, but decrease in the involutive phase and mTOR inhibition resulted in cell apoptosis and cell cycle arrest in proliferating hemangioma samples [62].
Kaposi’s sarcoma is a connective tissue cancer often associated with HIV infections and activates the PI3K/Alt/mTOR pathway [7]. PI3K and Akt were shown to be active in mice induced with KS tumors. Inhibition of the KS-associated herpesvirus used to infect the mice decreased Akt phosphorylation suggesting that the mTOR pathway may be involved in this disease as well [75]. Furthermore, infection of human umbilical vein endothelial cells with Kaposi’s sarcoma herpesvirus resulted in increased phosphorylation of PI3K, Akt, and mTOR [86]. Similarly, transfection with the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor (KSHV-GPCR) promoted human umbilical endothelial cell survival via activation of Akt [55]. Expression of the K1 glycoprotein encoded by Karposi’s sarcoma-associated herpesvirus, in B-lymphocytes and endothelial cells increased PI3K phosphorylation and Akt phosphorylation at Ser473 and Thr308 [81, 87]. Furthermore, K1 expression in human endothelial cells also increased mTOR phosphorylation [87].
mTOR has also been shown to be involved in hemangiosarcomas (HSA) which are tumors derived from endothelial cells. Phosphorylated Akt at serine473, the mTORC2-specific phosphorylation site, was present in 86.5 % of HSA’s compared to just 15 % of hemangiomas (HA) in canines. Phosphorylated mTOR at Ser2448, the Akt specific phosphorylation site for mTORC1, was found to be present in 35 % of HSA’s and 7 % of HA’s. mTOR’s downstream target 4E-BP1 was phosphorylated in 81 % of HSA’s and 26 % of HA’s [56]. Similar levels of phosphorylated Akt were observed in human patients with angiosarcoma [40]. This data suggests that mTORC2 is more heavily involved in the development of tumors in HSA and HA than mTORC1.
Mycosis fungoides
Mycosis fungoides (MF) is a type of cutaneous T cell lymphoma. mTOR has been shown to directly phosphorylate the signal transducers and activators of transcription factor 3 (STAT3) [88], which has been implicated in mycosis fungoides [51]. Accordingly, active mTOR was found to be present with phosphorylated S6K1 in 67 % of lesions taken from 50 patients with MF [44].
Pemphigus vulgaris
Pemphigus vulgaris is an autoimmune disease caused by recognition of the transmembrane glycoprotein desmoglein 3 by IgG auto-antibodies. Phosphorylated mTOR was highly localized in the basal cells of mice injected with pemphigus vulgaris IgG and was scattered when the mice were injected with normal human serum. Treatment with rapamycin in mice injected with PV IgG showed no signs of suprabasal acantholysis showing that mTOR activity contributes to the development of lesions in pemphigus vulgaris [66]. Neural nitric oxide synthase (nNOS) has been linked to pemphigus vulgaris acantholysis as well as mTOR. Mice pre-treated with nNOS before injection of PV IgG showed a significantly decreased amount of phosphorylated mTOR compared to mice without the nNOS knockdown [23].
Psoriasis
Psoriasis, a disease of increased epidermal proliferation of keratinocytes, leads to a compromised barrier and inflammation due to an abnormal stratum corneum. Analysis of punch biopsies from patients with severe plaque psoriasis revealed elevated levels of phosphorylated mTOR at Ser2448 in the stratum basale as well as its downstream target S6K1 in suprabasal layers [11]. This suggests that mTORC1 plays a role in the pathogenesis of psoriasis.
Infections can trigger acute guttate psoriasis or exacerbate chronic plaque psoriasis. The chemokine (C–C motif) ligand 3 (CCL3) downregulates forkhead/winged helix transcription factor 3 (FOXP3) which is believed to have a role in psoriasis development [13]. The ratio of FOXP3 to activated effector T cells was reduced in progressive psoriasis skin samples as well as in severe psoriatic samples [13]. CCL3 regulates FOXP3, found in lower levels in patients with psoriasis, through the PI3K/Akt/mTOR pathway, which decreases FOXP3 stability. Akt phosphorylation at Ser473 by mTORC2 was required for the degradation of FOXP3 and overexpression of the mSin1 component of mTORC2 reduced the stability of FOXP3. Furthermore, CCL3 did not affect 4E-BP1 phosphorylation and rapamycin treatment did not affect FOXP3 stability suggesting that mTORC1 does not affect FOXP3 [14]. TNFα modulates inflammatory cytokines and activates mTORC1 as well and rapamycin treatment has been shown to inhibit the expression of TNFα mRNA in human keratinocytes [89]. Since TNFα is upregulated in psoriatic lesions, this further suggests that the mTOR pathway is involved in the pathogenesis of psoriasis. Taken together, psoriasis appears to have increased activity of both mTORC1 and mTORC2 activity.
