Abstract
In this manuscript, we report observations of the effects of rapamycin in an organotypic culture of human skin explants. The tissues were cultured for 5 days at the air–liquid interface or in submersed conditions with media with and without rapamycin at 2 nM concentration. Histological analysis of tissue sections indicated that rapamycin-treated samples maintained a better epidermal structure in the upper layers of the tissue than untreated samples, mostly evident when skin was cultured in submersed conditions. A significant decrease in the number of positive proliferative cells using the Ki67 antigen was observed when specimens were treated with rapamycin, in both air–liquid and submersed conditions but apoptosis differences between treated and untreated specimens, as seen by cleaved caspase-3 positive cells, were only observed in submersed specimens. Finally, a decrease and variability in the location in the expression of the differentiation marker involucrin and in E-cadherin were also evident in submersed samples. These results suggest that the development of topical applications containing rapamycin, instead of systemic delivery, may be a useful tool in the treatment of skin diseases that require reduction of proliferation and modulation or control of keratinocyte differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapamycin (sirolimus) has been extensively studied and used in a variety of areas, including organ transplantation [13] or cancer studies [17, 35], but non-cancer related or direct studies of its effects on skin are less common and the published literature relating rapamycin and keratinocytes is scarce. A recent study summarizing the dermatologic and mucosal problems associated with systemic treatments of mTOR inhibitors indicates that further studies are needed in this area [7].
While rapamycin action mechanism is fairly complex, there appears to be ample evidence that one of its effects is related to reduction in cellular proliferation, as it has recently been found in human hepatocytes after organ transplantation [38] and can also regulate differentiation [26]. It has also recently been shown that administration of rapamycin, as an adjuvant in pulsed-dye laser in treatments of port-wine stains reduced Ki67 (a marker of proliferation) expression in dermal endothelial cells [24], and may be effective in other diseases [28, 31].
These indications point in the direction that rapamycin can be an effective agent in controlling or reducing keratinocyte proliferation and could potentially be used as anti-proliferation agent in a number of skin diseases. In this study, we present results indicating that concentrations of 2 nM rapamycin in regular skin culture medium reduce Ki67 and involucrin expression in an in vitro human skin explant model while maintaining or slightly reducing apoptosis as measured by cleaved caspase-3 positive cells. These experiments show that ex vivo full-thickness organ culture skin experiments can be an effective model to study mechanisms of action of rapamycin on human skin.
Methods
Skin explant culture
Human skin was obtained from elective surgeries and cultured as previously reported [32], with variations. Briefly, full thickness human breast or abdominal skin explants from discarded material from surgeries performed at the University of Michigan Health System were used. The specimens were received from healthy human subjects under University of Michigan informed consent and used immediately. After removal of subcutaneous fat, the tissue was rinsed abundantly with PBS 1× containing 125 μg/ml of Gentamicin (Invitrogen/GIBCO, Carlsbad, CA) and 1.87 μg/ml of Amphotericin B (Sigma-Aldrich, Milwaukee, WI). The culture medium used was EpiLife (Cascade Biologics, Portland, OR), supplemented with EpiLife defined Growth Supplement EDGS (Cascade Biologics, Portland, OR) (EDGS is an ionically balanced supplement containing purified bovine serum albumin (BSA), purified bovine transferrin, hydrocortisone, recombinant human insulin-like growth factor type-1 (rhIGF-1), prostaglandin E2 (PGE2) and recombinant human epidermal growth factor (rhEGF)). In addition, the medium was supplemented with 75 μg/ml of Gentamicin and 1.125 μg/ml of Amphotericin B. The final concentration of calcium used in the culture medium was 1.2 mM. Rapamycin-treated specimens (Catalog #R-5000, LC Laboratories, Woburn, MA, USA) were cultured with the same medium containing rapamycin at 2 nM concentration. In an additional, separate experiment (unrepeated), we also used rapamycin at 100 nM and 2 μM concentrations both at the air–liquid and in submersed conditions for 5 days to briefly evaluate cellular response at high concentrations of rapamycin.
