Abstract
Introduction
Postoperative complications after hip fractures in osteoporotic bone such as implant cutout can be reduced by the use of specially designed implants or additional cement augmentation. It is not yet clear at which degree of osteoporosis, patients will profit from implant augmentation or specially designed implants for geriatric patients. As the surgeon ideally should obtain information on local bone quality at the site of implant anchorage already preoperatively, the aim of the study was to develop an easily applicable radiographic method to estimate bone quality in those patients.
Materials and methods
75 patients with unilateral hip fracture were included. Preoperatively, a CT scan with a calibration device was conducted. Postoperatively, DXA scans were performed. The proposed method measures local cancellous bone mineral density in the contralateral and uninjured femoral head. As a control, 15 young and healthy non-osteoporotic subjects were included. Inter- and intraobserver reliability was investigated for a subgroup of 20 patients.
Results
Study group patients had a mean BMD measured by CT scans of 194.2 mg/cm3 (SD 40.4). There was a statistically significant correlation with data from DXA scans (r = 0.706, p < 0.001). The control group was significantly younger and showed a significantly higher BMD when compared to the study group (p < 0.001). Reliability evaluation showed no statistically significant difference in inter- and intraobserver measurements. Interclass correlation proved to be very high.
Conclusion
The proposed method is an easily applicable, reliable and useful tool to estimate bone quality preoperatively using the contralateral hip as a reference. Obtained data may facilitate the decision-making towards the use of further therapeutic measures to improve implant anchorage in osteoporotic bone such as bone cement augmentation. Thus, our method allows for a more individualized surgical treatment of hip fracture patients adapted to the estimated cancellous bone quality of the patient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fractures of the proximal femur are the most severe consequences of osteoporosis. Generally, the incidence of hip fractures has been increasing recently and due to aging of the population, the number will still grow in the future [16]. Although the quality of fracture treatment is consistently rising, implant-related complications after surgical fracture fixation are reported to be high. Failures such as cutout, lateral protrusion or varus collapse are most commonly reported to be caused by poor bone quality as well as malplacement of the implant material [1, 6, 15, 25, 26]. To diminish complications, new implants have been developed and additive options such as cement augmentation of the implant tip have been proposed [10, 15, 19, 20]. However, it is not yet reported, which patients exactly would profit from these additional measures. To facilitate the decision making process for the surgeon, the individual bone mineral density should be known prior to surgery. The gold standard to obtain this information still is dual X-ray absorptiometry (DXA). This, however, is rarely performable preoperatively due to reasonability to the fractured patient, as the latter has to be thoroughly positioned on the investigation table which is not feasible due to pain. Moreover, DXA scans are only available on appointment in specialized centers and cannot be performed immediately in the preoperative setting [4]. Furthermore, the technique measures areal BMD of combined cortical as well as cancellous bone. As implant anchorage is only in the cancellous portion of the femoral head, DXA values cannot provide the necessary information. Additionally, no osteoporotic BMD values could be detected in DXA scans in more than half of the patients with hip fractures [29]. Experimental procedures such as quantitative ultrasound [7, 28] are not yet implemented in the clinical routine and well-known X-ray indices for bone quality prediction such as the Singh Index (SI) are prone to failure [8, 12]. In biomechanical tests, CT data were reported to have a good informative value concerning load to failure, Young’s modulus or compressive resistance [3, 18]. Recently, a clinical study preoperatively evaluated local bone quality at the humeral head using conventional CT scans of patients with proximal humerus fractures. The authors described a good correlation with DXA measurements [13]. Also investigations comparing qCT and DXA in an elderly population showed a high correlation for obtained qCT values concerning areal bone mineral density (aBMD) [11, 21].
Hence, the aim of our study was to investigate whether CT scans performed with an additional calibration device could be a tool to preoperatively estimate local bone quality also in patients with proximal femur fractures. Thus, the surgeon would have a further tool in preoperative planning to facilitate the decision towards a certain treatment option.
Methods
To investigate the proposed method for bone mineral density evaluation, 75 patients aged 65 years or older (“study group”, 54 female, 21 male) were included in the study within 2 years. All patients sustained an acute unilateral proximal femur fracture after a low-energy trauma. Only patients without a history of contralateral proximal femur fracture were included. As a control we used CT scans of 15 non-osteoporotic subjects aged 50 years or younger without hip pathology (“control group”, 7 male, 8 female). The control group underwent CT scans to rule out hip fractures in trauma patients from our department. All scans of the control group were diagnosed as uneventful by a radiologist. Prior to inclusion, all patients gave informed consent to participation in the study. The study plan was approved by the local ethics committee board.