Certain vitamins have demonstrated therapeutic effects on psoriasis. Studies have examined the efficacy of 1α,25-dihydroxyvitamin D3 (Vit-D) and 1α,25-dihydroxyvitamin D3-3-bromoacetate (BE) for the treatment of psoriasis [20]. Both Vit-D and BE exhibited antiproliferative effects and induced apoptosis in keratinocytes through downregulation of phosphorylated Akt and mTOR with BE being significantly more potent. Furthermore, Vit-D and BE treatment inhibited IL-22 induced psoriasis-like characteristics in a reconstructed human epidermis model with BE producing a more potent effect again [20]. BE was discovered to decrease the IL-22 induced expression of Akt and mTOR by nearly 17-fold. This suggests that both BE and Vit-D may be potential therapeutic agents for psoriasis through inhibition of Akt/mTOR inhibition. These cell culture findings warrant further examination in animal and human tissue to assess the clinical significance.
Acne vulgaris
Acne vulgaris is a widely prevalent skin disease. Insulin growth factor 1 (IGF-1) is involved in stimulating sebaceous gland lipogenesis through upregulating sterol response element-binding protein-1 (SREBP-1) [74]. Stimulation of the Seb-1 human sebocyte cell line with IGF-1 increased Akt phosphorylation as well as lipogenesis in sebocytes. Furthermore, inhibition of PI3K inhibited Akt phosphorylation as well as the production of SREBP-1 mRNA and protein thereby revealing the importance of the PI3K/Akt pathway to lipogenesis in sebocytes [74]. Since the PI3K/Akt pathway is an upstream modulator of mTORC1 and Akt is a downstream target of mTORC2, the mTOR pathway is likely to play a role in the pathogenesis of acne vulgaris although no direct evaluations of mTOR in regards to acne vulgaris have been examined. It has also been recently suggested that a deficiency of the forkhead box O1 transcription factor (FoxO1) is responsible for the pathogenesis involved in acne vulgaris [50]. This evidence strongly suggests that mTORC2 activation is vital to the development of acne since decreased Akt phosphorylation by mTORC2 decreased the phosphorylation of certain downstream targets of Akt such as forkhead box O1/3a leading to a deficiency of FoxO1 [28, 34].
Wound healing
Considering that the mTOR pathway promotes cellular growth and proliferation, the pathway likely plays a role in wound healing. Increased expansion of phosphorylated Akt at serine473 and S6K1 from the granular layer to the spinous layer was observed in the transitional epithelium of mice with incisional skin wounds undergoing the healing process [77]. Furthermore, knockdown of phosphatase and tensin (PTEN) gene which is a tumor suppressor gene that negatively regulates PI3K and Akt led to increased phosphorylated Akt and accelerated wound healing. Treatment with rapamycin delayed wound healing [77]. To further validate the significance of mTOR in wound healing, excision of TSC1, the upstream inhibitor of mTORC1, accelerated wound healing in mice [77]. These results are consistent with another study that determined that the mTOR inhibitor everolimus decreased the proliferation of squamous cell carcinomas, but also increased wound-healing times in vivo in humans [24]. Table 1 reviews the target of the PI3K/Akt/mTOR pathways for the previously mentioned diseases.
Phytochemical regulation of the PI3K/Akt/mTOR pathway
Rapamycin (sirolimus)
Rapamycin is a macrolide isolated from Streptomyces hygroscopicus in 1972 and it specifically targets mTOR and was originally developed to be an antifungal agent [73]. It was shown to have numerous properties including immunosuppressive effects, antibacterial and antifungal capabilities. Rapamycin is mainly used as an immunosuppressive medication in patients with renal transplants. Rapamycin appears to primarily target mTORC1 although it also exhibits cell-specific mTORC2 inhibition [71]. Furthermore, clinical studies have demonstrated rapamycin to be a promising treatment for psoriasis [61, 67]. Two derivatives of rapamycin: temsirolimus and everolimus are FDA approved mTOR inhibitors for oncological indications [27].