After preparation, skin specimens of approximately 1.5 cm2 were cut using a scalpel and cultured for 5 days at 37 °C and 5 % CO2 atmosphere, either epidermal side up at the air–liquid interface, in a Transwell system consisting of 6-well Corning Costar supports (Fisher Scientific, Pittsburgh, PA, USA) or in submersed conditions in 6-well plates. In this later condition, the skin was left free-floating on the well with the medium. Culture medium was changed every 24 h and the stratum corneum remained constantly exposed to the air for the air–liquid interface specimens. Experiments were performed five times, with skin from five different individuals. For 100 nM and 2 μM concentrations only one experiment was performed. The specimens are labeled as follows: 5DCNT-AIR, as control specimen cultured at the air–liquid interface in regular medium; 5DRAPA-AIR, cultured at the air–liquid interface in 2 nM rapamycin medium; 5DCNT-SUB, as control specimen cultured submersed in regular medium and 5DRAPA-SUB cultured submersed in 2 nM rapamycin medium. In one of the initial, preliminary experiments, in addition to the regular 5-day specimens, the culture period was extended to 10 days.
Histology and immunohistochemistry
Histological evaluation with hematoxylin and eosin (H&E) staining was performed for all specimens collected. For histological analysis, biopsies were fixed in 4 % phosphate buffered paraformaldehyde for 24 h, routinely dehydrated and paraffin embedded. Serial sections were obtained at 5 μm. For light microscopy analysis, images were taken using a Nikon E800 microscope. Proliferation and differentiation were analyzed with the epithelial markers Ki67 and involucrin. Apoptosis was analyzed with cleaved caspase-3 and calcium-dependent cell contact with E-cadherin.
For Ki67 (1:200; DAKO, Cat #M7240), paraffin sections were cut at 5 μm and heated for 40 min at 65 °C, deparaffinized in Xylene, three changes of 2 min each. Slides are then rehydrated through graduated alcohols of 2 min each and end in tap water (100 % alcohol, 95 % alcohol, 70 % alcohol, water). Slides are then placed in wash buffer until ready to begin IHC staining. Antigen retrieval was performed in the microwave for 20 min with citrate buffer, pH 6.0 at 95 °C. Immunoperoxidase staining was performed on a DAKO AutoStainer at room temperature using peroxidase blocking for 5 min followed by primary antibody incubation for 30 min, followed by secondary antibody (Rabbit HRP Polymer from Biocare #RMR622) for 30 min, DAB chromogen for 5 min, IP Sparkle (Biocare #DS830G) for 1 min and H&E counterstain.
For involucrin (1:100; Novus Biologicals, Cat #NB100-2774) paraffin sections were cut at 5 μm and heated for 40 min at 65 °C, deparaffinized in Xylene, three changes of 2 min each. Slides are then rehydrated through graduated alcohols of 2 min each and end in tap water (100 % alcohol, 95 % alcohol, 70 % alcohol, water). Slides are then placed in wash buffer until ready to begin IHC staining. Antigen retrieval was performed in the microwave for 20 min with citrate buffer, pH 6.0 at 95 °C. Immunoperoxidase staining was performed on a DAKO AutoStainer at room temperature using peroxidase blocking for 5 min followed by primary antibody incubation for 30 min, followed by secondary antibody (Mouse Secondary Reagent from Biocare #IPSC5001Secondary Reagent from Biocare #IPSC5001) for 10 min; tertiary reagent/HRP (Universal HRP Tertiarty from Biocare #IPT5002) for 10 min; followed by buffer rinse, DAB chromogen for 5 min and H&E counterstain.
For cleaved caspase-3 (1:1,000; Cell Signaling; CAT. NO 9661), paraffin sections were cut at 5 μm and heated for 20 min at 65 °C, deparaffinized in Xylene, three changes of 2 min each. Slides are then rehydrated through graduated alcohols of 2 min each and end in tap water (100 % alcohol, 95 % alcohol, 70 % alcohol, water). Slides are then placed in wash buffer until ready to begin IHC staining. Antigen retrieval was performed in the microwave for 40 s at 125 °C and then at 95 °C for 10 s with citrate buffer, pH 6.0. Immunoperoxidase staining was performed on a DAKO AutoStainer at room temperature using peroxidase blocking for 5 min followed by primary antibody incubation for 1 h, followed by secondary antibody (Rabbit HRP Polymer from Biocare #RMR622) for 30 min, DAB chromogen for 5 min and H&E counterstain.