Preoperatively, patients and control subjects were subjected to a CT scan of both hips with a 64-slice GE Lightspeed VCT (GE Healthcare, Milwaukee, WI, USA) using a standardized protocol (120 kV, mA Auto, noise index 50, 0.625 mm slice thickness, standard bone kernel). For calibration, a density phantom (EFP, QRM GmbH, Möhrendorf, Germany) was placed on the patients’ symphysis to allow for the conversion of Hounsfield Units (HU) to bone mineral density (BMD) during data analysis. The phantom itself consists of three different density zones as well as a water equivalent allows for correction of obtained BMD values for fatty tissue in the measured region [13]. Postoperatively within 10 days of the surgical procedure, the patients’ BMD was additionally investigated by means of dual X-ray absorptiometry (DXA) for the total hip at the uninjured side. CT data of all patients were then evaluated with the proposed method by one observer (S.E.). To investigate for inter- and intraobserver reliability, 20 arbitrary chosen patient data sets were evaluated two times with an interval of at least 4 weeks by 3 different observers (S.E., T.R., M.Z.). Control group data were measured by one observer (S.E.).
Measurements were conducted on the uninjured hip in multiplanar CT reconstructions with the JVision software package (JVision, Version 3.3.16, Agfa, Belgium) as follows. It was reported that measurements on the contralateral femoral head show very high correlations in all femoral regions for DXA measurements [31, 32].
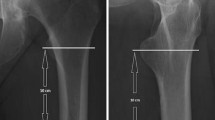
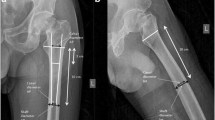
In every CT scan, first, the different regions of the calibration device (50, 100 and 200 mg/cm3 hydroxylapatite) and the water equivalent region were measured to obtain a calibration equation. Therefore, three measurements of HU were conducted and the mean HU value calculated in each density region. With all regions measured, the linear calibration equation could be automatically calculated and a water equivalent value could be obtained to correct for bone marrow fat as previously described ([14]; Fig. 1). Afterwards, the three-dimensional axes were aligned with the femoral neck in a standardized fashion (Fig. 2). Then, in a plane perpendicular to the femoral neck axis, the height of the femoral head was measured from the cartilage bone border to the apex of the femoral head and divided by three. In every third, a region of interest was placed as a best fit circle inside the cortical bone (Fig. 2) and its diameter diminished by 10 % to exclude the possibility of cortical overlap. HU values were obtained and corrected for fatty bone marrow using the water equivalent value (Fig. 3). Measurements of all three-thirds were then converted to BMD in mg/cm3 with the calibration equation and mean values were calculated for the femoral head.
Regions of interest placed in the phantom to obtain a calibration equation. The real hydroxylapatite value is stated in the phantom. The value for water would be 0. Hounsfield units were measured in three specified spots in each section. Then values were calculated and used to obtain a calibration equation together with the original values
In the study group, BMD measurements were conducted at the uninjured femoral head. In the control group, measurements were taken in both hips.
Statistical analysis was performed with the PASW 18.0 software package (SPSS, Chicago, IL, USA). Data are reported as mean and standard deviation. Correlations of investigated data were calculated using the Pearson coefficient. Data comparison was accomplished by using an unpaired t test. Normal distribution of the data was confirmed with a Kolmogorov–Smirnov test. The level for statistical significance was set at p < 0.05. To analyze for inter- and intraobserver reliability, interclass correlation coefficients (ICC) and the corresponding 95 % confidence intervals were used.
Results
Mean age in the study group was 77.5 years (SD 10.5). 35 patients showed a fracture of the left proximal femur and 40 injured their right hip. DXA measurements of the contralateral total hip region revealed a mean T score of −2.1 (SD 0.9) and a two-dimensional BMDdx of 0.7 g/cm2 (SD 0.1). Calculated BMD of the contralateral hip was derived from the analysis of HU in preoperative CT scans. It showed a mean BMDct of 194.2 mg/cm3 (SD 40.4).
There was a statistically significant correlation between BMDct from CT scans and obtained T scores from DXA measurements (r = 0.706, p < 0.001, Pearson). Furthermore, a significant correlation of age and BMDct as well as T score was confirmed (p < 0.002, Pearson). T scores of female patients (−2.23; SD 0.91) were significantly lower than the T scores of investigated male patients (−1.75; SD 9.13, p = 0.045, t test). The same was true for BMDct, but without statistical significance (female 198.8 mg/cm3; SD 33.9, male 205.3 mg/cm3, SD 53.2, p = 0.137, t test).
