Abstract
Introduction
The risk for late periprosthetic femoral fractures is higher in patients treated for a neck of femur fracture compared to osteoarthritis. It has been hypothesised that osteopaenia and consequent decreased stiffness of the proximal femur are responsible for this. We investigated whether a femoral component with a bigger body would increase the torque to failure in a biaxially loaded composite Sawbone model.
Materials and methods
A biomechanical bone analogue was used. Two different body sizes (Exeter 44-1 versus 44-4) of a polished tapered cemented femoral stem were implanted by an experienced surgeon in seven bone analogues each and internally rotated at 40°/s until failure. Torque to fracture and fracture energy were measured using a biaxial materials testing device (Instron 8874, MI, USA). The data were non-parametric and therefore tested with the Mann–Whitney U test.
Results
The median torque to fracture was 156.7 Nm (IQR 19.7) for the 44-1 stem and 237.1 Nm (IQR 52.9) for the 44-4 stem (p = 0.001). The median fracture energy was 8.5 J (IQR 7.3) for the 44-1 stem and 19.5 J (IQR 8.8) for the 44-4 stem (p = 0.014).
Conclusion
The use of large body polished tapered cemented stems for neck of femur fractures increases the torque to failure in a biomechanical model and therefore is likely to reduce late periprosthetic fracture risk in this vulnerable cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing age of people in Western society is unfortunately accompanied with an increasing incidence of displaced neck of femur fractures (NOF). Modern medical science has spent many resources in studying the treatment options for these fractures. There is more and more evidence that cemented femoral components outperform uncemented components in patients with a neck of femur fracture, with less pain, less complication rates, better functionality and with the same or even lower mortality rates [1–5]. However, some complications are still associated with this treatment option. There is an increased risk of late periprosthetic femoral fracture in patients undergoing surgery for a fractured neck of femur for cemented and uncemented femoral components compared to patients who receive the same components electively for hip arthritis [6–8]. The literature on the true prevalence of periprosthetic fractures of cemented and uncemented stems is difficult to interpret because of heterogeneity in the patient population and different end points in the cohorts, but has been reported to be up to 3.5 % at 10 years [9–11].
Patient suffering from an NOF often have capacious canals and osteopaenic bone [12, 13]. We believe this results in a reduction of torsional stiffness in the proximal femur that makes it susceptible to torsional loads [14].
The torsional mode of failure matches the findings of Lindahl et al. and Young et al. [15, 16], who showed that 82–90 % of periprosthetic proximal femur fractures were of the Vancouver B1 and B2 type [17] that are associated with a spiral fracture pattern.
We hypothesise that increasing the stiffness of the proximal femur with a larger body-cemented implant will increase both the torsional load to failure and total energy to fracture.
Materials and methods
Ethics approval was not required for this bone analogue study. Power analysis using figures obtained for the 44 mm offset stem in a previous Sawbones study [18] with an expected difference of 30 Nm, SD of 18, power of 80 % and significance level of 5 % indicated that a minimum of seven femurs were required in each group.
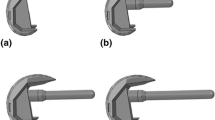
Fourteen bone analogues, divided into two groups of seven (Sawbones medium left femur model 3403; Pacific Research Laboratories, Vashon WA USA) were prepared for implantation of an Exeter cemented femoral component (Stryker Orthopedics, Mahwah, NJ). Two femoral stems were used, the Exeter 44 no. 1 stem (Stainless steel 165 gram, standard proximal body) and the Exeter 44 no. 4 stem (Stainless steel 215 gram, large proximal body) (Fig. 1). All implant constructs were prepared by an experienced orthopaedic surgeon (BG). A standard femoral neck cut was made approximately 1 cm proximal to the lesser trochanter, using a premade cutting guide to standardise the neck cuts. The bone analogues were broached using the appropriate broaching rasp for the stem. Broaching was performed to allow the stem to seat with the middle marking at the level of the neck cut (Fig. 2).
