Abstract
Neuronal loss in numerous neurodegenerative disorders has been linked to protein aggregation and oxidative stress. Emerging data regarding overlapping proteinopathy in traditionally distinct neurodegenerative diseases suggest that disease-modifying treatments targeting these pathological features may exhibit efficacy across multiple disorders. Here, we describe proteinopathy distinct from classic synucleinopathy, predominantly comprised of the anti-oxidant enzyme superoxide dismutase-1 (SOD1), in the Parkinson’s disease brain. Significant expression of this pathology closely reflected the regional pattern of neuronal loss. The protein composition and non-amyloid macrostructure of these novel aggregates closely resembles that of neurotoxic SOD1 deposits in SOD1-associated familial amyotrophic lateral sclerosis (fALS). Consistent with the hypothesis that deposition of protein aggregates in neurodegenerative disorders reflects upstream dysfunction, we demonstrated that SOD1 in the Parkinson’s disease brain exhibits evidence of misfolding and metal deficiency, similar to that seen in mutant SOD1 in fALS. Our data suggest common mechanisms of toxic SOD1 aggregation in both disorders and a potential role for SOD1 dysfunction in neuronal loss in the Parkinson’s disease brain. This shared restricted proteinopathy highlights the potential translation of therapeutic approaches targeting SOD1 toxicity, already in clinical trials for ALS, into disease-modifying treatments for Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormal protein aggregation constitutes a central pathophysiology within the degenerative cascades of multiple neurodegenerative disorders, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). These disorders are conventionally categorized based upon a single prominent proteinopathy, although more recent research demonstrates a substantial overlap between the pathological proteomes of several of these disorders [15, 30, 34, 44], suggesting that current disease-modifying treatments may exhibit efficacy across multiple disorders.
Deposition of α-synuclein protein is one of the two primary features required for a confirmed pathological diagnosis of Parkinson’s disease [7], the other being the death of dopaminergic neurons within the substantia nigra (SN), the loss of which underlies the development of movement dysfunction [22]. While the aggregation of α-synuclein no doubt plays an important role in disease pathology, the disparity between the widespread deposition of α-synuclein and relatively selective pattern of neuronal loss within Parkinson’s disease brains [7, 8] suggests that other factors also underlie neuronal vulnerability in this disorder. This hypothesis is supported by the disappointing results of clinical trials targeting potential pathways of α-synuclein toxicity [19, 59, 60]. Identification of additional neurotoxic mechanisms that align more closely with nigrostriatal degeneration may reveal novel targets for the development of vulnerability-specific neuroprotective therapies. Two components of the Parkinson’s disease degenerative cascade are oxidative stress [6] and a regional deficiency in copper [18], both of which occur specifically within vulnerable regions of the Parkinson’s disease brain.
Alterations in the anti-oxidant enzyme superoxide dismutase 1 (SOD1) underlie around 20% of familial (f)ALS cases [1], where mutations to the SOD1 gene result in functional [57] and/or structural defects [21], including misfolding and loss of copper binding capacity [33]. Wild-type SOD1 protein is highly oxidized in a proportion of sporadic (s)ALS patients [27], conferring molecular characteristics similar to that observed in mutant SOD1. Despite this data, the role of atypical wild-type SOD1 in neurodegeneration in sALS is a matter of debate [2, 10, 16, 37].
Here, we report a pathological SOD1 fingerprint restricted to regions of neuronal loss in the Parkinson’s disease brain. Similarities between our findings and those described for SOD1 in ALS suggests shared mechanisms of deposition and toxicity. Therapies targeting SOD1 aggregation in murine models of ALS have resulted in substantial improvements in motor function and survival time, prompting their progression into human clinical trials [46] (ClinicalTrials.gov Identifier: NCT02870634). One such compound, diacetyl-bis (4-ethylthiosemicarbazonato) copperII (CuII[atsm]), also improves dopamine neuron survival in multiple animal models of Parkinson’s disease [32]. We argue that such an approach may also be beneficial in Parkinson’s disease by modulating a novel pathway in degenerating brain regions in this disorder that we describe here for the first time.
Materials and methods
Human post-mortem tissues
Seven-micron paraffin-embedded formalin-fixed post-mortem human tissue sections from idiopathic Parkinson’s disease (n = 5–6) and age-matched control (n = 6–9) substantia nigra pars compacta (SNc), anterior cingulate cortex (ACC), fusiform cortex (FUS), entorhinal cortex (EC), locus coeruleus (LC), and occipital cortex (OCx) were sourced from the New South Wales Brain Banks, Australia. Brain tissue sections from corresponding regions of incidental Lewy body disease (ILBD; n = 2) and SOD1-associated fALS cases (n = 3) cases were sourced from the King’s College Brain Bank, London, UK. Furthermore, tissue sections of post-mortem spinal cord from the same fALS cases, as well as matching spinal cord and LC from three age-matched control cases, were also sourced from the King’s College Brain Bank, London, UK. Fresh frozen brain tissue (229–593 mg) from the SNc, ACC and OCx of seven cases of Parkinson’s disease and eight age-matched control cases was obtained from the New South Wales Brain Banks, Australia for isoelectric focusing and immunoblot experiments.
Neuropathological diagnosis of Parkinson’s disease was made according to published criteria [22] and next-of-kin consent obtained. All cases were free of other neurological or neuropathological conditions, including the motor neuron diseases. None of the Parkinson’s disease cases were early onset or had a family history of any movement disorder, including the motor neuron diseases. Both cases of ILBD were free of neurological and psychiatric diseases, and were diagnosed pathologically according to the presence of Lewy bodies in the midbrain and pons, in the absence of substantial neuronal loss. ALS cases (familial) were pathologically diagnosed by substantial neuronal loss in the anterior horn of the spinal cord. SOD1-associated fALS cases were identified through SOD1 genotyping. Normal control cases were free of any clinically diagnosed neurological disorders and neuropathological abnormalities. Ethics approval was obtained from the University of Sydney Human Research Ethics Committee (approval number 2015/202). Demographic and clinical information for all cases are detailed in Supplementary Tables 1 and 2.