For E-cadherin (1:200, Invitrogen, Cat #13-1700) paraffin sections were cut at 5 μm and heated for 20 min at 65 °C, deparaffinized in Xylene, rehydrated through graduated series of alcohols. Antigen retrieval was performed in the microwave for 10 min with citrate buffer, pH 6.0. Immunoperoxidase staining was performed on a DAKO AutoStainer at room temperature using peroxidase blocking for 5 min followed by primary antibody incubation for 30 min, secondary antibody for 60 min, streptavidin label tertiary reagent for 30 min, DAB chromogen for 5 min and H&E counterstain.
To determine Ki67 and cleaved caspase-3 positively stained cells, six randomly selected areas per slide were examined for analysis. The number of Ki67 or cleaved caspase-3 positive cells per microscopic 400X fields was recorded. Student’s t tests were performed between different groups. A p < 0.05 was considered significant.
Results
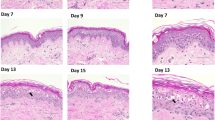
Some morphological changes between controls (CNT specimens) and rapamycin-treated specimens were observed as well as between air–liquid cultured samples and submersed samples. These are presented in Fig. 1 using H&E-stained tissue sections, Fig. 1a for 5 days and Fig. 1b for 10 days. With respect to the air–liquid samples, submersed samples showed a thinning of the epidermis with a reduction in the viable epidermal thickness (nucleated, non-pyknotic cells). This reduction was seen in both regular medium and rapamycin-cultured samples, as evidenced comparing left and right images of Fig. 1a and happened also for the specimens cultured for 10 days, as seen comparing left and right images in Fig. 1b. An evaluation of the viable epidermal thickness as number of cell layers as an average of specimens from four experiments is included in Table 1. We already mentioned that there appears to be a slight thinning of the epidermis when samples are submersed versus air–liquid interface. The measurements showed a reduction in the number of cells when they are submersed, which was less when using submersed samples with rapamycin. Little reduction in viable epidermis was seen when culturing at the air–liquid interface. In the additional experiment with rapamycin at higher concentrations, there was no reduction in epidermal thickness when using 100 nM rapamycin at the air–liquid interface (~3–4 viable cells) and a slight reduction to 2–4 cells with 2 μM rapamycin. Increased reduction was again observed when submersed, with an average of 1–3 viable cells for 100 nM rapamycin and 1–2 viable cells at 2 μM concentration.
In general, air–liquid samples showed only minimal epidermal degeneration with only occasional epidermal pyknosis (black arrows in Fig. 1) or dermal interstitial cell pyknosis (green arrows in Fig. 1). No significant morphological changes were observed between 5DCNT-AIR and 5DRAPA-AIR. However, moderate to severe epidermal degeneration was observed for 5DCNT-SUB specimens, with multifocal dermo-epidermal separation. Extended vacuolation (big red arrows) and pyknosis were observed. In contrast, submersed specimens treated with rapamycin showed a better preservation of the epidermal architecture and clear reduction in pyknosis and vacuolation. Particularly affected in the 5DCNT-SUB specimens were the granular layer and the lower stratum corneum, which showed extended number of vacuolated cells with indistinct nuclei indicative of severe autolysis. When samples were cultured for 10 days to assess epidermal changes with longer culture periods, overall, these differences in morphology between different specimens were maintained, and the general epidermal architecture was severely disrupted, but to a lesser extent in rapamycin-treated specimens (bottom images of Fig. 1b). In general, basal keratinocytes were disorganized and both regular medium specimens (air liquid and submersed) were very severely autolyzed: nearly all the nuclei in the upper epidermal layers were pyknotic with extended vacuolation and multifocal cell death, manifested by hypereosinophilia and dissociation of keratinocytes. In general, these observations are consistent with widespread multifocal epithelial degeneration and necrosis, that was more limited in rapamycin-treated samples. When samples were cultured at higher rapamycin concentrations (100 nM and 2 μM) in the additional experiment, the 100 nM rapamycin-treated specimens showed an epidermal architecture similar to 2 nM while the 2 μM was more similar to the untreated specimen, again this was particularly evident in submersed specimens (Figures S1 and S2 in Supplementary Material).
