Abstract
Introduction
Studies have reported various effects of autologous chondrocyte implantation (ACI) on osteochondral defects of the talus. Therefore, to assess the effectiveness of ACI for osteochondral defects of the talus, we used the meta-analytic approach.
Materials and methods
Electronic databases PubMed, Embase, and the Cochrane Library were systematically searched to identify eligible studies from their inception until November 2020. The random-effects model was used to calculate the incidence of success rate and American Orthopaedic Foot and Ankle Society (AOFAS) score for patients after ACI treatment. Subgroup analyses were also conducted based on age, technique, indication, size, and follow-up duration.
Results
For the final meta-analysis, we selected 23 case series studies with a total of 458 patients with osteochondral defects of the talus. Overall, after ACI for patients with osteochondral defects of the talus, we noted that the incidence of success rate was 89% (95% confidence interval (95% CI) 85%–92%; P < 0.001). Moreover, after ACI for patients with osteochondral defects of the talus, the AOFAS score was 86.33 (95% CI 83.33–89.33; P < 0.001). Subgroup analysis showed that the AOFAS score after ACI is significantly different when stratified by the mean age of the patients (P = 0.006).
Conclusions
This study revealed that the use of ACI could provide a relatively high success rate and improve the AOFAS score for patients with osteochondral defects of the talus, which should be recommended in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankle sprains as a common joint injury and nearly 27,000 injuries per day occurred in the United States [1]. Moreover, nearly 70% of sprains and fractures involving the ankle could cause osteochondral lesions [2]. The entire body weight was supported, and stabilisation by the ankle and the small area of distribution caused the joint to be sensible to shearing stresses [3]. Osteochondral defects of the talus contained the lesion in the subchondral bone and its overlying cartilage, and mostly osteochondral defects of the talus occurred after an ankle fracture or lateral ankle ligament rupture [2, 4]. Moreover, osteochondral defects could progress to cystic lesion and induce deep ankle pain during activity, prolonged swelling, diminished range of motion, and synovitis [5, 6].
Recently, autologous chondrocyte implantation (ACI) is widely used to cover the anatomical defects for repairing osteochondral defects, which is based on two-time surgical procedures. The first procedure includes revision arthroscopy of the joint with the lesion area as well as a trephine of healthy cartilage tissue and then graft obtained by stimulating chondrocyte mitosis. The second procedure is conducted by implant matrix using arthroscopy or arthrotomy of the medial malleolus to expose the injury area [7, 8]. The effectiveness of ACI for patients with osteochondral defects of the talus has already been demonstrated, whereas, the treatment effectiveness was variable across studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Therefore, this systematic review and meta-analysis aimed to assess the effectiveness of ACI for patients with osteochondral defects of the talus.
Methods
Data sources, search strategy, and selection criteria
This systematic review and meta-analysis were performed and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement [32]. Studies that assessed the effectiveness of ACI for patients with osteochondral defects of the talus were eligible in our study. Electronic databases PubMed, Embase, and the Cochrane Library were systematically searched to identify eligible studies using the following search terms until November 2020: (“Chondrocytes” AND “Transplantation, Autologous” OR “autologous chondrocyte transplantation” OR “autologous chondrocyte implantation”) AND (“Ankle” OR “Ankle Joint” OR “Ankle injuries” OR “Talus” OR “talus” OR “talar”). Furthermore, the reference lists of potentially relevant reviews and original articles were also manually searched to identify any new eligible study.
The literature search and study selection were independently performed by two reviewers, and the inconsistency was settled by a group discussion. Studies were included if they met the following criteria: (1) patients: osteochondral defects of the talus; (2) intervention: ACI; (3) outcome: success rate (defined as American Orthopedic Foot and Ankle Society (AOFAS) score > 80) and AOFAS score; and (4) study design: case series and observational and randomised controlled trials.
Data collection and quality assessment
The data collection and quality assessment were independently conducted by two reviewers, and any disagreement between reviewers was settled by discussion mutually until a consensus was reached. The items collected from each study included the first author’s name, publication year, country, evidence level, sample size, age, number of males and females, technique, first-line or revision ACI, subchondral bone grafting, indication, size, follow-up, success rate, assessment tool, and reported outcomes. The modified Coleman methodology score was determined for each study to assess the study quality and the different types of detected bias [33].