Immunohistochemistry
Fixed tissue sections used for fluoresence microscopy were dewaxed, and antigen retrieval was performed [99% formic acid (Sigma), 3 min]. Sections were then rinsed with PBS and washed with 50% ethanol and PBS. After blocking with casein (0.5% in PBS, 1.5 h), sections were incubated with appropriate primary antibodies (Supplementary Table 3) diluted in blocking solution overnight at 4 °C. Following PBS washes, sections were incubated with appropriate non-spectrally overlapping fluorescently labelled secondary antibodies (Alexa-Fluor 594, 488, or 405 nm conjugated anti-host) for 3 h at room temperature and washed with PBS. Sections were then incubated with Sudan Black (Sigma), Congo Red (Sigma), or Thioflavin-S (Sigma), washed, and coverslipped (Supplementary Table 4). To validate the absence of spectral overlap between secondary fluorescent antibodies, tissues were labelled individually with a single secondary-conjugated fluorophore and images captured using sequential acquisition. All three secondary conjugates exhibited no fluorescence in any channel other than that being observed, indicating an absence of fluorophore cross-contamination across channels (Supplementary Fig. 1). The specificity of primary antibodies for SOD1 and α-synuclein was determined through the inclusion of multiple primary antibodies against each protein, each with distinct class, host-isotype, species reactivity, and immunogen properties (Supplementary Table 3). The specificity of the principal SOD1 primary antibody (Merck Millipore) was confirmed by immunoelectrophoresis against human red blood cell lysate and confirmed by immunodiffusion against a human red blood cell lysate and a known anti-SOD (Cu/Zn) antibody. Images were collected under high magnification (40–100×) with oil immersion using a Zeiss LSM 710 Confocal Laser Scanning/Multi-photon Microscope (Carl Zeiss Inc., Oberkochen, Germany) and ZEN 2010 software (Carl Zeiss Inc), and were viewed and analyzed using Fiji software (National Institute of Health, Bethesda, MD, USA). During acquisition of double- or triple-labelled fluorescent sections, a sequential protocol was used where each fluorophore was excited and the corresponding channel was individually acquired, before moving onto the next fluorophore and channel, to prevent any spectral overlap between channels.
Fixed tissue sections used for 3,3′-diaminobenzidine (DAB) staining were deparaffinated and antigen retrieved as above, and were then incubated with 3% H2O2 (Fronine, Australia) in 50% ethanol to quench non-specific peroxidase activity. Sections were then blocked and incubated with appropriate primary antibodies as above. Primary antibodies were detected using biotinylated IgG secondary antibodies Vector Laboratories, Burlingame, CA) (1:200), followed by Vector Elite Kit tertiary antibody complex (Vector Laboratories, Burlingame, CA) (1:100), and visualized using DAB (Sigma). Sections were finally dehydrated and mounted with DPX, and visualized using an Olympus VS 120 Slide Scanner (Olympus-life science, Shinjuku, Tokyo, Japan) and Fiji software (National Institute of Health) [58].
Proteinopathy and neuronal quantifications and correlations
For quantification of proteinopathies of interest and neurons, one of four primary SOD1 antibodies (Merck Millipore, Supplementary Table 3) and one of two primary α-synuclein antibodies (BD transductions, Supplementary Table 3) were employed, based on superior stain specificity, signal intensity, and consistency of staining throughout the method optimisation process. We quantified Lewy body, Lewy neurite, SOD1 aggregate, and neuronal densities in 5–6 Parkinson’s disease cases and 6–9 controls in regions known to exhibit synucleinopathy and progressive neuronal loss (SNc and LC), synucleinopathy with no neuronal loss (ACC, EC, and FUS), and neither synucleinopathy nor neuronal loss (OCx). Corresponding regions of the ILBD cases (n = 2), a rare disorder believed to represent pre-clinical Parkinson’s disease [22], were included to indicate whether SOD1 aggregation represents an early pathological change in Parkinson’s disease. Corresponding regions of the fALS brain were investigated to determine if SOD1 aggregation and neuronal loss were present in these same regions, in addition to the well-documented pathology reported in the fALS spinal cord. Twelve to twenty-one images were obtained at 20× magnification, in randomised areas of the neuroanatomical region of interest using the Zeiss LSM 710 Confocal Laser Scanning/Multi-photon Microscope (Carl Zeiss Inc.) and ZEN software (Carl Zeiss Inc.). Aggregates immunopositive for SOD1, but not α-synuclein, were manually counted in each quadrant of each image using the ImageJ software (National Institute of Health, Bethesda, MD, USA). The primary investigator was blinded to the case identities; each case was assigned a randomised number by a secondary investigator. This procedure was repeated to calculate α-synuclein immunopositive Lewy body and Lewy neurite densities, as well as neuronal densities, in each quadrant of each image. The image scale within ZEN software was applied to images in the ImageJ software to enable calculation of aggregate and neuron density per mm2.
Neither neuron nor SOD1 aggregate, Lewy body, or Lewy neurite densities were significantly correlated with post-mortem delay or age in any brain region of the Parkinson’s disease or control groups. These variables were also not correlated with disease duration, although all Parkinson’s disease cases investigated died at similar disease durations and disease severity, as indicated by Braak staging (Supplementary Table 1). To account for natural variations in neuronal density between brain regions, an index of neuronal loss was calculated for each Parkinson’s disease case/brain region, by normalizing neuronal density for each region/case to the mean neuronal density of the same brain region for the healthy control group. Indexed neuronal loss data were used to explore correlations with each proteinopathy across all Parkinson’s disease brain regions. Sample sizes for each brain region for each diagnostic group are shown in Supplementary Table 5.
Non-denaturing isoelectric focusing
Protein extraction was performed by homogenisation of human brain tissue samples (120–288 mg) in ten volumes of buffer solution (20 mM Tris-hydrochloride, pH 7.4, 1 mM phenylmethanesulfonyl fluoride, and 1 mM benzamidine) at 4 °C, using a hand-held electric homogeniser with a polycarbonate probe (OmniTH, Kelly Scientific, New South Wales, Australia). DNAse I (Sigma) and RNAse A (Sigma) were each added to a final concentration of 10 μg mL−1, with an incubation for 1 min at 4 °C. Each sample was then centrifuged at 50,000×g for 1 h at 4 °C. The supernatant was collected and stored at −80 °C until further analysis. BCA Protein Assay Kit (Thermo Scientific Pierce) was used to determine sample protein concentrations, which lay in the range 1.9–2.9 mg mL−1.
Triplicate immobilized pH gradient strips (pH 4.7–5.9, 17 cm; ReadyStrip™ IPG Strip, Bio-Rad) were rehydrated overnight at 10 °C with 300 μL human brain protein extract (neat; 570–870 μg total protein). The backing of the strips was covered in mineral oil (Bio-Rad) to prevent drying. Isoelectric focusing was performed as previously described [12] under non-denaturing conditions to preserve SOD1 metal status. SOD1 was detected on IPG strips using nitroblue tetrazolium as previously described [12] and the pI value of each sample determined.
SOD1 protein levels and specific activity
Initial immunohistochemical investigations revealed that most SOD1 protein in the SNc was localized within tyrosine hydroxylase-immunopositive dopaminergic neurons (Supplementary Fig. 2). SOD1 protein levels and SOD1-specific activity levels in the Parkinson’s disease and control SNc, previously reported by our group [18], were thus normalized to β-actin-corrected tyrosine hydroxylase protein levels in these same cases to adjust for dopamine neuron loss in the Parkinson’s disease SNc.
Copper chaperone for SOD1 (CCS) immunoblots
Proteins were extracted via homogenisation in 17 volumes of buffer solution (10 mM Tris-hydrochloride, 0.25 M sucrose, and 1 mM EDTA, pH 7.4; all from Sigma) using a hand-held electric homogeniser with a polycarbonate probe (OmniTH, Kelly Scientific, New South Wales, Australia). 1% SDS and 1:50 dilution of protease inhibitor cocktail (Sigma) were then added to homogenates, and the supernatant collected via centrifugation (10,000×g for 15 min at 4 °C, Eppendorf, Hamburg, Germany). BCA Protein Assay Kit (Thermo Scientific Pierce) was used to determine sample protein concentration.