To investigate proliferation and apoptosis, the 5-day cultured specimens were analyzed against Ki67 and cleaved caspase-3. Preliminary data (not shown) from one experiment indicated no visible differences in the expression of p63 and keratin 14 between control and rapamycin-treated samples, and further analysis of the expression of these markers was not pursued. In general, Ki67 cells were present mostly in the suprabasal compartment, and there were no discernible differences in their locations between air–liquid and submersed or between rapamycin or regular medium specimens. Representative examples of Ki67-positive cells (stained dark brown) for these specimens are shown in the block of images of Fig. 2a. The notable difference was in the number of cells expressing Ki67 (Fig. 2b). There was a significant decrease of proliferative cells for specimens cultured with rapamycin versus specimens cultured in regular medium, and this happened for both 5DCNT-AIR versus 5D2R-AIR (p < 0.001) and 5DCNT-AIR versus 5D2R-AIR (p < 0.001). The effect of rapamycin was visible in both air and submersed samples. In the case of cleaved caspase-3 (Fig. 2c for H&E and Fig. 2d for number of positive cells), however, the effect of rapamycin was mostly visible in submersed specimens, with a substantial decrease in the number of cleaved caspase-3 positive cells with respect to untreated specimens. This difference was not evident at the air–liquid interface. Almost in all cases cleaved caspase-3 positive cells appeared in the basal layer and occasionally in the suprabasal layer. Preliminary evaluation of cleaved caspase-3 using 100 nM and 2 μM rapamycin showed an increase in the number of cleaved caspase-3 cells when the specimens were cultured in submersion with respect to the air–liquid interface (Figure S3, Supplementary Material).
Representative examples of Ki67 expression in skin specimens (a) and cleaved caspase-3 expression (c). The specimens treated with rapamycin showed a significant decrease in Ki67 expression in both air–liquid and submersed specimens (b). Cleaved caspase-3 was low and similar at the air–liquid interface, but was reduced with 2 nM rapamycin when culturing in submersed conditions
Changes in involucrin expression were also noticed (Fig. 3a). For 5DCNT-AIR, 5D2R-AIR and 5DCNT-SUB involucrin showed intense expression in the upper spinous and granular layers, but was generally weaker for 5D2R-AIR. 5DCNT-SUB samples showed areas of weaker expression among strongly expressing involucrin areas. There was, however, a marked, decrease of involucrin all along the spinous and granular layers of 5D2R-SUB samples (noted with red arrows). This trend in the weaker expression of involucrin in rapamycin-treated specimens was also seen in the samples of the 10-day experiment (Fig. 3b). With respect to E-cadherin expression, in specimens cultured at the air–liquid interface in regular medium (Fig. 4a) or with 2 nM rapamycin (Fig. 4c), E-cadherin showed a regular pattern of expression in all cellular layers but that was disrupted in specimens cultured in submersed conditions (Fig. 4b, d). With regular medium (Fig. 4b), there was a general decrease in the expression in all layers, as shown in Fig. 4b1 and b2 or was reduced to the basal layer on certain areas with more severe epidermal degeneration (Fig. 4b3 and b4). However, with 2 nM rapamycin medium (Fig. 4d) E-cadherin was better preserved along the basal and spinous layers. Occasional loss of expression was seen multi-focally (black arrow in Fig. 4d3 and d4).