Curcumin
Curcumin (diferuloylmethane) is a phytochemical derived from turmeric (Fig. 2). Curcumin arrests the growth of rhabdomyosarcoma cells in the G1/G0 phase by inhibiting S6K1 and 4E-BP1 phosphorylation, which are both downstream targets for mTOR, in Rh1 and Rh30 cells [6]. Similar inhibition of S6K1 was seen with curcumin pretreatment in mice prior to the induction of squamous cell carcinomas with an associated decrease in average tumor volume [64]. Dose-dependent inhibition of mTOR phosphorylation by Akt and at its auto-phosphorylation site was induced [6]. Curcumin inhibits Akt at high concentrations (50–75 mM) [6] and at low concentrations (0.5–2.7 μM) when combined with UVA or visible light exposure [21]. However, curcumin did not inhibit Akt phosphorylation in mice [64]. This discrepancy may be due to the differences in species-related responses between mice and human cells. In addition, the systemic concentration in the mice may have been too low to inhibit Akt, although the authors do not report this [64].
Curcumin’s mechanistic inhibition of mTOR is due to its ability to disrupt the stability of mTORC1 through dissociating raptor from mTOR at low concentrations, and it inhibits mTORC2 by disrupting rapamycin-insensitive companion of mTOR (rictor) binding to mTOR at higher concentrations (Table 2) [5]. This is consistent with decreases in the phosphorylation of S6K1 and 4E-BP1 by mTORC1 at lower concentrations and Akt phosphorylation by mTORC2 at higher concentrations [6]. Overall, curcumin represents a viable inhibitor of the mTOR pathway by disrupting mTORC1 at low concentrations and mTORC2 at higher concentrations.
Resveratrol
Resveratrol (trans-3,4,5-trihydroxystilbene) (RSV) is a naturally occurring phytochemical found in more than 70 plant species (Fig. 2) including grapes, cranberries and peanuts [1]. Resveratrol inhibits PI3K and Akt phosphorylation. Topical application of RSV decreased 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced phosphorylation of PI3K and Akt and the average number and incidence of papillomas in mice induced by DMBA and promoted by TPA [82]. Furthermore, RSV inhibits the migratory and invasive properties of melanoma cells in mice by inhibiting Akt phosphorylation at Ser473 and Thr308 [8].
RSV inhibits mTOR activation and function. RSV inhibited mTOR phosphorylation in TNFα-treated mouse embryonic fibroblast cells [91]. RSV was determined to inhibit the mTOR pathway through enhancing the binding of the mTOR inhibitory subunit, DEP domain-containing mTOR-interacting protein (DEPTOR), to the catalytic mTOR kinase but had no effect on the expression of DEPTOR (Table 2) [45]. Furthermore, RNAi knockdown of DEPTOR decreased RSV’s inhibitory effects on mTOR [45]. RSV also significantly decreased S6K1 and 4E-BP1 phosphorylation in mouse fibroblasts since mTOR was inhibited [45]. In vitro human studies using RSV treatment decreased phosphorylated S6K1 levels in HeLa cervical cancer, HepG2 hepatocarcinoma, and MCF-7 breast cancer cell lines [33]. Likewise in human U251 glioma cells, RSV treatment produced a dose-dependent reduction in phosphorylated mTOR and Akt at serine473, the mTORC2 target site [36]. Furthermore, another study determined that RSV treatment increased the phosphorylation of the raptor subunit of mTORC1, thereby inhibiting the complex in human embryonic kidney cells transfected with Alzheimer’s disease amyloid protein [85]. However, another study performed with esophageal squamous carcinoma cells (ESCC) reported opposing results in terms of raptor phosphorylation [79]. Both studies reported that RSV treatment induced autophagy in their respective cell lines, and the ESCC cells were also apoptotic upon treatment. This discrepancy is likely due to RSV’s differing mode of action in different cell lines considering that RSV induced AMPK-dependent autophagy in myelogenous leukemia cells, but was AMPK-independent in esophageal squamous carcinoma cells [79].