Example of how a stem is fitted in the composite sawbone. The three markings on the stem can be used to determine the position of the stem. Reprinted from Morishima et al. [18]. Copyright (2014), with permission from Elsevier
A distal cement plug (Stryker 13–17 mm Artisan plug, Stryker Orthopedics, Mahwah, NJ) was introduced to sit just distal to the tip of the implant. The implant was then cemented with the use of a stem centraliser into the Sawbone using two mixes of Simplex cement (Stryker Orthopedics, Mahwah, NJ). The distal femoral condyle was resected 40 mm into the supracondylar region to allow the femur to fit within the testing mechanism.
The proximal femur was attached at the centre of rotation of the implant head by means of a hydraulic clamp (Fig. 3). The centre of the femoral head and the intercondylar notch (located by the longitudinal hole in the bone analogue) were positioned in the vertical loading axis of the machine (Instron 8874) (Fig. 4). Distally, the femur was fixed with Paladur dental acrylic (Heraeus-Kulzer GmbH, Wertheim, Germany), prepared as per the manufacturer’s instructions.
The femoral head and intercondylar notch are located in the vertical loading axis of the machine to replicate the natural loading axis of the femur. The proximal femur is attached at the centre of rotation of the implant head by means of a hydraulic clamp. Reprinted from Morishima et al. [18]. Copyright (2014), with permission from Elsevier
The testing mechanism. The composite femur and stem are fitted in the Instron. Reprinted from Morishima et al. [18]. Copyright (2014), with permission from Elsevier
The femurs were tested in a combined compression force with torque to replicate clinically observed fracture patterns in cadaveric and analogue femurs. Clinical fractures are typically spiral in nature with a crack starting angle more than 45° indicating both torque and compressive forces being applied at fracture initiation. In a single leg stance, the resultant force at the hip is approximated by F = 2.5 × BW, where body weight is assumed to be 80 kg giving a compressive force of 2000 N [19]. A preload of 2 Nm in the internal rotation direction and 2 kN of compression were applied. The compressive load was then maintained and the implant internally rotated 40° in 1 s to simulate falling with internal rotation with a single leg stance. The angle of 40° was chosen to ensure that fracture had occurred fully (Fig. 5).
Fracture torque was defined as the maximum torque measured. Fracture energy was calculated by numerically integrating the torque and angle measurements against time. All calculations were made with Matlab 2011(b) (Mathworks, USA) and statistical analysis performed using IBM SPSS for Windows version 21 (IBM Corp, released 2012, Armok, NY). Normality testing indicated that the data were not normally distributed and so non-parametric methods were used for data analysis (Mann–Whitney U test for comparisons). Accordingly, summary statistics are presented as medians and interquartile ranges (IQR). Box plots are also presented. The primary end point was fracture torque, with fracture energy as the secondary end point. The significance level was set at 5 %.
Results
In all samples, the fracture pattern produced was the same as commonly observed in clinical practice. They are consistent with unstable fractures occurring around previously well fixed implants—a Vancouver B-type periprosthetic fracture, with fracture around the previously well-fixed stem resulting in a loose implant [20, 21]. In all of our samples, the fracture propagated from the posterior proximal femur in the calcar and metaphyseal region and spiralled distally into the diaphysis to exit close to the tip of the stem. A varying degree of fracture comminution was produced.
The median torque to fracture was 237.1 Nm (IQR 52.9) for the 44-4 stem, which was statistically significantly higher than the mean torque to fracture for the 44-1 stem of 156.7 Nm (IQR 19.7) (p = 0.001, Mann–Whitney U test) (Fig. 6). The median fracture energy was 19.5 J (IQR 8.8) for the 44-4 stem, statistically significantly higher than for the 44-1 stem of 8.5 J (IQR 7.3) (p = 0.014, Mann–Whitney U test) (Fig. 7).