Changes in involucrin expression on human skin explants after a 5- or b 10-day incubation with 2 nM rapamycin medium. A general decrease in involucrin in the upper spinous layer and in the granular layer was observed for submersed specimens treated with rapamycin (broadly indicated with red arrows and in magnified inset images). Regular medium specimens showed areas of weaker expression among strongly expressing involucrin areas
Changes in E-cadherin expression on human skin explants after 5-day incubation with 2 nM rapamycin medium. In specimens cultured at the air–liquid interface in regular medium (a and higher magnification inset in a) or with 2 nM rapamycin (c and higher magnification inset in c), E-cadherin showed a normal pattern of expression but that was disrupted in specimens cultured in submersed conditions. With regular medium (b), there was a general decrease in the expression (b1 and magnification b2) or was reduced to the basal layer on certain areas (b3 and magnification b4). With 2 nM rapamycin medium (d), the expression was more similar to the air–liquid interface specimens (d1 and magnification d2), but it was lost multi-focally (black arrow in d3 and d4 and magnification in d4)
Discussion
In this study, we undertook an analysis of the effects of rapamycin in human skin in vitro using organotypic cultures of human skin explants as a model system; an experimental setting that has been used previously to carry out a variety of studies in human skin, including toxicological and drug effect [23]. While rapamycin is an FDA approved drug that has been used historically in organ transplantation as immunosupressor [6], most of the published studies of the effect of rapamycin on human skin are indirect studies of the side effects observed on patients due to rapamycin treatment for other diseases. In most of these studies rapamycin is administered systemically, either in animal models or in humans [11, 36]. Recently, other studies in diseases involving the skin have shown promising results, for example, in the treatment of scleroderma [14] and possibly in keloids and excessive scars [29], by way of affecting fibroblast proliferation.
As we have shown in previous reports [32, 33], skin explants are well maintained for 5 days in culture at the air–liquid interface, hence it was not surprising that there were no substantial visual morphological differences between control and rapamycin-cultured specimens at the air–liquid interface for 5 days. Another model of culturing skin explants is by submersion [1]. In our experiments, it was clearly noticed that culturing the whole tissue submersed in medium at 37 °C led to substantial epidermal degeneration both at 5 and 10 days (Fig. 1). This degeneration was reduced for rapamycin-treated submersed samples (except for 2 μM rapamycin, Figure S2 Supplementary Material), indicating that this culturing method may be preferable to morphologically assess the effect of the drug. The preservation of morphological features was confirmed when the samples were cultured for 10 days. The observation that rapamycin eliminated signs of suprabasal acantholysis (in submersed specimens) was in line with results reported by Pretel [34] in an animal model. Rapamycin, then, appears to maintain a better epidermal structure when skin is submersed, particularly in the upper spinous and granular layer. Perhaps this opens-up the possibility of studying the interactions of the drug with the molecules that facilitate cell–cell attachment and that are disrupted in diseases like pemphigus vulgaris, like desmoglein. This correlation may exist in view of recent reports [15] of the effect of systemic administration of rapamycin in pemphigus vulgaris. In part, for this reason, we analyzed expression of E-cadherin, which is a molecule typically expressed in adherens junctions and interdigitating membrane structures in normal epidermis [22]. Its expression was normal and did not appear to be affected by rapamycin when cultured at the air–liquid interface. It is known that keratinocytes undergoing apoptosis show reduced expression of E-cadherin and disruption of the epidermal barrier [12], much as we suspect is happening in semi-submersed samples in regular medium in our experiments. Rapamycin, however, preserved its expression (except in some localized foci) when skin was cultured in submersed conditions, where E-cadherin showed no expression or was limited to the basal layer. These results are in line with previous information indicating that rapamycin can indirectly block the loss of E-cadherin expression in normal rat kidney epithelial cell line [39] as well as the fact that cell morphology of rapamycin-cultured cells in vitro changes to a cuboidal type with increased cell–cell adhesion, associated with increased expression of E-cadherin [25].
In our study, it was found that the use of rapamycin at low concentrations restricted Ki67 expression (Fig. 2), demonstrating that rapamycin is effective in reducing cell proliferation in keratinocytes in whole tissues, as it does in other cells and in keratinocytes in culture, for instance inhibiting PCNA and T cell activation [20].