Overall, RSV holds promise as a phytochemical inhibitor of both complexes of the mTOR pathway although its mode of action is complex and includes upstream modulation of Akt and PI3K as well.
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is the most abundant phytochemical catechin extract from green tea (Camellia sinensis) (Fig. 2). EGCG increases Akt phosphorylation through PI3K regulation in normal human epidermal keratinocytes at low concentrations (0.5 μM), but demonstrated no significant effect at high concentrations (50 μM) [17]. PI3K inhibitors affected EGCG’s ability to phosphorylate Akt. EGCG also stimulates hair growth and in vitro studies using EGCG formulations of various concentrations up to 0.5 μM demonstrated a 2.5-fold increase in Akt phosphorylation compared to the ethanol control in human dermal papilla cells [39].
In contrast to the experiments demonstrating that EGCG increases PI3K and Akt phosphorylation, peracetylated EGCG downregulated PI3K and Akt phosphorylation in mouse skin when induced by DMBA/TPA [16]. Although no studies were done on mice skin using just EGCG to measure P-Akt, it is likely that EGCG alone without peracetylation also downregulates PI3K and Akt, since both EGCG and peracetylated EGCG treatment decreased the percentage of mice with tumors and the average number of tumors present in mice, except that peracetylated EGCG had a more pronounced effect. EGCG also appears to inhibit keloid fibroblast proliferation and collagen production via inhibition of the PI3K/Akt-signaling pathways [90]. Furthermore, EGCG decreases Akt phosphorylation in a dose-dependent manner in human squamous carcinoma cells [17]. EGCG was later determined to be an ATP-competitive inhibitor of PI3K and mTOR in both mammary epithelial adenocarcinoma and alveolar basal epithelial adenocarcinoma cell lines [83]. Phosphorylation of Akt at Ser473 was inhibited by EGCG treatment, and immunoprecipitation studies showed that EGCG inhibited both mTORC1 and mTORC2 (Table 2) [83].
Some studies have also demonstrated that EGCG had no effect on Akt phosphorylation in the PC3 and LNCaP prostate adenocarcinoma cell lines, which may be due to the fact that the cell lines were also unresponsive to insulin-like growth factor 1 (IGF-1) stimulation that is involved in activating the mTOR pathway [83]. This is likely due to the fact that LNCaP and PC3 have mutations in the phosphatase and tensin (PTEN) gene, which is a tumor suppressor gene that negatively regulates PI3K and Akt.
The discrepancy in EGCG’s different effects may be cell specific. As mentioned above, rapamycin treatment had varying efficacy on decreasing Akt phosphorylation depending on cell type [71]. The cause behind this cell type specificity to treatments is still unknown. However, there is a general trend that EGCG tends to downregulate the PI3K/Akt/mTOR pathways in carcinogenic cell lines or cells induced to proliferate by various chemicals such as DMBA/TPA or other processes. In contrast, EGCG stimulated Akt phosphorylation in normal proliferating cell lines such as dermal papilla cells and normal human epidermal keratinocytes. EGCG is a complex regulator of the mTOR pathway and appears to produce differing effects depending on the baseline proliferative state of the cell. Further studies are needed to better assess how EGCG interacts with different cell lines.
Caffeine
Caffeine is a popular xanthine alkaloid found in various products such as coffee, tea, cocoa beans, and soft drinks (Fig. 2). In mouse epidermal JB6 cells induced by epidermal growth factor (EGF) and TPA, caffeine decreased the phosphorylation of Akt at Ser473 and its downstream target S6K1 (Table 2). However, caffeine did not affect PI3K activation and did not directly inhibit Akt activation as shown through immunoprecipitation assays [59]. This suggests that caffeine interacts with the mTOR pathway since Akt phosphorylation at Ser473 was affected, a characteristic effect of mTORC2. These results are supported by Han et al. [29] who demonstrated that caffeine increases UVB-induced apoptosis in human HaCaT keratinocytes by inhibiting Akt activation. Furthermore, caffeine inhibits formation of phosphorylated Akt in sarcoma cells [52]. Caffeine also inhibits the downstream targets of mTOR, 4E-BP1 and S6K1, in cervical cancer cells (HeLa) and rat neuroendocrine tumor cells (PC12D) [69]. Similarly, treatment with caffeine suppressed Akt/mTOR/S6K1 activity in human osteosarcoma cells [53]. Taken together, caffeine inhibits mTORC1 and mTORC2 activity.