20 μg protein was loaded onto 4–12% Bis–Tris Criterion pre-cast gels (Bio-Rad, Hercules, CA) and separated by SDS-PAGE in MOPS buffer (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). Separated proteins were transferred to Immobilon-PSQ PVDF (Millipore, Billerica, MA) membranes. Following transfer, membranes were blocked in 5% skim milk (Bio-Rad, Hercules, CA) in PBS containing 0.1% Tween® 20 (Sigma, St. Louis, MO), and incubated with primary antibody against CCS (1:250, Santa Cruz sc-20141) diluted in PBS containing 0.1% Tween® 20 (Sigma, St. Louis, MO) and 1% skim milk (Bio-Rad, Hercules, CA) for 2 h at room temperature. Following incubation with appropriate horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000), protein signals were obtained using an ECL western blotting detection system (Bio-Rad, Hercules, CA) as per the manufacturer’s instructions, and developed using the Chemi-Doc XRS (Bio-Rad, Hercules, CA). CCS signal intensities were quantified by densitometry using Quantity One® software (Version 4.6.3, Bio-Rad, Hercules, CA) and normalized to either β-actin (1:10,000, ACC and OCx) or β-actin-adjusted tyrosine hydroxylase (SNc only) protein levels. Tyrosine hydroxylase was again used to control for dopaminergic neuron loss in the SNc, as CCS protein was also determined to exist primarily inside dopaminergic neurons within the SNc (Supplementary Fig. 2). An internal standard (a single human brain sample) was run on every western blot to allow for between-blot comparisons.
Statistical analysis
Statistical analyses were performed using PASW v18 (SPSS Inc., Chicago, IL, USA), Prism 6.0 h (GraphPad, La Jolla, CA, USA), and R. Parametric tests or descriptive statistics with parametric assumptions (standard two-way and one-way ANOVA, Pearson’s r and t test) were used for variables (neuronal density, SOD1 aggregate density, SOD1 specific activity, and isoelectric point) meeting the associated assumptions. Non-parametric tests or statistics (Spearman’s r, Kruskal–Wallis test) were used for variables (Lewy Body and Lewy neurite density, SOD1, and CCS protein expression) where the observed data did not fit the assumptions of parametric tests. For these latter variables, where an interaction between the region and disease status was examined, a robust two-way ANOVA was performed using the percentile bootstrap method for medians implemented in the WRS package [69]. The observations for the OCx regions of both control and Parkinson’s disease brains are graphically presented as a negative control for comparison but are not included in the statistical analysis as they were entirely negative for Lewy bodies and neurites. Similarly, the observations from the ILBD cases are presented but not included in the statistical analysis as there were only two cases representing this group. Final values were reported as mean ± SEM. A p value of less than 0.05 was accepted as the level of significance. For image correlation analysis and calculation of Li’s ICQ [40], images were imported into ImageJ (National Institutes of Health, Bethesda, MD, USA), converted into 16-bit greyscale images and analyzed using the Image Correlation Analysis plugin (http://wwwfacilities.uhnresearch.ca/wcif/imagej/colour_analysis.htm). Product of differences of the mean (PDM) images represent pixel-wise calculation of Σ N (A i − a)(B i − b), with more positive PDM values representing co-dependent intensities and negative values representing asynchronous intensities. The ICQ value for an image represents the ratio of the number of positive values to the total number of pixel pairs, with dependent staining 0 < ICQ ≤ +0.5.
Results
Novel SOD1 proteinopathy, distinct from classic synucleinopathy, in the Parkinson’s disease brain is associated with neuronal loss
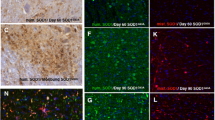
As Parkinson’s disease is associated with elevated oxidative stress [6], we examined degenerating and non-degenerating regions of the Parkinson’s disease brain for synucleinopathy and a primary neuronal anti-oxidant enzyme, SOD1. SOD1 immunoreactivity was observed within Lewy neurites (Fig. 1a), Lewy bodies, and punctate intraneuronal α-synuclein deposits (Fig. 1b) in the SNc and LC [48] of the Parkinson’s disease brain. Co-deposition of SOD1 and α-synuclein proteins was absent in these regions of age-matched control brains (Fig. 1c). Although approximately 90% of Lewy bodies contained SOD1 protein, the variable intensity of SOD1 immunoreactivity between Lewy bodies suggests that the SOD1 content varies between Lewy bodies. This is consistent with an established interaction between SOD1 and α-synuclein [30, 39]. In the degenerating SNc and LC of the Parkinson’s disease brain, low levels of classic amyloid-like Lewy pathology were confirmed (Fig. 1a–i), as previously described [47, 62, 63]. Phosphorylated TAR DNA-binding protein 43 (pTDP43) immunopositivity was also observed in a proportion of Lewy bodies within the SNc (12%) and LC (29%) of all 11 investigated Parkinson’s disease cases (Fig. 1j, Supplementary Fig. 3). No pTDP43 immunoreactivity was observed within Lewy neurites in these brain regions. Absolute neuronal density varied significantly between corresponding regions of the Parkinson’s disease and control brain (two-way ANOVA, p < 0.0001), and was markedly decreased in the Parkinson’s disease SNc (76%) and LC (83%) compared with age-matched controls (Bonferroni post hoc test, both p < 0.001; Fig. 1k). Although the density of Lewy neurites was significantly elevated in the degenerating Parkinson’s disease SNc and LC (Fig. 1l), Lewy body density in the SNc was equivalent to that in non-degenerating cortical regions in Parkinson’s disease (Fig. 1m) confirming existing reports [7, 8] (Supplementary Fig. 4). Lewy neurite, but not Lewy body, densities were inversely correlated with neuronal loss throughout the Parkinson’s disease brain (Spearman’s r = −0.473, p = 0.013; Fig. 1n). Two cases of ILBD (considered a prodromal form of Parkinson’s disease [20]) were also examined and exhibited Lewy pathology (Supplementary Fig. 4, Fig. 1l, m) but no neuronal loss (Fig. 1k). Our data confirm reports of co-deposition of SOD1 and α-synuclein within Lewy pathology in the Parkinson’s disease brain [48].