Normally, in vitro studies using rapamycin show that the drug has been used typically in the low to mid-nanomolar concentrations. Use of rapamycin in dissociated keratinocytes cultures has been reported in low nanomolar concentrations, except for Duncan [11] that used low micromolar concentrations. We decided to use rapamycin at 2 nM concentration as previous reports have shown that anti-proliferative effects appear at low concentrations [4, 9, 18, 40]. However, in contrast to most previously published experiments, where cells are cultured for short periods of time (24–48 h, except for the study by Izumi), we have used rapamycin for mid or longer periods of time.
Our study was complemented with the analysis of cleaved caspase-3 in the epidermis, which is normally activated in skin insults [2, 21, 27] to evaluate whether the decrease in proliferation was also associated with an increase in apoptosis. Cellular toxicity associated with rapamycin does not seem to be linked to inhibition of TORC1 [3], so if there is cellular cytotoxicity is likely to be dependent on other factors.
Caspase-3 activation in skin is normally seen in the basal layer (as seen in Fig. 2c). At 2 nM concentration rapamycin appeared to limit apoptosis when the cultures were submersed compared to specimens cultured in regular medium, but this difference was not observed at the air–liquid interface. In any case, at the air liquid interface, the number of positive cells were generally low for all specimens. However, when using samples at 2 μM in submersed conditions (Figure S3 in Supplemental Material) the number of positive cells was similar to the specimen cultured in regular medium, suggesting that at high concentrations rapamycin may indirectly contribute to keratinocyte apoptosis in our model of culture in submersed conditions. This indicates that at low concentrations rapamycin inhibits proliferation without concomitant increase in apoptosis (perhaps even limiting apoptosis), but that it is unable to do so at high concentrations when culturing in submersed conditions.
Our results regarding involucrin (a marker of terminal differentiation) show (Fig. 3) that rapamycin reduces the presence of involucrin, particularly in the granular layer, indicating that rapamycin may affect more keratinocytes committed to terminal differentiation rather than early differentiation. This reduction in cell differentiation by rapamycin has been shown before in cultured keratinocytes (Peramo, personal communication) and in human endothelial progenitor cells [5]. With respect to the possible mechanism of action of rapamycin, a possibility is that rapamycin may restrict keratinocyte proliferation and differentiation by blocking or reducing Akt activation. There is evidence that activation of Akt is involved in the initiation of keratinocyte terminal differentiation [8, 19, 37].
Our results point toward applications of rapamycin in skin in which restriction or modulation of keratinocyte differentiation and proliferation is necessary. Given that Akt/mTOR signaling pathway is up regulated in wound healing, Akt-mediated rapamycin pathway may be a potential therapeutic target in extramammary Paget’s disease, by reducing cell cycle progression [10]. As mentioned, another possible application is in pemphigus vulgaris as it has been shown by Pretel [34] that an imbalance in Akt/mTOR is involved in acantholitic processes in a mouse model of pemphigus vulgaris. Topical formulations containing rapamycin, instead of oral administration, could be developed to be used in treatment of other diseases, for instance pachyonychia congenital [16] or psoriasis [30].