Other phytochemicals
The flavonoid quercetin (Fig. 3) was shown to reduce phosphorylation of mTOR at Ser2448 and 4E-BP1 and S6K1 in UVB-induced HaCaT cells [60]. Similarly, irradiated riboflavin (Fig. 3) inhibits mTOR phosphorylation at Ser2448 in B16F10 mouse melanoma cells and reduces melanoma metastasis in vivo in mice [49]. Gartanin, a xanthone from mangosteen juice (Fig. 3), was shown to inhibit the downstream targets of the mTOR pathway 4E-BP1 and S6K1 but not mTOR itself [46]. Continued research is necessary to determine the direct effect of these phytochemicals specifically on mTOR to extend the implications of these previous studies. All the reviewed phytochemicals can be purchased from the suppliers listed in Table 3.
Conclusion
The mTOR pathway is an important regulator of many physiological processes and is active in several dermatological diseases. As shown in Table 1, all the inflammatory and proliferative diseases discussed in this review upregulated PI3K/Akt and mTORC1. This association is suggestive that mTOR pathway activation may be a commonality for both cutaneous inflammation and proliferation. However, there may be some specificity as to how mTORC1 and mTORC2 are balanced, but this will require further research to better assess the commonalities and the differences in mTOR activation between cutaneous inflammation and proliferation. Furthermore, all the phytochemicals discussed here were shown to downregulate the mTOR pathway when applied in cells transfected or induced into a disease state that has upregulated mTOR pathway activation. EGCG was the only phytochemical tested in cells in a normal state that increased Akt phosphorylation at low concentrations only. This suggests that the mTOR pathway in diseased cells may respond differently to phytochemical treatment than normal cells. The majority of the studies conducted on the mTOR pathway have been in vitro and few in vivo studies have evaluated the use of phytochemicals within dermatology. Future animal studies will help elucidate in vivo mechanisms more clearly, but the gold standard will be clinical testing. Botanical and phytochemical evaluation for the modification of skin structure/function or disease will require either an investigational new drug (IND) approval or an IND exemption for clinical testing. A careful reconsideration of the requirements and impediments to topical botanical testing may need to occur to move clinical research forward more quickly. On the other hand, phytochemicals are unlikely to receive any other distinction when tested in purified form. Regardless, further interaction between dermatologists, cell biologists, and plant scientists will help continue to move this field forward in hopes of developing novel therapies for cutaneous disease.
References
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24(5A):2783–2840
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P et al (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15(23):6541–6551
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB et al (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7(4):261–269
Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A et al (2006) Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci USA 103(42):15552–15557
Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S (2009) Curcumin disrupts the mammalian target of rapamycin–raptor complex. Cancer Res 69(3):1000–1008
Beevers CS, Li F, Liu L, Huang S (2006) Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer 119(4):757–764
Bhatt AP, Damania B (2012) AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front Immunol 3:401
Bhattacharya S, Darjatmoko SR, Polans AS (2011) Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res 21(3):180–187
Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL (1995) Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377(6548):441–446
Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ et al (1997) Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277(5322):99–101
Buerger C, Malisiewicz B, Eiser A, Hardt K, Boehncke WH (2013) Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br J Dermatol 169(1):156–159
Carr TD, DiGiovanni J, Lynch CJ, Shantz LM (2012) Inhibition of mTOR suppresses UVB-induced keratinocyte proliferation and survival. Cancer Prev Res (Phila) 5(12):1394–1404
Chen L, Shen Z, Wang G, Fan P, Liu Y (2008) Dynamic frequency of CD4 + CD25 + Foxp3 + Treg cells in psoriasis vulgaris. J Dermatol Sci 51(3):200–203