SOD1 aggregation and dysfunction is a pathological feature independent of Lewy bodies in Parkinson’s disease substantia nigra pars compacta (SNc). SOD1 showed strong co-localization with α-synuclein in Lewy neurites (a open white arrowheads), fully formed Lewy bodies (a, b white arrowheads), as well as with smaller punctate α-synuclein deposits (b white double arrowheads). Li’s intensity correlation quotient (ICQ)46 = 0.239 (a) and 0.269 (b); product of distance from the mean [PDM] of +0.5 = perfect correlation. c No evidence of α-synuclein immunopositive deposits was observed in control brains. d Lewy bodies contained SOD1 immunoreactivity, but not CCS immunoreactivity. e Presence of phosphorylated α-synuclein (pS129) and poly-ubiquitin chains further confirmed classical Lewy body chemical composition. Thioflavin-S (f) and Congo red (g) histological dyes confirmed an amyloid structure. Low levels of pS129-immunopositive Lewy pathology were confirmed in the SNc and LC of the Parkinson’s disease brain (h), though not in these regions of the age-matched control brain (i). j Lewy bodies were also found to contain phosphorylated TDP-43. Scale bars 20 μm. k In Parkinson’s disease neuronal density was significantly and selectively decreased in the SNc and LC, though not in other regions that typically display synucleinopathy [anterior cingulate cortex (ACC); entorhinal cortex (EC); fusiform cortex (FUS)] or in the occipital cortex (OCx, our negative internal control region). ILBD neuronal densities remained unchanged in all regions. l In Parkinson’s disease Lewy neurite densities were significantly higher in the degenerating SNc and LC, whereas m Lewy bodies were present in significantly higher densities in the LC (p = 0.0007–0.0001). Data represent mean ± SEM. *Comparisons to Parkinson’s disease SNc; #comparisons to Parkinson’s disease LC. Deposition of α-synuclein was absent in all brain regions in all control cases. n Lewy neurite densities throughout the Parkinson’s disease brain were inversely correlated with an index of neuronal loss (Spearman’s r = −0.473, p = 0.013)
Aggregated SOD1 was, however, not solely co-localized with α-synuclein proteinopathy. Multiple primary antibodies (Supplementary Fig. 5; Supplementary Table 3) confirmed the presence of previously undescribed SOD1-containing aggregates in the Parkinson’s disease and ILBD brain, which were morphologically distinct from corpora amylacea [2] in these brain regions. In contrast to Lewy bodies, these aggregates contained negligible amounts of α-synuclein (Fig. 2a), no pTDP43 (Fig. 2b), and exhibited a non-amyloid structure (Fig. 2c, d). Unlike wholly spherical Lewy bodies with a typical diameter >15 µm, these SOD1 aggregates presented both amorphous and spherical structures with a more variable size range (5–30 µm diameter). The absence of pS129 α-synuclein (Fig. 2b,e), which was highly enriched in Lewy bodies [68], and the presence of CCS protein (Fig. 2f) are also indicative of a unique biochemical composition. Ubiquitin accumulation in these newly described pathological features (Fig. 2e) suggests either dysfunctional proteins marked for proteasomal degradation [41], or ubiquitin co-aggregation within these aggregates.
In Parkinson’s disease, amorphous and spherical SOD1 immunopositive aggregates (white arrows) in the SNc contained minimal non-phosphorylated α-synuclein (a Li’s ICQ = 0.049), phosphorylated α-synuclein (pS129; b), and phosphorylated TDP-43 (pTDP43; b), and displayed a non-amyloid structure (c, d). e Ubiquitin and f CCS were identified in these inclusions. The control SNc contained a negligible density of SOD1 aggregates compared with the Parkinson’s disease SNc (g). Scale bars 20 μm (a–g). h Density of SOD1 aggregates was significantly and selectively elevated in the SNc and LC in Parkinson’s disease (11 cases examined; SNc, n = 5; LC, n = 6), though only in the LC of both ILBD cases (2.8-fold elevation), compared to age-matched controls. Data represent mean ± SEM. SOD1 aggregate density throughout the Parkinson’s disease brain was also strongly inversely correlated with an index of neuronal loss (i Pearson’s r = −0.903, p < 0.001) and positively correlated with Lewy neurite density (j Spearman’s r = 0.590, p = 0.0024), but not Lewy body density (a, c Spearman’s r = 0.119). Across the ILBD brain, however, SOD1 aggregates were positively correlated with both Lewy body (k Spearman’s r = 0.722, p = 0.022) and Lewy neurite (k Spearman’s r = 0.768, p = 0.012) densities
Aggregated proteins are a feature of normal aging [17]; occasional SOD1 aggregates were observed in all regions of age-matched control brains (Fig. 2g), and possessed a morphology, protein composition, and macrostructure identical to that observed for SOD1 aggregates in the Parkinson’s disease SNc. Based on these findings, we speculate that a very low level of SOD1 aggregation may be inherent to the healthy aging process. Aggregate density was, however, significantly higher within the degenerating SNc and LC of Parkinson’s disease cases (Fig. 2g) compared with equivalent brain regions of age-matched controls (two-way ANOVA, p < 0.001). SOD1 aggregates were found within, and adjacent to, surviving catecholaminergic neurons in these brain regions. The density of SOD1 aggregates was on average eightfold higher in the SNc and fourfold higher in the LC compared to age-matched controls (Bonferroni post hoc test, both p < 0.001), and was significantly greater than all non-degenerating regions of the same Parkinson’s disease brains (Bonferroni post hoc test, all p < 0.001; Fig. 2h). These data suggest an association between SOD1 aggregation and neuronal loss in Parkinson’s disease, reinforced by the presence of a strong inverse correlation between SOD1 aggregate and neuronal densities in the Parkinson’s disease brain (Pearson’s r = −0.903, p < 0.001; Fig. 2i). In the ILBD cases, substantial SOD1 deposition was restricted to the LC, suggesting that SOD1 aggregation in Parkinson’s disease develops in the LC prior to the SNc, mirroring the documented topographical and temporal pattern of α-synuclein deposition in Parkinson’s disease [7, 8, 22]. SOD1 aggregate density was positively correlated with Lewy neurite (Spearman’s r = 0.590, p = 0.0024; Fig. 2j), but not Lewy body, density throughout the Parkinson’s disease brain, reinforcing a proposed link between α-synuclein and SOD1 deposition [39]. The presence of a positive correlation between SOD1 aggregate and both Lewy body (Spearman’s r = 0.722, p = 0.022; Fig. 2k) and neurite (Spearman’s r = 0.768, p = 0.012; Fig. 2k) densities in the ILBD brain further implies that the deposition of SOD1 and α-synuclein proteins may be linked in early stage Parkinson’s disease.
Similarities between SOD1 proteinopathy in Parkinson’s disease and fALS
The aggregation of mutant SOD1 is believed to underlie motor neuron death in another neurodegenerative disorder, SOD1-associated fALS [38]. The discovery of a similar association between SOD1 aggregation and neuronal loss in the Parkinson’s disease brain suggests coincident degenerative pathways in these disorders. Examination of fALS spinal cord identified aggregated SOD1 in the spinal cord of all three fALS cases examined (I113T and D101G mutations; Fig. 3a). As observed in the Parkinson’s disease brain, fALS SOD1 aggregates contained ubiquitin (Fig. 3b) and CCS (Fig. 3c) but were negative for pTDP43 (Fig. 3d). Immunoreactivity for pS129 and non-phosphorylated α-synuclein was also detected in approximately 50% of these aggregates (Fig. 3e), consistent with a reported interaction between mutant SOD1 and α-synuclein [30]. All aggregates were negative for Thioflavin-S, demonstrative of non-amyloid-type aggregation (Fig. 3f). Interestingly, one fALS case with an I113T SOD1 mutation also possessed SOD1-immunonegative aggregated CCS protein inside several motor neurons (Fig. 3g), signifying dysfunction of CCS protein is associated with SOD1 protein dysfunction in SOD1-associated fALS. The similarities in SOD1 aggregate protein composition and non-amyloid macrostructure suggest shared pathways of SOD1 aggregation within degenerating regions in both disorders.