Control group subjects had a mean age of 37.4 years (SD 8.4). Measured mean BMDct of both femoral heads was 282.7 mg/cm3 (SD 36.7). There was a statistically significant difference between age (p < 0.001, t test), and BMDct (p < 0.001, t test) when data of the control group were compared to the study group. Comparison of the left and right femoral heads in the control group revealed a statistically significant correlation (p = 0.776, p < 0.001).
To investigate inter- and intraobserver reliabilities, ICC and confidence intervals were calculated for a subgroup of 20 study group patients. Mean BMDct of each observer was calculated for both measurement occasions (Table 1). There was neither a statistically significant difference between the three observers nor between the measurements of each single observer (p > 0.05). Inter- and intraobserver reliability proved to be very high (Table 2). All ICC values were >0.99 in all investigated cases (Table 2). For further illustration of inter- and intraobserver reliability Bland–Altman diagrams shows the distribution of measurement differences (Fig. 4).
All investigated subjects were then subdivided into two groups: those with a normal DXA T score and those with an osteopenic or osteoporotic bone status (Fig. 5). Mean BMDct and mean age was calculated for the three above-described groups (Table 3).
Discussion
We investigated the feasibility of a new method to determine local bone quality at the contralateral and uninjured femoral head from preoperative CT scans in patients with a proximal femur fracture. Obtained data were compared to DXA scans. Especially bone quality in the femoral head is thought to be crucial for implant anchorage as this is the region where the threads of the screw or the blade wings are placed. Preoperatively, the information on local bone quality is important for the surgeon, as appropriate implant material can be chosen in advance. We assume that the unfractured hip is a good estimate for the fractured side concerning bone quality, as there was a significant correlation for measurements in the left and right femoral head. Also other authors described a high correlation between BMD of both proximal femurs. Thus, it is suspected that the BMD of the contralateral femoral head can be used to estimate the BMD of the contralateral hip. However, hip osteoarthritis or immobilization may lead to outlining results in few cases [31, 32]. The proposed method proved to be easy to accomplish and very comparable for different measurements in the same patient as well as between different observers. Comparison with DXA, the diagnostic gold standard for osteoporosis also showed very good correlations with the proposed method.
However, there were differences between BMD determined by CT scans and the ones obtained from DXA measurements. This is thought to be due to the fact that DXA is an areal, two-dimensional method where cortical and cancellous bone are superimposed on the final image. Implant anchorage at the femoral head, however, is mainly dependent on cancellous bone quality [2]. Therefore, the proposed method might give more accurate information on the local bone situation than the mere evaluation of a T score by DXA scans, as cortical and trabecular compartments contribute differentially to bone strength and resistance to fracture [17]. It was also shown that areal BMD (aBMD) as investigated by DXA is highly influenced by bone size. Thus, larger bones display higher aBMD values, although vBMD (volumetric BMD as obtained by CT scans) proves to be identical [17, 27]. Using vBMD the confounding factor of the femoral head size could be reduced. CT scans furthermore provide a more accurate assessment of the fracture situation. BMD measurements in the uninjured femoral head can be a valuable estimate for cancellous bone quality at the particular localization of implant anchorage. We consider preoperative CT scans a feasible investigation technique as only little manipulation is necessary to place the patient on the examination table. Furthermore, we assume that the amount of radiation is negligible with the majority of our patients being older than 70 years. Thus, the risk–benefit profile for elderly patients is assumed to be very good. In a biomechanical investigation, measurements of bone quality with CT scans could predict failure load variances even more accurately than DXA measurements [3]. An advantage of CT measurements is that its enables one to evaluate trabecular compartments in selected volumes of interest [9].
The limitation of the study is the fact that measurements could only be conducted at the contralateral and uninjured hip. Measurements at the fractured hip were not feasible, as axis alignment was too difficult due to the fracture. Thus, the real bone quality at the fracture site cannot be specified. However, we assume that values between left and right are comparable and thus a conclusion can be drawn for the injured hip.
The information obtained by preoperative CT scans could facilitate preoperative planning for additional therapeutic measures, such as the use of implants specially designed for osteoporotic bone [30], implant cement augmentation or primary prosthetic surgery. Bone cement augmentation of the PFNa has proven to be a valuable option for enhanced implant anchorage in biomechanical studies as well as in clinical trials [5, 10, 23, 24]. However, the indication for implant augmentation is not yet clear. Biomechanically, bone cement augmentation of femoral heads with a high BMD is hardly to achieve and there is no benefit in mechanical stability of the implant [5].