Statistical analysis
After ACI, the success rate and AOFAS score were assigned as categorical and continuous data, respectively. Then, the random-effects model was used to calculate the pooled incidence of success rate and AOFAS score [34, 35]. After this, I2 and Q statistics were applied to assess the heterogeneity across the included studies, and significant heterogeneity was defined as I2 > 50.0% or P < 0.10 [36, 37]. Sensitivity analyses for success rate and AOFAS score were also performed to assess the impact of a single study on the overall conclusion [38]. Subgroup analyses for success rate and AOFAS score were also performed based on age, technique, indication, size, or follow-up duration, and the interaction P test was performed to assess the difference between subgroups [39]. Furthermore, Funnel plot, Egger, and Begg tests were performed to assess publication bias for success rate and AOFAS score [40, 41]. All reported P values are two-sided, and a significant difference was defined as P < 0.05. The STATA software (version 10.0; Stata Corporation, College Station, TX, USA) was used to perform all of the analyses in this study.
Results
Literature search
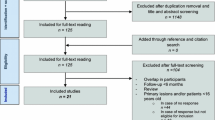
By initial electronic searches, a total of 786 articles were identified, and 381 studies were retained after exclusion of the duplicate articles. After this, 311 studies were removed because of irrelevant topics. The remaining 70 studies were retrieved for further full-text evaluations, and 47 studies were excluded because of osteochondral defects in knee (n = 21), no sufficient data (n = 15), and other interventions (n = 11). After reviewing the reference lists of relevant studies, three potentially included studies were found, and all of these studies were included in electronic searches. Therefore, the remaining 23 studies were selected for final meta-analysis [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] (Fig. 1).
Study characteristics
Table 1 shows the characteristics of the included studies and patients. All of the 23 included studies were designed as case series, and a total of 458 patients with osteochondral defects of the talus were recruited. The sample size ranged from 7 to 46, and the follow-up duration ranged from 12.0 to 154.8 months. For all of the included studies, the evidence level was IV. Six studies applied periosteum-covered ACI, and the remaining 17 studies applied matrix-associated ACI. All of the included studies had a level of evidence of IV. The mean modified Coleman methodology score was 48.1, and the score in each study ranged from 35 to 65, which suggested that all of the included studies were of low to moderate quality.
Success rate
A total of 17 studies reported the incidence of success rate for patients after ACI. We noted that the pooled incidence for success rate was 89% (95% confidence interval (95%CI) 85%–92%; P < 0.001; Fig. 2), and unimportant heterogeneity was detected across the included studies (I2 = 30.1%; P = 0.117). Sensitivity analysis indicated that the incidence of success rate was robust after sequentially excluding individual study (Online Resource 1). Subgroup analysis indicated that the pooled incidence of success rate was > 80.0% in all subgroups, and age, technique, indication, size, or follow-up duration was not affecting the incidence of success rate (Table 2). Furthermore, a significant publication bias for success rate was found (P value for Egger: < 0.001; P value for Begg: 0.002; Online Resource 2), and the pooled incidence of success rate was not altered by adjusted using the trim and fill method [42].
AOFAS score
The AOFAS score for patients after ACI was reported in a total of 19 studies. We noted that the AOFAS score after ACI was 86.33 (95% CI 83.33–89.33; P < 0.001; Fig. 3), and no evidence of heterogeneity was seen among the included studies (I2 = 0.0%; P = 0.603). The pooled AOFAS score after ACI was stable after sequentially excluding single study (Online Resource 1). Subgroup analyses revealed that the pooled AOFAS score was lower when pooling studies for patients with chondral lesion (Table 2). Moreover, we noted that the AOFAS score could be affected after ACI for patients with osteochondral defects of the talus (P = 0.006). There was no significant publication bias for the AOFAS score after ACI (P value for Egger: 0.794; P value for Begg: 0.069; Online Resource 2).
Adverse events
Ten out of the included studies reported complications after ACI [11,12,13, 15,16,17,18, 23,24,25, 28]. Six studies indicated no intraoperative or postoperative complications [11, 13, 15, 16, 24, 28]. Whittaker et al. reported one patient (10.0%) who presented with superficial infection of the ankle [12]. Schneider et al. reported two patients (10.0%) with anterior graft impingement, two patients (10.0%) with recurrent pain associated with hardware, and two patients (10.0%) who presented with clear failures combined with persistent pain and synovitis [18]. Lee et al. showed that the prevalence of nonunion and delayed unions of the osteotomy sites was 2.6% and 5.3%, respectively. Moreover, nine ankles (29.0%) sustained damaged medial malleolar cartilage [23]. Finally, Buda et al. reported three patients (15.0%) who presented with adhesions or joint effusion [25].