SOD1 aggregation in fALS shares compositional and structural features with those observed in Parkinson’s disease. a fALS spinal cord tissue was immunoreactive for SOD1, showing aggregates with varying morphology. SOD1 aggregates were rich in ubiquitin (b) and CCS (c) proteins; however, phosphorylated (pS129) and non-phosphorylated α-synuclein proteins were present in approximately 50% of aggregates (b–e). Phosphorylated TDP-43 protein was absent in all aggregates (d). All aggregates were negative for Thioflavin-S (f), indicative of non-amyloid aggregation. Interestingly, one case with an I113T sod1 mutation demonstrated CCS protein aggregation independent of SOD1 immunopositivity (g)
Evidence of fALS-like SOD1 misfolding and metal deficiency in the Parkinson’s disease brain
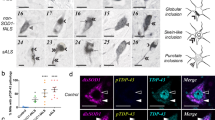
Insoluble proteinaceous deposits in neurodegenerative disease are an end-stage feature subsequent to dysfunction of the active soluble protein [14]. As SOD1 dysfunction in fALS is directly linked to altered protein structure [56], we examined structural abnormalities in SOD1 extracted from post-mortem Parkinson’s disease brains. Using native isoelectric focusing and mass spectrometry, we demonstrated a positive shift in the isoelectric point of soluble SOD1 in Parkinson’s disease compared to controls, reflecting a reduction in net protein charge (t test p < 0.05; Fig. 4a, b). We have previously demonstrated that a reduction in SOD1 charge is associated with decreased binding of its copper enzymatic cofactor [56], suggesting that reduced copper metallation of SOD1 protein occurs in Parkinson’s disease. This is consistent with our finding of reduced intraneuronal copper in the SNc and LC in Parkinson’s disease [18], associated with a reduction in the copper transport protein Ctr1, which has directly been correlated with elevated levels of metal-deficient SOD1 [5].
SOD1 proteins levels are increased in Parkinson’s disease, and SOD1 shows evidence of misfolding and metal deficiency. a Isoelectric focusing and mass spectrometry analysis of SOD1 from Parkinson’s disease and age-matched control brain homogenates showed a positive shift in SOD1 pI in Parkinson’s disease compared to SOD1 in age-matched control brains (b 5.067 ± 0.007 vs 5.042 ± 0.006, respectively; n PD = 21; n control = 23 p < 0.01), consistent with protein misfolding and mismetallation. Mitochondrial SOD2 activity was also identified (red arrowheads), confirmed by mass spectrometry. Immunohistochemical analyses of misfolded SOD1 in the spinal cord (SpC) of three fALS cases with either D101G or I113T sod1 mutations and three healthy control cases (c), and in the substantia nigra (SNc) and locus coeruleus (LC) of five cases of Parkinson’s disease (PD; d), six healthy control cases (e), and two cases of incidental Lewy body disease (ILBD; f), using B8H10 and exposed dimer interface (EDI) misfolded SOD1 antibodies. Arrows indicate the presence of globular intracellular misfolded SOD1 inclusions. Solid arrowheads indicate the presence of extracellular misfolded SOD1 deposits. Open arrowheads indicate the presence of intraneuronal misfolded SOD1 amorphous puncta. Single downward arrowhead indicates diffuse cytoplasmic staining and double downward arrowheads indicate neuritic misfolded SOD1. Scale bars 20 µm (c–f). g In the degenerating Parkinson’s disease, SNc SOD1 (574597 Merck Millipore) and CCS (sc-20141 Santa Cruz) protein expression levels were significantly correlated (Spearman’s r = 0.857, p = 0.024). h SOD1-specific activity was reduced in the SNc (p = 0.004) despite an elevation in SOD1 protein expression in this brain region (p = 0.016; i). *p < 0.05; **p < 0.01
To confirm the presence of misfolded, metal-deficient SOD1 in degenerating Parkinson’s disease brain regions, we employed two conformation-specific misfolded SOD1 antibodies. The first more broadly recognizes misfolded, metal-deficient mutant, and wild-type SOD1 (B8H10, Medimabs Inc) [52], whereas the second specifically recognizes SOD1 with an exposed dimer interface (EDI, StressMarq Biosciences), resulting from a lack of copper bound to SOD1 [3, 53]. Misfolded SOD1 was detected in spinal cord motor neurons within all three fALS cases (both I113T and D101G mutations) using the B8H10 misfolded SOD1 antibody, whilst control spinal cords were immunonegative (Fig. 4c). A variety of intra- and extracellular spherical and amorphous B8H10-immunopositive SOD1 deposits were also identified in the Parkinson’s disease SNc and LC (Fig. 4d), which were absent in these regions of control brains (Fig. 4e). Corresponding results were obtained using the EDI misfolded SOD1 antibody (Fig. 4c–e). Approximately 70% of pigmented neurons within the SNc and LC of the control brain exhibited diffuse cytoplasmic staining for EDI-SOD1. This is unsurprising given that 35% of human SOD1 is known to exist as metal-deficient SOD1 [51], where molecular modelling has shown the dimer interface to be exposed [3, 53]. These data strongly indicate that SOD1 is misfolded and copper-deficient in vulnerable regions of the Parkinson’s disease brain, similar to misfolded mutant SOD1 in the fALS spinal cord.
Intracellular, amorphous B8H10- and EDI-immunoreactive deposits and puncta were also identified in the ILBD SNc and LC (Fig. 4f), as well as extracellular immunoreactive deposits. Interestingly, B8H10 immunoreactivity was also observed in neurites of melanised neurons in the SNc and LC. The absence of diffuse EDI-SOD1 neuronal staining, and the presence of intraneuronal EDI-SOD1 puncta, within the ILBD and Parkinson’s disease SNc and LC indicates changes in the intraneuronal localization of the metal-deficient SOD1 pool within these neurons during early–late-stage Parkinson’s disease.
Copper loading in SOD1 protein occurs primarily via a heterodimeric interaction with CCS. Given that CCS and SOD1 expression were highly positively correlated in the Parkinson’s disease SNc (Spearman’s r = 0.857, p = 0.024; Fig. 4d), it is unlikely that inadequate copper delivery by CCS underlies the deficiency in copper in SOD1 in vulnerable brain regions.