Chen L, Wu J, Pier E, Zhao Y, Shen Z (2013) mTORC2-PKBalpha/Akt1 Serine 473 phosphorylation axis is essential for regulation of FOXP3 stability by chemokine CCL3 in psoriasis. J Invest Dermatol 133(2):418–428
Chen SJ, Nakahara T, Takahara M, Kido M, Dugu L, Uchi H et al (2009) Activation of the mammalian target of rapamycin signalling pathway in epidermal tumours and its correlation with cyclin-dependent kinase 2. Br J Dermatol 160(2):442–445
Chiou YS, Sang S, Cheng KH, Ho CT, Wang YJ, Pan MH (2013) Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis 34(6):1315–1322
Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH et al (2003) Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J 17(13):1913–1915
Curatolo P, Moavero R (2012) mTOR inhibitors in tuberous sclerosis complex. Curr Neuropharmacol 10(4):404–415
Cybulski N, Polak P, Auwerx J, Ruegg MA, Hall MN (2009) mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA 106(24):9902–9907
Datta Mitra A, Raychaudhuri SP, Abria CJ, Mitra A, Wright R, Ray R et al (2013) 1alpha,25-dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR signaling cascades: a therapeutic agent for psoriasis. J Invest Dermatol 133(6):1556–1564
Dujic J, Kippenberger S, Hoffmann S, Ramirez-Bosca A, Miquel J, Diaz-Alperi J et al (2007) Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J Invest Dermatol 127(8):1992–2000
Einspahr JG, Calvert V, Alberts DS, Curiel-Lewandrowski C, Warneke J, Krouse R et al (2012) Functional protein pathway activation mapping of the progression of normal skin to squamous cell carcinoma. Cancer Prev Res (Phila) 5(3):403–413
Espana A, Modol T, Gil MP, Lopez-Zabalza MJ (2013) Neural nitric oxide synthase participates in pemphigus vulgaris acantholysis through upregulation of Rous sarcoma, mammalian target of rapamycin and focal adhesion kinase. Exp Dermatol 22(2):125–130
Feldmeyer L, Hofbauer GF, Boni T, French LE, Hafner J (2012) Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol 166(2):422–424
Garcia-Martinez JM, Alessi DR (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416(3):375–385
Gray-Schopfer VC, da Rocha Dias S, Marais R (2005) The role of B-RAF in melanoma. Cancer Metastasis Rev 24(1):165–183
Guertin DA, Sabatini DM (2009) The pharmacology of mTOR inhibition. Sci Signal 2((67)):pe24
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J et al (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11(6):859–871
Han W, Ming M, He YY (2011) Caffeine promotes ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J Biol Chem 286(26):22825–22832
Hartman TR, Nicolas E, Klein-Szanto A, Al-Saleem T, Cash TP, Simon MC et al (2009) The role of the Birt–Hogg–Dube protein in mTOR activation and renal tumorigenesis. Oncogene 28(13):1594–1604
Huang C, Li J, Ke Q, Leonard SS, Jiang BH, Zhong XS et al (2002) Ultraviolet-induced phosphorylation of p70(S6K) at Thr(389) and Thr(421)/Ser(424) involves hydrogen peroxide and mammalian target of rapamycin but not Akt and atypical protein kinase C. Cancer Res 62(20):5689–5697
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590
Iqbal MA, Bamezai RN (2012) Resveratrol inhibits cancer cell metabolism by down regulating pyruvate kinase M2 via inhibition of mammalian target of rapamycin. PLoS One 7(5):e36764
Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY et al (2006) SIN1/MIP1 maintains rictor–mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127(1):125–137
Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A et al (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6(11):1122–1128
Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C et al (2009) Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol 8(1):25–33
Karayannopoulou G, Euvrard S, Kanitakis J (2013) Differential expression of p-mTOR in cutaneous basal and squamous cell carcinomas likely explains their different response to mTOR inhibitors in organ-transplant recipients. Anticancer Res 33(9):3711–3714
Karbowniczek M, Spittle CS, Morrison T, Wu H, Henske EP (2008) mTOR is activated in the majority of malignant melanomas. J Invest Dermatol 128(4):980–987
Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC et al (2007) Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 14(7–8):551–555
Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD et al (2010) Angiosarcoma: clinical and molecular insights. Ann Surg 251(6):1098–1106
Laplante M, Sabatini DM (2009) An emerging role of mTOR in lipid biosynthesis. Curr Biol 19(22):R1046–R1052
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293
Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y et al (2007) IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130(3):440–455
Levidou G, Siakantaris M, Papadaki T, Papadavid E, Vassilakopoulos TP, Angelopoulou MK et al (2013) A comprehensive immunohistochemical approach of AKT/mTOR pathway and p-STAT3 in mycosis fungoides. J Am Acad Dermatol 69(3):375–384
Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W et al (2010) Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem 285(47):36387–36394
Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A et al (2013) The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutr Cancer 65(Suppl 1):68–77
Loffing J, Flores SY, Staub O (2006) Sgk kinases and their role in epithelial transport. Annu Rev Physiol 68:461–490
Lu ZH, Shvartsman MB, Lee AY, Shao JM, Murray MM, Kladney RD et al (2010) Mammalian target of rapamycin activator RHEB is frequently overexpressed in human carcinomas and is critical and sufficient for skin epithelial carcinogenesis. Cancer Res 70(8):3287–3298
Machado D, Shishido SM, Queiroz KC, Oliveira DN, Faria AL, Catharino RR et al (2013) Irradiated riboflavin diminishes the aggressiveness of melanoma in vitro and in vivo. PLoS One 8(1):e54269
Melnik BC (2010) FoxO1––the key for the pathogenesis and therapy of acne? J Dtsch Dermatol Ges 8(2):105–114
Mitchell TJ, John S (2005) Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas. Immunology 114(3):301–312
Miwa S, Sugimoto N, Shirai T, Hayashi K, Nishida H, Ohnari I et al (2011) Caffeine activates tumor suppressor PTEN in sarcoma cells. Int J Oncol 39(2):465–472
Miwa S, Sugimoto N, Yamamoto N, Shirai T, Nishida H, Hayashi K et al (2012) Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-kappaB and MAPK pathways. Anticancer Res 32(9):3643–3649
Molhoek KR, Brautigan DL, Slingluff CL Jr (2005) Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor rapamycin. J Transl Med 3:39
Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS (2001) The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res 61(6):2641–2648
Murai A, Abou Asa S, Kodama A, Sakai H, Hirata A, Yanai T (2012) Immunohistochemical analysis of the Akt/mTOR/4E-BP1 signalling pathway in canine haemangiomas and haemangiosarcomas. J Comp Pathol 147(4):430–440
Nardi V, Song Y, Santamaria-Barria JA, Cosper AK, Lam Q, Faber AC et al (2012) Activation of PI3K signaling in Merkel cell carcinoma. Clin Cancer Res 18(5):1227–1236
Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344(Pt 2):427–431
Nomura M, Ichimatsu D, Moritani S, Koyama I, Dong Z, Yokogawa K et al (2005) Inhibition of epidermal growth factor-induced cell transformation and Akt activation by caffeine. Mol Carcinog 44(1):67–76
Olson ER, Melton T, Dickinson SE, Dong Z, Alberts DS, Bowden GT (2010) Quercetin potentiates UVB-induced c-Fos expression: implications for its use as a chemopreventive agent. Cancer Prev Res (Phila) 3(7):876–884
Ormerod AD, Shah SA, Copeland P, Omar G, Winfield A (2005) Treatment of psoriasis with topical sirolimus: preclinical development and a randomized, double-blind trial. Br J Dermatol 152(4):758–764
Ou JM, Qui MK, Dai YX, Dong Q, Shen J, Dong P et al (2012) Combined blockade of AKT/mTOR pathway inhibits growth of human hemangioma via downregulation of proliferating cell nuclear antigen. Int J Immunopathol Pharmacol 25(4):945–953
Perry B, Banyard J, McLaughlin ER, Watnick R, Sohn A, Brindley DN et al (2007) AKT1 overexpression in endothelial cells leads to the development of cutaneous vascular malformations in vivo. Arch Dermatol 143(4):504–506
Phillips JM, Clark C, Herman-Ferdinandez L, Moore-Medlin T, Rong X, Gill JR et al (2011) Curcumin inhibits skin squamous cell carcinoma tumor growth in vivo. Otolaryngol Head Neck Surg 145(1):58–63
Potter CJ, Pedraza LG, Xu T (2002) Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4(9):658–665
Pretel M, Espana A, Marquina M, Pelacho B, Lopez-Picazo JM, Lopez-Zabalza MJ (2009) An imbalance in Akt/mTOR is involved in the apoptotic and acantholytic processes in a mouse model of pemphigus vulgaris. Exp Dermatol 18(9):771–780
Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths CE (2001) Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. Br J Dermatol 145(3):438–445
Romeo Y, Moreau J, Zindy PJ, Saba-El-Leil M, Lavoie G, Dandachi F et al (2013) RSK regulates activated BRAF signalling to mTORC1 and promotes melanoma growth. Oncogene 32(24):2917–2926