Discussion
The treatment effectiveness of ACI for patients with osteochondral defects of the talus has already been illustrated in numerous studies, while the effect was variable and not confirmed to date. This systematic review and meta-analysis was performed and assessed the effectiveness of ACI on the incidence of success rate and AOFAS score. A total of 458 patients with osteochondral defects of the talus were identified from 23 case series studies, and the characteristics of patients were broad across the included studies. We noted that the pooled success rate was high, and the AOFAS score was improved after ACI. Moreover, the AOFAS score after ACI for patients with osteochondral defects of the talus could be affected by age.
In a previous systematic review, 16 studies were identified and revealed that the ACI should be considered as a promising treatment for osteochondral and chondral defects of the talus [43]. Erickson et al. conducted a systematic review of 19 studies and found that there were no significant differences among the combination of open or arthroscopic matrix-associated ACI and periosteum-covered ACI for talar osteochondral lesions less than 2.5 cm2 [44]. However, these two studies have just given the qualitative analysis for the included studies, and according to patients’ characteristics, the quantitative analysis was not illustrated. Therefore, this systematic review and meta-analysis were performed to assess the treatment effectiveness of ACI on success rate and AOFAS score for patients with osteochondral defects of the talus.
The summary success rate for the effect of ACI was 89% (95% CI 85%–92%; P < 0.001), and the success rate in each study ranged from 50 to 100%. In Giza et al.’s study, 10 patients with osteochondral defects of the talus were recruited and only five patients showed significant improvement in AOFAS score [19]. Five of the included studies presented 100% of success rate for patients treated with ACI [9,10,11, 25, 29]. Although subgroup analyses revealed that age, technique, indication, size, or follow-up duration did not affect the success rate for patients with osteochondral defects, we noted that the success rate was higher when the age of patients was < 35.0 years, patients were treated with periosteum-covered ACI, patients were with osteochondral defects, and the lesion size was ≥ 2.0 cm2. These results suggested the ACI might give a superior effect on the success rate in patients with specific characteristics.
We noted that the pooled AOFAS score after ACI was 86.33 (95% CI 83.33–89.33) for patients with osteochondral defects of the talus, and the AOFAS score in the individual study ranged from 74.7 to 92.7. Subgroup analysis suggested the AOFAS score after ACI was high in the subgroups of the age of patients < 35.0 years, patients treated with matrix-associated ACI, patients with osteochondral defects, lesion size ≥ 2.0 cm2, and follow-up duration ≥ 36.0 months. Moreover, after ACI, there was a significant difference in AOFAS score when stratified by age of patients. Studies have already revealed that patients’ age could affect cartilage repair and the clinical outcome after ACI [15, 45], while this result was not consistent [46]. The potential reason for a beneficial effect of ACI on younger patients could be the restore ability of younger patients was stronger than elderly patients.
Several shortcomings of this study should be discussed. First, all of the included studies were designed as case series, and the evidence level was lower (IV). Second, in a smaller number of studies, the comparisons of various treatment strategies were reported [47], and in this study, the lack of controlled treatment strategies and the superiority or inferiority effects of ACI compared with other techniques were not addressed. Third, the analysis based on crude data and the potential role of other characteristics was not adjusted. Fourth, the background therapies including physical therapy, bracing, casting, and nonsteroidal anti-inflammatory medication were not mentioned, which could affect the treatment effectiveness of ACI. Fifth, the current study was not registered, and the transparency was restricted. Sixth, the AOFAS score is not a validated score, which could affect the treatment effects of ACI. Finally, inherent limitations for meta-analysis based on pooled data, including inevitable publication bias, and the restricted detail analyses.