In addition to protein misfolding and aggregation, reduced copper metallation of SOD1 decreases enzymatic function [31]. The pattern of SOD1 specific activity between the three experimental brain regions varied between the Parkinson’s disease and healthy control groups (two-way ANOVA, p = 0.026). Notably, we observed a 66% reduction in SOD1 specific activity in the copper-deficient degenerating SNc compared with the ACC (Bonferroni post hoc test, p = 0.004; Fig. 4e), in which neuronal loss is not observed despite equivalent synucleinopathy (Fig. 1i–k). These data indicate a decreased ability of SOD1 to manage oxidative stress in copper-deficient degenerating brain regions [28, 57], which we associate with a reduction in copper bound to SOD1. In turn, elevated oxidative stress stimulates SOD1 expression [25]. Similar to SOD1 specific activity, we observed that the pattern of SOD1 protein expression between the three experimental brain regions varied between the Parkinson’s disease and healthy control groups (robust two-way ANOVA, p = 0.026). Furthermore, a significant elevation in SOD1 protein expression was identified in the degenerating Parkinson’s disease SNc compared with the control SNc (Bonferroni post hoc test, p = 0.016; Fig. 4f). Elevated SOD1 protein levels likely further increase the demand for copper in these copper-deficient neurons [18, 31], driving production of copper-deficient, misfolded SOD1 in degenerating Parkinson’s disease brain regions.
SOD1 proteinopathy and neuronal loss observed in Parkinson’s disease-linked regions of the ALS brain
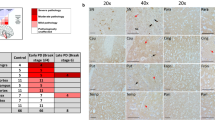
Whilst misfolded mutant SOD1 has long been implicated in motor neuron degeneration in the spinal cords of fALS patients [1], there has been minimal investigation into SOD1 dysfunction in the fALS brain, despite reports of moderate neuronal loss [36, 50]. Considering the strong biochemical similarities of Parkinson’s disease brain SOD1 proteinopathy to neurotoxic fALS spinal cord SOD1 aggregates, we investigated whether SOD1 aggregation may also be associated with neuronal loss in Parkinson’s disease-linked regions of fALS brains. We identified SOD1 aggregates in the SNc and LC of fALS brains which, matching SOD1 aggregates in these regions of the Parkinson’s disease brain, were α-synuclein-negative (Fig. 5a, b). Consistent with previous reports [36, 50], substantial neuronal loss was observed in the fALS SNc (29% loss) and LC (31% loss; Fig. 5c). Importantly, SOD1 aggregation was again greatest within these degenerating fALS brain regions (Fig. 5d). Although neuronal loss and SOD1 aggregate density in fALS brains were proportionally less than in corresponding regions of Parkinson’s disease brains, these results imply that SOD1 aggregation may underlie moderate catecholaminergic neuron loss in the fALS brain.
SOD1 aggregates in the fALS SNc (a Li’s ICQ = 0.162) and LC (b Li’s ICQ = 0.146) mirrored the composition of similar aggregates in these brain regions in Parkinson’s disease. c Neuronal density in the fALS brain was decreased in the SNc (29% loss) and LC (31% loss), and d SOD1 aggregate density was substantially elevated in the SNc (3.1-fold elevation)
Discussion
This study presents the first demonstration of aggregated, misfolded SOD1 protein associated with neuronal loss in the Parkinson’s disease brain. We show that significant accumulation of deposited copper-deficient SOD1, as well as reduced anti-oxidant activity of SOD1, is present in degenerating brain regions. This suggests reduced oxidant buffering capacity within these vulnerable neurons as well as a potential toxic gain-of-function associated with the aggregation of SOD1, similar to that proposed in fALS [54].
Inadequate copper metallation of SOD1 results in the observed shift in isoelectric point [13] (Fig. 4a, b) and also increases protein flexibility, allowing oxidation of exposed free cysteine residues (Cys-6 and Cys-111) within the normally solvent-inaccessible dimer interface (Fig. 4c) [3, 53]. Cysteine oxidation disrupts the stabilizing influence of the intramolecular disulfide bond in SOD1 monomers, stimulating the oligomerization and aggregation of metal-deficient monomeric SOD1 [26, 65]. This pathway to SOD1 aggregation explains why the quiescent metal-deficient pool of dimeric SOD1 is not prone to aggregation in the healthy brain, where normal levels of oxidative stress and sufficient cellular copper enable maturation of the protein to the holo form when required. In degenerating regions of the Parkinson’s disease brain, however, higher levels of oxidative stress results in increased production of SOD1 protein, resulting in the growth of the metal-deficient SOD1 pool, which is unable to mature to holo-SOD1 due to inadequate intracellular copper. This growing pool of metal-deficient SOD1 is prone to oxidation, which promotes monomerization and subsequently oligomerization of the protein.
In addition to promoting the accumulation of metal-deficient SOD1 protein, both oxidative stress [23] and low intraneuronal copper [49] disrupt signalling pathways in protein clearance systems, further exacerbating the accumulation of misfolded SOD1. Together, these data describe a self-perpetuating cycle of metal dyshomeostasis, oxidative stress, and protein dysfunction restricted to the SNc and LC of the Parkinson’s disease brain (Fig. 6). The presence of both a copper deficit [18] and of misfolded SOD1 in the ILBD SNc (Fig. 4c), where neuronal loss is absent (Fig. 1i), suggests that the molecular changes within this cycle are early events in Parkinson’s disease. In SOD1-associated fALS, aggregation of SOD1 protein is believed to represent a toxic gain-of-function underlying motor neuron death. The presence of a similar pathway resulting in SOD1 aggregation in the Parkinson’s disease brain, therefore, suggests that analogous molecular mechanisms may also contribute to catecholaminergic neuron loss in Parkinson’s disease. The current data suggest that both loss- and gain-of-SOD1-function pathways, similar to those proposed in fALS [57], are present within vulnerable neurons in the Parkinson’s disease brain, and may contribute to the regional pattern of neuronal death in this disorder [24].
Proposed mechanism of SOD1-mediated neuron death in Parkinson’s disease. Decreased expression of Ctr1 restricts neuronal copper import, limiting bioavailable copper for maturing SOD1. As copper is intricately linked to the formation of the stabilizing intramolecular disulphide bond in SOD1 protein, a lack of this essential cofactor creates structurally unstable forms of the protein, including copper-deficient, disulphide reduced monomers, and inactive dimers. Inability to properly synthesise active SOD1 may be exacerbated by sod1 mutations. Failure to produce functional SOD1 reduces mitigation of oxidative stress in Parkinson’s disease. Increased oxidative load in the neuron perpetuates a cycle of dysfunctional SOD1 production by stimulating increased SOD1 protein expression in these copper-deficient environments. Copper-deficient SOD1 oligomerizes and eventually forms insoluble aggregates within degenerating neurons. The presence of ubiquitin within these aggregates indicates that normal proteasomal degradation pathways are also impaired, consistent with the influence of oxidative stress and metal dyshomeostasis on these systems. A loss of SOD1 activity additionally contributes to α-synuclein oligomerization (Lewy neurites) through peroxynitrite generation, which likely contributes to neuronal degeneration. Ultimately, a loss of capacity to manage oxidative stress by SOD1, in addition to mismetallation of SOD1, contributes to neuronal death in Parkinson’s disease
Reduced SOD1 specific activity also results in elevated production of peroxynitrite, which is reported to promote nitration and oligomerization of α-synuclein [9]. Such a mechanism could explain the correlation between SOD1 and Lewy neurite proteinopathy, and why both concentrate in degenerating regions of the Parkinson’s disease brain. Our data support a reported interaction between these proteins [29, 30], proposed to trigger seeding and deposition of both proteins [39], possibly disrupting axonal transport [67]. The presence of a correlation between SOD1 aggregates and Lewy neurites and Lewy bodies in the ILBD brain further suggests that these changes occur in early stage Parkinson’s disease.