References
Ameglio F, Bonifati C, Fazio M, Mussi A, Trento E, Cordial Fei P, Donati P, Pimpinelli F, D’Auria L, Carducci M (1997) Interleukin-11 production is increased in organ cultures of lesional skin of patients with active plaque-type psoriasis as compared with nonlesional and normal skin. Similarity to interleukin-1 beta, interleukin-6 and interleukin-8. Arch Dermatol Res 289:399–403
Bacqueville D, Mavon A (2008) Caspase-3 activation and DNA damage in pig skin organ culture after solar irradiation. Photochem Photobiol 84:1164–1171
Bansbach C, Wancio D, Caccese RG, Shen CF, Sehgal SN (1993) Rapamycin’s inhibition of thymocyte proliferation, unlike that of cyclosporin A or prednisolone, is not associated with cytotoxicity. Ann N Y Acad Sci 685:114–116
Bridges D, Fisher K, Zolov SN, Xiong T, Inoki K, Weisman LS, Saltiel AR (2012) Rab5 proteins regulate activation and localization of target of rapamycin complex 1. J Biol Chem 287:20913–20921
Butzal M, Loges S, Schweizer M, Fischer U, Gehling UM, Hossfeld DK, Fiedler W (2004) Rapamycin inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Exp Cell Res 300:65–71
Calne RY, Lim S, Samaan AD, Collier STJ, Pollard SG, White DJG, Thiru S (1989) Rapamycin for immunosuppression in organ allografting. Lancet 334:227
Campistol JM, de Fijter JW, Flechner SM, Langone A, Morelon E, Stockfleth E (2010) mTOR inhibitor-associated dermatologic and mucosal problems. Clin Transpl 24:149–156
Calautti E, Li J, Saoncella S, Brissete JL, Goetinck PF (2005) Phosphoinositide 3-kinase signalling to Akt promotes keratinocyte differentiation versus death. J Biol Chem 280:32856–32865
Canning MT, Brown DA, Yarosh DB (2003) A bicyclic monoterpene diol and UVB stimulate BRCA1 phosphorylation in human keratinocytes. Photochem Photobiol 77:46–51
Chen S, Nakahara T, Uchi H, Takeuchi S, Takahara M, Kido M, Dugu L, Tu Y, Moroi Y, Furue M (2009) Immunohistochemical analysis of the mammalian target of rapamycin signalling pathway in extramammary Paget’s disease. Br J Dermatol 161:357–363
Duncan JI (1994) Differential inhibition of cutaneous T-cell mediated reactions and epidermal cell proliferation by cyclosporin A, FK-506 and rapamycin. J Invest Dermatol 102:84–88
Engelhart K, El Hindi T, Biesalski HK, Pfitzner I (2005) In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Arch Dermatol Res 297:1–9
Ferrer IR, Araki K, Ford ML (2011) Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transpl 11:654–659
Fried L, Kirsner RS, Bhandarkar S, Arbirser JL (2008) Efficacy of rapamycin in scleroderma: a case study. Lymphat Res Biol 6:217–219
Grando SA, Laquer VT, Le HM (2011) Sirolimus for acute pemphigus vulgaris: a case report and discussion of dualistic action providing for both immunosuppression and keratinocyte protection. J Am Acad Dermatol 65:684–686
Hickerson RP, Leake D, Pho LN, Leachman SA, Kaspar RL (2009) Rapamycin selectively inhibits expression of an inducible keratin (K6a) in human keratinocytes and improves symptoms in pachyonychia congenita patients. J Dermatol Sci 56:82–88
Inoki K, Ouyang H, Li Y, Guan KL (2005) Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 69:79–100
Izumi K, Inoki K, Fujimori Y, Marcelo CL, Feinberg SE (2009) Pharmacological retention of oral mucosa progenitor/stem cells. J Dent Res 88:1113–1118
Janes SM, Ofstad TA, Campbell DH, Eddaoudi A, Warnes G, Davies D, Watt FM (2009) PI3-kinase-dependent activation of apoptotic machinery occurs on commitment of epidermal keratinocytes to terminal differentiation. Cell Res 19:328–339
Javier AF, Bata-Csorgo Z, Ellis CN, Kang S, Voorhes JJ (1997) Rapamycin (Sirolimus) inhibits proliferating cell nuclear antigen expression and blocks cell cycle in the G1 phase in human keratinocyte stem cells. J Clin Invest 99:2094–2099
Kleszczynski k, Fischer TW (2012) Development of a short-term human full-thickness skin organ culture model in vitro under serum-free conditions. Arch Dermatol Res. doi:10.1007/s00403-012-1239-z