Conclusions
The pooled success rate and AOFAS score after ACI for patients with osteochondral defects of the talus were 89% (95% CI 85%–92%) and 86.33 (95% CI 83.33–89.33), respectively. Moreover, the treatment effectiveness of ACI on the AOFAS score could be affected by age of patients. Further controlled compared studies should be conducted to compare the efficacy and safety of ACI with other techniques for patients with osteochondral defects of the talus.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG (2014) Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec 7:414–422. https://doi.org/10.1177/1938640014543362
Hannon CP, Smyth NA, Murawski CD, Savage-Elliott I, Deyer TW, Calder JD et al (2014) Osteochondral lesions of the talus: aspects of current management. Bone Joint J 96-b:164–171. https://doi.org/10.1302/0301-620x.96b2.31637
Correa Bellido P, Wadhwani J, Gil Monzo E (2019) Matrix-induced autologous chondrocyte implantation grafting in osteochondral lesions of the talus: evaluation of cartilage repair using T2 mapping. J Orthop 16:500–503. https://doi.org/10.1016/j.jor.2019.04.002
Reilingh M, Van Bergen C, Van Dijk C (2009) Diagnosis and treatment of osteochondral defects of the ankle. SA Orthopaedic Journal 8:44–50
Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR et al (2008) Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med 36:1750–1762. https://doi.org/10.1177/0363546508316773
van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ (2010) Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc 18:570–580. https://doi.org/10.1007/s00167-010-1064-x
Flick AB, Gould N (1985) Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle 5:165–185. https://doi.org/10.1177/107110078500500403
Shimozono Y, Yasui Y, Ross AW, Kennedy JG (2017) Osteochondral lesions of the talus in the athlete: up to date review. Curr Rev Musculoskelet Med 10:131–140. https://doi.org/10.1007/s12178-017-9393-8
Giannini S, Buda R, Grigolo B, Vannini F (2001) Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int 22:513–517. https://doi.org/10.1177/107110070102200612
Dorotka R, Kotz R, Trattnig S, Nehrer S (2004) Mid-term results of autologous chondrocyte transplantation in knee and ankle. A one- to six-year follow-up study. Z Rheumatol 63:385–392. https://doi.org/10.1007/s00393-004-0602-7
Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A (2005) The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage 13:601–607. https://doi.org/10.1016/j.joca.2005.02.010
Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P et al (2005) Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br 87:179–183. https://doi.org/10.1302/0301-620x.87b2.15376
Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM (2006) Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am 88:303–308. https://doi.org/10.2106/jbjs.E.00033
Caumo F, Russo A, Faccioli N, Vecchini E, Costa A, Ricci M et al (2007) Autologous chondrocyte implantation: prospective MRI evaluation with clinical correlation. Radiol Med 112:722–731. https://doi.org/10.1007/s11547-007-0175-z
Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B (2008) Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med 36:873–880. https://doi.org/10.1177/0363546507312644
Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F (2009) Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med 37(Suppl 1):112s–118s. https://doi.org/10.1177/0363546509349928
Nam EK, Ferkel RD, Applegate GR (2009) Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med 37:274–284. https://doi.org/10.1177/0363546508325670
Schneider TE, Karaikudi S (2009) Matrix-Induced Autologous Chondrocyte Implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int 30:810–814. https://doi.org/10.3113/fai.2009.0810
Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L et al (2010) Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int 31:747–753. https://doi.org/10.3113/fai.2010.0747
Lee KT, Choi YS, Lee YK, Kim JS, Young KW, Kim JH (2010) Comparison of MRI and arthroscopy after autologous chondrocyte implantation in patients with osteochondral lesion of the talus. Orthopedics 33. https://doi.org/10.3928/01477447-20100625-12
Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C et al (2011) Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc 19:1376–1384. https://doi.org/10.1007/s00167-011-1509-x
Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR (2012) Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am 94:1105–1110. https://doi.org/10.2106/jbjs.J.02010
Lee KT, Kim JS, Young KW, Lee YK, Park YU, Kim YH et al (2013) The use of fibrin matrix-mixed gel-type autologous chondrocyte implantation in the treatment for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 21:1251–1260. https://doi.org/10.1007/s00167-012-2096-1
Kwak SK, Kern BS, Ferkel RD, Chan KW, Kasraeian S, Applegate GR (2014) Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med 42:2156–2164. https://doi.org/10.1177/0363546514540587