Discussion
Patients treated with a cemented femoral stem for a fractured neck of femur have an increased risk of periprosthetic fracture when compared with hip arthritis patients. Bone quality in this cohort is often compromised due to osteoporosis and large capacious canals [8, 9]. The stiffness of the proximal femur is reduced and this makes these femurs more susceptible to periprosthetic fractures, as typically there is no corresponding reduction in loads to match the reduction in stiffness. A larger femoral implant can reduce this risk, as this study showed, by increasing the torsional stiffness of the proximal femur.
Fractures in the proximal femur occur when the bone is strained beyond its capacity. In a healthy young adult, bone remodels to increase stiffness under external stimulus such as weight training. In the elderly where the osteogenic potential is much lower, the likelihood of exceeding the strain limit is dramatically increased, as seen in greater fracture risk in this cohort. In structural loading scenarios, such as buildings, stiffer materials are used to prevent failure/fracture, which in a clinical sense translates to implanting a stiffer femoral component. The easiest option to increase the proximal femur stiffness is to use an implant with a larger proximal body.
Selection of the most appropriate femoral implant size is a clinical compromise between stress shielding and avoiding fractures in any patient [22]. The cumulative percent mortality in this patient group is reported to be between 73.8 and 92.0 % for partial hip replacement (58.4 % for conventional total hip replacement) [23]. Increasing the proximal stiffness in these elderly patients and thereby increasing the mean torque to fracture by 1.7 times as shown in this study seem more important than the long-term risk of stress shielding due to an immediate fracture risk reduction and the decreased 10 year life expectancy of this patient cohort.
The femoral shaft should not be broached aggressively in fractured neck of femurs. Aggressive broaching will lead to removal of cancellous bone (which is required for cement digitisation) and to an increased risk of intra-operative fracture. Our study does suggest that surgeons should not undersize the stem relative to the broach that is first stable in the canal or they will increase the risk of periprosthetic fracture.
There are a number of factors that have been shown to influence the fracture behaviour of cemented stems. Erhardt et al. [24] showed that a cemented double-polished tapered stem (CPT Zimmer) when loaded to failure resulted in a significantly different fracture pattern to a triple-tapered stem (C-stem). They showed that the fracture pattern was more around the body of the double-tapered stem. This result is consistent with the fracture pattern seen in our work and that described by Morishima et al. [18].
In one of our previous studies [18], we showed that the length of an implant has an influence on the load to failure. A shorter implant has a lower load to failure compared to a stem with a similar proximal geometry. Morishima showed a decrease in torque of 16 % for the shorter stem compared with the standard length size 1 stem. This difference was hypothesised to be due to a difference in the stiffness of the construct and the energy absorbed by the implant prior to fracture.
Our findings in this current study showed that the body size of the implant significantly affects the load to fracture in stems of the same length. A size 4 (larger bodied) stem of the same length (150 mm) and offset (44 mm) as a size 1 (small bodied) stem showed a 51 % increase in torque to fracture. Increasing the volume of an implant will increase the rigidity of the proximal femur and will influence bone remodelling after implantation as noted by Pepke et al. [25] Their study supports the hypothesis that larger implants will lead to less physiological bone remodelling. In a younger population of patients undergoing primary THR the use of less bulky implants will lead to better long-term bone remodelling. In elderly patients undergoing prosthetic treatment for femoral neck fracture, we believe that the protection against periprosthetic fracture conferred by larger-bodied implants outweighs any concerns about bone remodelling and stress shielding in the long term.
The biomechanical model used in this study controlled the moment and the axial force through a validated Sawbone model. We tested seven Sawbones in each group. Because all composite Sawbones are similarly manufactured and are proven to have identical biomechanical properties, no larger groups are needed. The only variation between the test object is created by the protocolled implantation technique and positioning in the test model. All fractures generated by the model resulted in a consistent Vancouver B2 fracture (unstable fracture around a previously well-fixed implant) type proving that it is an accurate representation of the fracture mechanism [15, 16, 18, 20, 21].