Kooy AJ, Tank B, de Jong AA, Vuzevski VD, van der Kwast TH, van Joost T (1999) Expression of E-cadherin, alpha- & beta-catenin, and CD44V6 and the subcellular localization of E-cadherin and CD44V6 in normal epidermis and basal cell carcinoma. Hum Pathol 30:1328–1335
Lebonvallet N, Jeanmaire C, Danoux L, Sibille P, Pauly G, Misery L (2010) The evolution and use of skin explants: potential and limitations for dermatological research. Eur J Dermatol 20:671–684
Loewe R, Oble DA, Valero T, Zukerberg L, Mihm MC Jr, Nelson JS (2010) Stem cell marker upregulation in normal cutaneous vessels following pulsed-dye laser exposure and its abrogation by concurrent rapamycin administration: implications for treatment of port-wine stain birthmarks. J Cutan Pathol 37:76–82
Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M (2002) Rapamycin blocks tumor progression unlinking immunosuppression from antitumor efficacy. Transplantation 73:1565–1572
Ma J, Meng Y, Kwiatkowski DJ, Chen X, Peng H, Sun Q, Zha X, Wang F, Wang Y, Jing Y, Zhang S, Chen R, Wang L, Wu E, Cai G, Malinowska-Kolodziej I, Liao Q, Liu Y, Zhao Y, Sun Q, Xu K, Dai J, Han J, Wu L, Zhao RC, Shen H, Zhang H (2010) Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest 120:103–114
Mass P, Hoffmann K, Gambichler T, Altmeyer P, Mannherz HG (2003) Premature keratinocyte death and expression of marker proteins of apoptosis in human skin after UVB exposure. Arch Dermatol Res 295:71–79
Micozkadioglu H, Koc Z, Ozelsancak R, Yildiz I (2010) Rapamycin therapy for renal, brain, and skin lesions in a tuberous sclerosis patient. Ren Fail 32:1233–1236
Ong CT, Khoo YT, Mukhopadhyay A, Do DV, Lim IJ, Aalami O, Phan TT (2007) mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol 16:394–404
Ormerod AD, Shah SA, Copeland P, Omar G, Winfield A (2005) Treatment of psoriasis with topical sirolimus: preclinical development and a randomized, double-blind trial. Br J Dermatol 152:758–764
Paghdal KV, Schwartz RA (2007) Sirolimus (rapamycin): from the soil of Easter Island to a bright future. J Am Acad Dermatol 57:1046–1050
Peramo A, Marcelo CL, Goldstein SA, Martin DC (2009) Novel organotypic cultures of human skin explants with an implant-tissue biomaterial interface. Ann Biomed Eng 37:401–409
Peramo A, Marcelo CL, Goldstein SA, Martin DC (2010) Improved preservation of the tissue surrounding percutaneous devices by hyaluronic acid and dermatan sulfate in a human skin explant model. Ann Biomed Eng 38:1098–1110
Pretel M, Espana A, Marquina M, Pelacho B, Lopez-Picazo JM, Lopez-Zabalza MJ (2009) An imbalance in Akt/mTOR is involved in the apoptotic and acantholytic processes in a mouse model of pemphigus vulgaris. Exp Dermatol 18:771–780
Raimondi AR, Molinolo A, Gutkind JS (2009) Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res 69:4159–4166
Saggar S, Zeichner JA, Brown TT, Phelps RG, Cohen SR (2008) Kaposi’s sarcoma resolves after sirolimus therapy in a patient with Pemphigus Vulgaris. Arch Dermatol 144:654–657
Thrash B, Menges CW, Pierces RH, McCance DJ (2006) AKT1 provides an essential survival signal for differentiation and stratification of primary human keratinocytes. J Biol Chem 281:12155–12162
Toso C, Patel S, Asthana S, Kawahara T, Girgis S, Kneteman NN, Shapiro AMJ, Bigam DL (2010) The impact of sirolimus on hepatocyte proliferation after living donor liver transplantation. Clin Transpl 24:695–700
Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ (2006) Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int 69:2029–2036
Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR (2008) Reactive oxygen species in tumor necrosis factor-a-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol 128:2606–2614
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peramo, A., Marcelo, C.L. Visible effects of rapamycin (sirolimus) on human skin explants in vitro. Arch Dermatol Res 305, 163–171 (2013). https://doi.org/10.1007/s00403-012-1288-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-012-1288-3