Buda R, Vannini F, Castagnini F, Cavallo M, Ruffilli A, Ramponi L et al (2015) Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop 39:893–900. https://doi.org/10.1007/s00264-015-2685-y
Desando G, Bartolotti I, Vannini F, Cavallo C, Castagnini F, Buda R et al (2017) Repair potential of matrix-induced bone marrow aspirate concentrate and matrix-induced autologous chondrocyte implantation for talar osteochondral repair: patterns of some catabolic, inflammatory, and pain mediators. Cartilage 8:50–60. https://doi.org/10.1177/1947603516642573
Chan KW, Ferkel RD, Kern B, Chan SS, Applegate GR (2018) Correlation of MRI appearance of autologous chondrocyte implantation in the ankle with clinical outcome. Cartilage 9:21–29. https://doi.org/10.1177/1947603516681131
Pagliazzi G, Vannini F, Battaglia M, Ramponi L, Buda R (2018) Autologous chondrocyte implantation for talar osteochondral lesions: comparison between 5-year follow-up magnetic resonance imaging findings and 7-year follow-up clinical results. J Foot Ankle Surg 57:221–225. https://doi.org/10.1053/j.jfas.2017.05.013
Kreulen C, Giza E, Walton J, Sullivan M (2018) Seven-year follow-up of matrix-induced autologous implantation in talus articular defects. Foot Ankle Spec 11:133–137. https://doi.org/10.1177/1938640017713614
López-Alcorocho JM, Guillén-Vicente I, Rodríguez-Iñigo E, Navarro R, Caballero-Santos R, Guillén-Vicente M et al (2019) High-Density Autologous Chondrocyte Implantation as Treatment for Ankle Osteochondral Defects. Cartilage:1947603519835898. https://doi.org/10.1177/1947603519835898
Lenz CG, Tan S, Carey AL, Ang K, Schneider T (2020) Matrix-Induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int 41:1099–1105. https://doi.org/10.1177/1071100720935110
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2009. Ottawa Hospital Research Institute Web site. L’Hopital d’Ottawa Institut de Recherche, Ottawa, Ontario, Canada.
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Ades AE, Lu G, Higgins JP (2005) The interpretation of random-effects meta-analysis in decision models. Med Decis Making 25:646–654. https://doi.org/10.1177/0272989x05282643
Deeks JJ, Higgins JP, Altman DG (2008) Analysing Data and Undertaking Meta‐Analyses. In: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. pp 243–296
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Tobias A (1999) Assessing the influence of a single study in meta-analysis. Stata Technical Bulletin 8.
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219. https://doi.org/10.1136/bmj.326.7382.219
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Duval S, Tweedie R (2000) A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 95:89–98
Niemeyer P, Salzmann G, Schmal H, Mayr H, Südkamp NP (2012) Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc 20:1696–1703. https://doi.org/10.1007/s00167-011-1729-0
Erickson B, Fillingham Y, Hellman M, Parekh SG, Gross CE (2018) Surgical management of large talar osteochondral defects using autologous chondrocyte implantation. Foot Ankle Surg 24:131–136. https://doi.org/10.1016/j.fas.2017.01.002
Lee KT, Lee YK, Young KW, Park SY, Kim JS (2012) Factors influencing result of autologous chondrocyte implantation in osteochondral lesion of the talus using second look arthroscopy. Scand J Med Sci Sports 22:510–515. https://doi.org/10.1111/j.1600-0838.2010.01262.x
Körner D, Gueorguiev B, Niemeyer P, Bangert Y, Zinser W, Aurich M et al (2017) Parameters influencing complaints and joint function in patients with osteochondral lesions of the ankle-an investigation based on data from the German Cartilage Registry (KnorpelRegister DGOU). Arch Orthop Trauma Surg 137:367–373. https://doi.org/10.1007/s00402-017-2638-6
Ettinger S, Gottschalk O, Kostretzis L, Plaas C, Körner D, Walther M et al (2020) One-year follow-up data from the German Cartilage Registry (KnorpelRegister DGOU) in the treatment of chondral and osteochondral defects of the talus. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-020-03631-z
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: MH; methodology: MH, XL and XX; formal analysis and investigation: MH and XL; writing—original draft preparation: MH; writing—review and editing: XX.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
This study did not contain any participants’ data and the ethics approval is not applicable.
Consent to participate
This study did not contain any participants’ data and the Consent to participate is not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, M., Li, X. & Xu, X. Efficacy and safety of autologous chondrocyte implantation for osteochondral defects of the talus: a systematic review and meta-analysis. Arch Orthop Trauma Surg 143, 71–79 (2023). https://doi.org/10.1007/s00402-021-03990-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-03990-1