The current study was limited by its design. Firstly, the in vitro biomechanical model used in this experiment has some limitations [26]. It is not an osteoporotic bone model, but the representation of a normal femoral bone in a healthy young male. This model was used because at the time of testing there was no validated bone analogue available for osteoporotic bone, and cadaveric bone was not available. We predict that an osteoporotic model would show a lower fracture energy for both stem sizes, but expect an amplification of the difference between the two due to the now even larger influence of the increased stiffness of the larger body size implant [13]. Secondly in our model, we discarded the influence of the soft tissue envelope. In an in vivo model, several muscle groups would counter the internal torsion force applied in the model. This would potentially influence the direction of force and the fracture pattern. A third limitation is that in the model, the torsional load applied only extends to the distal femur, while in an in vivo model it is unclear how far down the load would extend.
The results of this study indicate that the use of a large body cemented stem could reduce late periprosthetic fracture rates in this frail elderly population. Further research is required to confirm these findings in a cadaveric or osteopaenic bone analogue model.
References
Parker MI, Pryor G, Gurusamy K (2010) Cemented versus uncemented hemiarthroplasty for intracapsular hip fractures: a randomised controlled trial in 400 patients. J Bone Joint Surg Br 92(1):116–122. doi:10.1302/0301-620X.92B1.22753
Luo X, He S, Li Z, Huang D (2012) Systematic review of cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures in older patients. Arch Orthop Trauma Surg 132(4):455–463. doi:10.1007/s00402-011-1436-9
Parker MJ, Gurusamy KS, Azegami S (2010) Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev 6:CD001706. doi:10.1002/14651858.CD001706.pub4
Timperley AJ, Whitehouse SL (2009) Mitigating surgical risk in patients undergoing hip arthroplasty for fractures of the proximal femur. J Bone Joint Surg Br 91(7):851–854
Costain DJ, Whitehouse SL, Pratt NL, Graves SE, Ryan P, Crawford RW (2011) Perioperative mortality after hemiarthroplasty related to fixation method. Acta Orthop 82(3):275–281. doi:10.3109/17453674.2011.584208
Franklin J, Malchau H (2007) Risk factors for periprosthetic femoral fracture. Injury 38(6):655–660. doi:10.1016/j.injury.2007.02.049
Lindahl H, Garellick G, Regner H, Herberts P, Malchau H (2006) Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg [Am] 88(6):1215–1222. doi:10.2106/jbjs.e.00457
Sarvilinna R, Huhtala HS, Sovelius RT, Halonen PJ, Nevalainen JK, Pajamaki KJ (2004) Factors predisposing to periprosthetic fracture after hip arthroplasty: a case (n = 31)-control study. Acta Orthop Scand 75(1):16–20. doi:10.1080/00016470410001708030
Cook RE, Jenkins PJ, Walmsley PJ, Patton JT, Robinson CM (2008) Risk factors for periprosthetic fractures of the hip: a survivorship analysis. Clin Orthop Relat Res 466(7):1652–1656. doi:10.1007/s11999-008-0289-1
Ohly NE, Whitehouse MR, Duncan CP (2014) Periprosthetic femoral fractures in total hip arthroplasty. Hip Int 24(6):556–567. doi:10.5301/hipint.5000155
Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR (2011) The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg [Br] 93(1):96–101. doi:10.1302/0301-620X.93B1.25087
Neander G, Adolphson P, Hedstrom M, von Sivers K, Dahlborn M, Dalen N (1997) Decrease in bone mineral density and muscle mass after femoral neck fracture. A quantitative computed tomography study in 25 patients. Acta Orthop Scand 68(5):451–455