Clinically, several methods have been proposed to preoperatively investigate bone quality. Although promising good results, sonographical methods such as speed of sound or broadband ultrasonic attenuation measurement are still in an experimental stage and not feasible to obtain local values for the proximal femur [7]. Furthermore, ultrasound techniques are highly dependent on the examiners experience. BMD measurements with CT-osteodensitometry, however, showed to be very reproducible with little variation between observers [22]. Direct mechanical measurement of the break away torque of cancellous bone at the femoral head would be an intraoperative option to determine local bone quality. This tool, however, cannot help in the preoperative decision concerning the surgical method. Furthermore, it is only available for the dynamic hip screw (DHS) [28]. The gold standard for BMD measurement still is dual energy X-ray absorptiometry. Preoperatively, conduction of this method is fairly imaginable due to the patients’ expected pain and the impossibility of exact positioning on the investigation table. Furthermore, DXA is only available in specialized hospitals and slots are usually rare. On the contrary, CT scans are widely available and three-dimensional reconstructions allow for informative image quality not dependent on patient positioning.
To use the proposed method, a cutoff value for BMD would be necessary. The investigated population, however, was too small to set a clear value under which additional measures such as bone cement augmentation should be performed. Figure 5 clearly shows that almost all investigated osteoporotic patients had a BMDct lower than 200 mg/cm3 at the femoral head and mean BMDct was even lower as shown in Fig. 5. None of the control group patients had a BMDct that low. For the determination of definite cutoff values, however, a larger population has to be investigated.
Conclusion
In this study, we propose an easy method for fast and easy preoperative estimation of bone mineral density at the contralateral femoral head in patients with hip fractures. CT measurements can be performed right before surgery without any difficulties and give an estimation on the local cancellous bone quality in the femoral head. The method is reproducible and shows a good correlation with the gold standard (DXA). Furthermore, it could be easily implemented in computerized software. Our method is meant to be an additional tool to facilitate the decision towards further therapeutic options such as the use of (more expensive) implants especially designed for osteoporotic bone, cement augmentation or initial prosthetic surgery in patients with hip fractures. Hence, implant-related complications could be diminished and surgical care of each individual patient could be adapted to the local bone quality for osteoporotic hip fracture patients.
References
Baumgaertner MR, Curtin SL, Lindskog DM, Keggi JM (1995) The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Joint Surg Am 77(7):1058–1064
Bonnaire F, Zenker H, Lill C, Weber AT, Linke B (2005) Treatment strategies for proximal femur fractures in osteoporotic patients. Osteoporos Int 16(Suppl 2):S93–S102
Bousson V, Le Bras A, Roqueplan F et al (2006) Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int 17(6):855–864
de Klerk G, van der Velde D, van der Palen J, van Bergeijk L, Hegeman JH (2009) The usefulness of dual energy X-ray and laser absorptiometry of the calcaneus versus dual energy X-ray absorptiometry of hip and spine in diagnosing manifest osteoporosis. Arch Orthop Trauma Surg 129(2):251–257
Erhart S, Schmoelz W, Blauth M, Lenich A (2011) Biomechanical effect of bone cement augmentation on rotational stability and pull-out strength of the Proximal Femur Nail Antirotation. Injury 42(11):1322–1327
Fogagnolo F, Kfuri M Jr, Paccola CA (2004) Intramedullary fixation of pertrochanteric hip fractures with the short AO-ASIF proximal femoral nail. Arch Orthop Trauma Surg 124(1):31–37
Haiat G, Padilla F, Barkmann R et al (2005) Optimal prediction of bone mineral density with ultrasonic measurements in excised human femur. Calcif Tissue Int 77(3):186–192
Hauschild O, Ghanem N, Oberst M et al (2009) Evaluation of Singh index for assessment of osteoporosis using digital radiography. Eur J Radiol 71(1):152–158
Huber MB, Carballido-Gamio J, Bauer JS et al (2008) Proximal femur specimens: automated 3D trabecular bone mineral density analysis at multidetector CT—correlation with biomechanical strength measurement. Radiology 247(2):472–481