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I et al (2011) Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 7(2):176–187
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H et al (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14(14):1296–1302
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF et al (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22(2):159–168
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307(5712):1098–1101
Sehgal SN, Baker H, Vezina C (1975) Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 28(10):727–732
Smith TM, Gilliland K, Clawson GA, Thiboutot D (2008) IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol 128(5):1286–1293
Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA et al (2004) Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci USA 101(14):4821–4826
Soliman GA (2011) The integral role of mTOR in lipid metabolism. Cell Cycle 10(6):861–862
Squarize CH, Castilho RM, Bugge TH, Gutkind JS (2010) Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS One 5(5):e10643
Syed DN, Afaq F, Mukhtar H (2012) Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem Photobiol 88(5):1184–1190
Tang Q, Li G, Wei X, Zhang J, Chiu JF, Hasenmayer D et al (2013) Resveratrol-induced apoptosis is enhanced by inhibition of autophagy in esophageal squamous cell carcinoma. Cancer Lett 336(2):325–337
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J (2003) Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13(15):1259–1268
Tomlinson CC, Damania B (2004) The K1 protein of Kaposi’s sarcoma-associated herpesvirus activates the Akt signaling pathway. J Virol 78(4):1918–1927
Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT, Pan MH (2012) Pterostilbene, a natural analogue of resveratrol, potently inhibits 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin carcinogenesis. Food Funct 3(11):1185–1194
Van Aller GS, Carson JD, Tang W, Peng H, Zhao L, Copeland RA et al (2011) Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem Biophys Res Commun 406(2):194–199
Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9(3):316–323
Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P (2011) Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J 25(1):219–231
Wang L, Damania B (2008) Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res 68(12):4640–4648
Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B (2006) Immortalization of primary endothelial cells by the K1 protein of Kaposi’s sarcoma-associated herpesvirus. Cancer Res 66(7):3658–3666
Yokogami K, Wakisaka S, Avruch J, Reeves SA (2000) Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol 10(1):47–50
Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR (2008) Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol 128(11):2606–2614
Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS et al (2006) Green tea extract and (−)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol 126(12):2607–2613
Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X et al (2011) Activation of Sirt1 by resveratrol inhibits TNF-alpha induced inflammation in fibroblasts. PLoS One 6(11):e27081
Zinzalla V, Stracka D, Oppliger W, Hall MN (2011) Activation of mTORC2 by association with the ribosome. Cell 144(5):757–768
Related articles recently published in Archives of Dermatological Research (selected by the journal’s editorial staff)
Deng S, May BH, Zhang AL, Lu C, Xue CC (2014) Phytotherapy in the management of psoriasis: a review of the efficacy and safety of oral interventions and the pharmacological actions of the main plants. Arch Dermatol Res 306:211–229
Deng S, May BH, Zhang AL, Lu C, Xue CC (2013) Topical herbal medicine combined with pharmacotherapy for psoriasis: a systematic review and meta-analysis. Arch Dermatol Res 305:179–189
Lee J, Jung E, Kim YS, Park D, Toyama K, Date A, Lee J (2013) Phloridzin isolated from Acanthopanax senticosus promotes proliferation of alpha6 integrin (CD 49f) and beta1 integrin (CD29) enriched for a primary keratinocyte population through the ERK-mediated mTOR pathway. Arch Dermatol Res 305:747–754
Peramo A, Marcelo CL (2013) Visible effects of rapamycin (sirolimus) on human skin explants in vitro. Arch Dermatol Res 305:163–171
Conflict of interests
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leo, M.S., Sivamani, R.K. Phytochemical modulation of the Akt/mTOR pathway and its potential use in cutaneous disease. Arch Dermatol Res 306, 861–871 (2014). https://doi.org/10.1007/s00403-014-1480-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1480-8