Neander G, Adolphson P, von Sivers K, Dahlborn M, Dalen N (1997) Bone and muscle mass after femoral neck fracture. A controlled quantitative computed tomography study of osteosynthesis versus primary total hip arthroplasty. Arch Orthop Trauma Surg 116(8):470–474
Lill CA, Fluegel AK, Schneider E (2000) Sheep model for fracture treatment in osteoporotic bone: a pilot study about different induction regimens. J Orthop Trauma 14(8):559–565 (discussion 565–556)
Young SW, Pandit S, Munro JT, Pitto RP (2007) Periprosthetic femoral fractures after total hip arthroplasty. ANZ J Surg 77(6):424–428. doi:10.1111/j.1445-2197.2007.04087.x
Lindahl H, Malchau H, Herberts P, Garellick G (2005) Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplasty 20(7):857–865. doi:10.1016/j.arth.2005.02.001
Masri BA, Meek RM, Duncan CP (2004) Periprosthetic fractures evaluation and treatment. Clin Orthop Relat Res 420:80–95. doi:10.1097/00003086-200403000-00012
Morishima T, Ginsel BL, Choy GG, Wilson LJ, Whitehouse SL, Crawford RW (2014) Periprosthetic fracture torque for short versus standard cemented hip stems: an experimental in vitro study. J Arthroplasty 29(5):1067–1071. doi:10.1016/j.arth.2013.10.016
Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN (2001) Hip contact forces and gait patterns from routine activities. J Biomech 34(7):859–871
Brew CJ, Wilson LJ, Whitehouse SL, Hubble MJ, Crawford RW (2013) Cement-in-cement revision for selected Vancouver type B1 femoral periprosthetic fractures: a biomechanical analysis. J Arthroplasty 28(3):521–525. doi:10.1016/j.arth.2012.08.016
Crawford RW, Whitehouse SL, Brew CJ, Wilson LJ, Hubble MJ (2013) Author reply: cement-in-cement revision for selected Vancouver type b1 femoral periprosthetic fractures: a biomechanical analysis. J Arthroplasty 28(8):1446–1447. doi:10.1016/j.arth.2013.05.021
Huiskes R, Weinans H, van Rietbergen B (1992) The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res 274:124–134
AOA (2013) Australian Orthopaedic Association national joint replacement registry Annual report, vol 13. Adelaide
Erhardt JB, Khoo PP, Stoffel KK, Yates PJ (2013) Periprosthetic fractures around polished collarless cemented stems: the effect of stem design on fracture pattern. Hip Int 23(5):459–464. doi:10.5301/hipint.5000052
Pepke W, Nadorf J, Ewerbeck V, Streit MR, Kinkel S, Gotterbarm T, Maier MW, Kretzer JP (2014) Primary stability of the Fitmore stem: biomechanical comparison. Int Orthop 38(3):483–488. doi:10.1007/s00264-013-2138-4
Topp T, Muller T, Huss S, Kann PH, Weihe E, Ruchholtz S, Zettl RP (2012) Embalmed and fresh frozen human bones in orthopedic cadaveric studies: which bone is authentic and feasible? Acta Orthop 83(5):543–547. doi:10.3109/17453674.2012.727079
Acknowledgments
Implants, implanting tools, cement and cementing tools for this project were provided by Stryker Australia. The authors maintain full control of all data and agree to allow the journal to review this data if requested. An Exeter 44-4 stem was made available by Stryker Inc. for this study; no other form of support was received. The study was funded by the Queensland University of Technology.
Conflict of interest
One author (RWC) receives royalties from Stryker Corporation in relation to products investigated in this paper, and Stryker partially supports three positions via external institutions (BLG, LJW and SLW).
Ethical standard
As this is a Sawbones study, ethics approval was not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ginsel, B.L., Morishima, T., Wilson, L.J. et al. Can larger-bodied cemented femoral components reduce periprosthetic fractures? A biomechanical study. Arch Orthop Trauma Surg 135, 517–522 (2015). https://doi.org/10.1007/s00402-015-2172-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2172-3