Kammerlander C, Gebhard F, Meier C et al (2011) Standardised cement augmentation of the PFNA using a perforated blade: a new technique and preliminary clinical results. A prospective multicentre trial. Injury 42:1484–1490
Khoo BC, Brown K, Cann C et al (2009) Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 20(9):1539–1545
Koot VC, Kesselaer SM, Clevers GJ, de Hooge P, Weits T, van der Werken C (1996) Evaluation of the Singh index for measuring osteoporosis. J Bone Joint Surg Br 78(5):831–834
Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS (2011) Predicting failure after surgical fixation of proximal humerus fractures. Injury 42(11):1283–1288
Krappinger D, Roth T, Gschwentner M et al (2011) Preoperative assessment of the cancellous bone mineral density of the proximal humerus using CT data. Skeletal Radiol 41:299–304
Lenich A, Mayr E, Ruter A, Mockl C, Fuchtmeier B (2006) First results with the trochanter fixation nail (TFN): a report on 120 cases. Arch Orthop Trauma Surg 126(10):706–712
Lips P (1997) Epidemiology and predictors of fractures associated with osteoporosis. Am J Med 103(2A):3S–8S (discussion 8S–11S)
Liu XS, Cohen A, Shane E et al (2010) Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25(10):2229–2238
Lotz JC, Gerhart TN, Hayes WC (1990) Mechanical properties of trabecular bone from the proximal femur: a quantitative CT study. J Comput Assist Tomogr 14(1):107–114
Mereddy P, Kamath S, Ramakrishnan M, Malik H, Donnachie N (2009) The AO/ASIF proximal femoral nail antirotation (PFNA): a new design for the treatment of unstable proximal femoral fractures. Injury 40(4):428–432
O’Neill F, Condon F, McGloughlin T, Lenehan B, Coffey JC, Walsh M (2011) Dynamic hip screw versus DHS blade: a biomechanical comparison of the fixation achieved by each implant in bone. J Bone Joint Surg Br 93(5):616–621
Ramamurthi K, Ahmad O, Engelke K et al (2012) An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos Int 23(2):543–551
Reilly K, Munro J, Pandit S, Kress A, Walker C, Pitto RP (2007) Inter-observer validation study of quantitative CT-osteodensitometry in total knee arthroplasty. Arch Orthop Trauma Surg 127(8):729–731
Sermon A, Boner V, Boger A et al (2011) Potential of polymethylmethacrylate cement-augmented helical proximal femoral nail antirotation blades to improve implant stability—a biomechanical investigation in human cadaveric femoral heads. J Trauma Acute Care Surg 72:E54–E59
Sermon A, Boner V, Schwieger K et al (2011) Biomechanical evaluation of bone-cement augmented Proximal Femoral Nail Antirotation blades in a polyurethane foam model with low density. Clin Biomech (Bristol, Avon) 27:71–76
Simmermacher RK, Bosch AM, Van der Werken C (1999) The AO/ASIF-proximal femoral nail (PFN): a new device for the treatment of unstable proximal femoral fractures. Injury 30(5):327–332
Simmermacher RK, Ljungqvist J, Bail H et al (2008) The new proximal femoral nail antirotation (PFNA) in daily practice: results of a multicentre clinical study. Injury 39(8):932–939
Srinivasan B, Kopperdahl DL, Amin S et al (2012) Relationship of femoral neck areal bone mineral density to volumetric bone mineral density, bone size, and femoral strength in men and women. Osteoporos Int 23(1):155–162
Suhm N, Haenni M, Schwyn R, Hirschmann M, Muller AM (2008) Quantification of bone strength by intraoperative torque measurement: a technical note. Arch Orthop Trauma Surg 128(6):613–620
Wainwright SA, Marshall LM, Ensrud KE et al (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90(5):2787–2793
Windolf M, Braunstein V, Dutoit C, Schwieger K (2009) Is a helical shaped implant a superior alternative to the Dynamic Hip Screw for unstable femoral neck fractures? A biomechanical investigation. Clin Biomech (Bristol, Avon) 24(1):59–64
Xu H, Gong J, Chen JX, Zhang TM, Wu QL (2007) Bilateral femoral bone mineral density measurements in Chinese women and men. J Clin Densitom 10(2):165–169
Yang RS, Chieng PU, Tsai KS, Liu TK (1996) Symmetry of bone mineral density in the hips is not affected by age. Nucl Med Commun 17(8):711–716
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Erhart, M. Zegg and T. Roth: None. F. Kralinger and C. Kammerlander: Educational activities for DePuy Synthes.
Rights and permissions
About this article
Cite this article
Erhart, S., Zegg, M., Kralinger, F. et al. Fast and easy preoperative estimation of cancellous bone mineral density in patients with proximal femur fractures. Arch Orthop Trauma Surg 135, 1683–1689 (2015). https://doi.org/10.1007/s00402-015-2340-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2340-5