Our data demonstrate that a well-characterized pathological pathway in fALS shares remarkably similar attributes to a novel protein aggregate concentrated only in regions undergoing cell loss in Parkinson’s disease brain. Similarities in SOD1 aggregate composition and macrostructure, and in soluble SOD1 protein conformation (measured as isoelectric point) [56] in Parkinson’s disease and fALS suggest shared mechanisms of SOD1 dysfunction and neurodegeneration. Although no exonic mutations in the SOD1 gene have been identified in Parkinson’s disease [4], wild-type SOD1 has been shown to recapitulate mutant SOD1 protein in situations of oxidative stress and/or metal dyshomeostasis [27, 55], with respect to both protein conformation and intracellular interactions. The identification of comparable misfolded conformations of SOD1 in the Parkinson’s disease brain and mutant SOD1 in the fALS spinal cord in this study reinforces the likelihood of parallel pathways of SOD1 aggregation in both disorders. The identification of an intronic SOD1 mutation [35, 66], manifesting clinically as either early onset Parkinson’s disease or ALS, lends further support to our hypothesis that misfolded SOD1 represents a point at which degenerative cascades converge in these disorders. Our data also demonstrate for the first time that SOD1 pathology is present in Parkinson’s disease-associated brain regions of SOD1-associated fALS patients, although nigrostriatal dopaminergic denervation [11, 64] and SNc synucleinopathy [45] are less severe than that seen in these same regions in Parkinson’s disease (Fig. 5c, d). The short disease duration of ALS likely precludes sufficient dopaminergic neuron loss necessary for expression of clinical parkinsonism, which is reported in only 5–17% of ALS cases [42].
Extensive biochemical characterization of dysfunctional SOD1 in fALS, and in murine models, has resulted in therapies that directly target SOD1 metal status [54]. CuII[atsm] increases holo SOD1 [54] and dramatically extends the lifespan of the CCSxSODG93A overexpressing transgenic fALS murine model [70], suggesting that SOD1 copper status is linked to motor neuron death, and represents a tractable therapeutic target. Shared SOD1 proteinopathy in Parkinson’s disease and ALS suggests that restoration of neuronal copper levels in Parkinson’s disease may have similar benefits to those reported for CuII[atsm] in ALS [54, 70]. In support of this idea, administration of CuII[atsm] to four distinct Parkinson’s disease animal models improved motor function, rescued SNc cell loss, and enhanced dopamine metabolism [32]. Levels of nitrated α-synuclein were also reduced [32], linked to an increase in SOD1 specific activity [61], suggesting that restoration of SOD1 activity with CuII[atsm] may attenuate α-synuclein accumulation by stimulating anti-oxidant defenses [43]. Importantly, phase I clinical trials of this compound in fALS are planned (ClinicalTrials.gov Identifier: NCT02870634); data from these trials will inform the potential use of this therapeutic approach in Parkinson’s disease.
References
Ajroud-Driss S, Siddique T (2015) Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochem Biophys Acta 1852:679–684. doi:10.1016/j.bbadis.2014.08.010
Ayers JI, Xu G, Pletnikova O, Troncoso JC, Hart PJ, Borchelt DR (2014) Conformational specificity of the C4F6 SOD1 antibody; low frequency of reactivity in sporadic ALS cases. Acta Neuropathol Commun 2:55. doi:10.1186/2051-5960-2-55
Banci L, Bertini I, Boca M, Calderone V, Cantini F, Girotto S, Vieru M (2009) Structural and dynamic aspects related to oligomerization of apo SOD1 and its mutants. Proc Natl Acad Sci USA 106:6980–6985. doi:10.1073/pnas.0809845106
Bandmann O, Davis MB, Marsden CD, Harding AE (1995) Sequence of the superoxide-dismutase 1 (SOD1) gene in familial Parkinson’s disease. J Neurol Neurosurg Psychiatry 59:90–91. doi:10.1136/jnnp.59.1.90
Bartnikas TB, Gitlin JD (2003) Mechanisms of biosynthesis of mammalian copper/zinc superoxide dismutase. J Biol Chem 278:33602–33608. doi:10.1074/jbc.M305435200
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91. doi:10.3389/fnana.2015.00091
Braak H, Del Tredici K, Rub U, de Vos RAI, Steur E, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. doi:10.1016/s0197-4580(02)00065-9
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134. doi:10.1007/s00441-004-0956-9
Breydo L, Wu JW, Uversky VN (2012) Alpha-synuclein misfolding and Parkinson’s disease. Biochem Biophys Acta 1822:261–285. doi:10.1016/j.bbadis.2011.10.002
Brotherton TE, Li Y, Cooper D, Gearing M, Julien JP, Rothstein JD, Boylan K, Glass JD (2012) Localization of a toxic form of superoxide dismutase 1 protein to pathologically affected tissues in familial ALS. Proc Natl Acad Sci USA 109:5505–5510. doi:10.1073/pnas.1115009109
Burrow JN, Blumbergs PC (1992) Substantia nigra degeneration in motor neurone disease: a quantitative study. Aust N Z J Med 22:469–472
Chevreux S, Roudeau S, Fraysse A, Carmona A, Deves G, Solari PL, Mounicou S, Lobinski R, Ortega R (2009) Multimodal analysis of metals in copper-zinc superoxide dismutase isoforms separated on electrophoresis gels. Biochimie 91:1324–1327. doi:10.1016/j.biochi.2009.05.016
Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L (2005) Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem 280:11648–11655. doi:10.1074/jbc.M414327200
Ciryam P, Kundra R, Morimoto RI, Dobson CM, Vendruscolo M (2015) Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol Sci 36:72–77. doi:10.1016/j.tips.2014.12.004
Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM (2010) Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. JNeurosci 30:7281–7289. doi:10.1523/JNEUROSCI.0490-10.2010
Da Cruz S, Bui A, Saberi S, Lee SK, Stauffer J, McAlonis-Downes M, Schulte D, Pizzo DP, Parone PA, Cleveland DW et al (2017) Misfolded SOD1 is not a primary component of sporadic ALS. Acta Neuropathologica. doi:10.1007/s00401-017-1688-8
David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C (2010) Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8:e1000450. doi:10.1371/journal.pbio.1000450
Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, Finberg JPM, Reyes S, Halliday GM, Mercer JFB et al (2014) Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging 35:858–866. doi:10.1016/j.neurobiolaging.2013.09.034
Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, Singleton A, Olanow CW, Merchant KM, Bezard E et al (2015) Targeting alpha-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14:855–866. doi:10.1016/S1474-4422(15)00006-X
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ et al (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080. doi:10.1001/archneur.65.8.1074
Deng HX, Shi Y, Furukawa Y, Zhai H, Fu RG, Liu ED, Gorrie GH, Khan MS, Hung WY, Bigio EH et al (2006) Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA 103:7142–7147. doi:10.1073/pnas.0602046103
Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK et al (2009) Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 8:1150–1157
Domenico FD, Head E, Butterfield A, Perluigi M (2014) Oxidative Stress and proteostasis network: culprit and casualty of Alzheimer’s-like neurodegeneration. Adv Geriatr 2014:14
Double KL, Reyes S, Werry EL, Halliday GM (2010) Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol 92:316–329. doi:10.1016/j.pneurobio.2010.06.001
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. doi:10.1038/35041687
Furukawa Y, Kaneko K, Yamanaka K, O’Halloran TV, Nukina N (2008) Complete loss of post-translational modifications triggers fibrillar aggregation of SOD1 in the familial form of amyotrophic lateral sclerosis. J Biol Chem 283:24167–24176. doi:10.1074/jbc.M802083200
Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, Trotti D, Pasinelli P (2012) An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci USA 109:5074–5079. doi:10.1073/pnas.1115402109
Hare DJ, Double KL (2016) Iron and dopamine: a toxic couple. Brain J Neurol 139:1026–1035. doi:10.1093/brain/aww022
Helferich AM, McLean PJ, Weishaupt JH, Danzer KM (2016) Commentary: alpha-synuclein interacts with SOD1 and promotes its oligomerization. J Neurol Neuromed 1:28–30
Helferich AM, Ruf WP, Grozdanov V, Freischmidt A, Feiler MS, Zondler L, Ludolph AC, McLean PJ, Weishaupt JH, Danzer KM (2015) alpha-synuclein interacts with SOD1 and promotes its oligomerization. Mol Neurodegener 10:66. doi:10.1186/s13024-015-0062-3
Hilton JB, White AR, Crouch PJ (2015) Metal-deficient SOD1 in amyotrophic lateral sclerosis. J Mol Med (Berl) 93:481–487. doi:10.1007/s00109-015-1273-3
Hung LW, Villemagne VL, Cheng L, Sherratt NA, Ayton S, White AR, Crouch PJ, Lim S, Leong SL, Wilkins S et al (2012) The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J Exp Med 209:837–854. doi:10.1084/jem.20112285
Ip P, Mulligan VK, Chakrabartty A (2011) ALS-causing SOD1 mutations promote production of copper-deficient misfolded species. J Mol Biol 409:839–852. doi:10.1016/j.jmb.2011.04.027
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Kacem I, Funalot B, Torny F, Lautrette G, Andersen PM, Couratier P (2012) Early onset Parkinsonism associated with an intronic SOD1 mutation. Amyotroph Lateral Scler 13:315–317. doi:10.3109/17482968.2011.623301
Kato S, Oda M, Tanabe H (1993) Diminution of dopaminergic neurons in the substantia nigra of sporadic amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 19:300–304
Kerman A, Liu H-N, Croul S, Bilbao J, Rogaeva E, Zinman L, Robertson J, Chakrabartty A (2010) Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol 119:335–344. doi:10.1007/s00401-010-0646-5
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955. doi:10.1016/S0140-6736(10)61156-7
Koch Y, Helferich AM, Steinacker P, Oeckl P, Walther P, Weishaupt JH, Danzer KM, Otto M (2016) Aggregated alpha-synuclein increases SOD1 oligomerization in a mouse model of amyotrophic lateral sclerosis. Am J Pathol 186:2152–2161. doi:10.1016/j.ajpath.2016.04.008
Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF (2004) A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24:4070–4081. doi:10.1523/JNEUROSCI.0346-04.2004
Li W, Ye Y (2008) Polyubiquitin chains: functions, structures, and mechanisms. Cell Mol Life Sci 65:2397–2406. doi:10.1007/s00018-008-8090-6
Manno C, Lipari A, Bono V, Taiello AC, La Bella V (2013) Sporadic Parkinson disease and amyotrophic lateral sclerosis complex (Brait–Fahn–Schwartz disease). J Neurol Sci 326:104–106. doi:10.1016/j.jns.2013.01.009
Martinez-Lazcano JC, Montes S, Sanchez-Mendoza MA, Rodriguez-Paez L, Perez-Neri I, Boll MC, Campos-Arroyo HD, Rios C, Perez-Severiano F (2014) Sub-chronic copper pretreatment reduces oxidative damage in an experimental Huntington’s disease model. Biol Trace Elem Res 162:211–218. doi:10.1007/s12011-014-0127-0
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L (2001) beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA 98:12245–12250. doi:10.1073/pnas.211412398
Mather K, Watts FZ, Carroll M, Whitehead P, Swash M, Cairn N, Burke J (1993) Antibody to an abnormal protein in amyotrophic lateral sclerosis identifies Lewy body-like inclusions in ALS and Lewy bodies in Parkinson’s disease. Neurosci Lett 160:13–16
Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K et al (2013) An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol 12:435–442. doi:10.1016/S1474-4422(13)70061-9
Nilsson MR (2004) Techniques to study amyloid fibril formation in vitro. Methods 34:151–160. doi:10.1016/j.ymeth.2004.03.012
Nishiyama K, Murayama S, Shimizu J, Ohya Y, Kwak S, Asayama K, Kanazawa I (1995) Cu/Zn superoxide dismutase-like immunoreactivity is present in Lewy bodies from Parkinson’s disease—a light and electron-microscopic immunocytochemical study. Acta Neuropathol 89:471–474
Opazo CM, Greenough MA, Bush AI (2014) Copper: from neurotransmission to neuroproteostasis. Front Aging Neurosci 6:143. doi:10.3389/fnagi.2014.00143
Orrell RW, King AW, Hilton DA, Campbell MJ, Lane RJ, de Belleroche JS (1995) Familial amyotrophic lateral sclerosis with a point mutation of SOD-1: intrafamilial heterogeneity of disease duration associated with neurofibrillary tangles. J Neurol Neurosurg Psychiatry 59:266–270
Petrovic N, Comi A, Ettinger MJ (1996) Identification of an apo-superoxide dismutase (Cu, Zn) pool in human lymphoblasts. J Biol Chem 271:28331–28334
Pickles S, Semmler S, Broom HR, Destroismaisons L, Legroux L, Arbour N, Meiering E, Cashman NR, Vande Velde C (2016) ALS-linked misfolded SOD1 species have divergent impacts on mitochondria. Acta Neuropathol Commun 4:43. doi:10.1186/s40478-016-0313-8
Pratt AJ, Shin DS, Merz GE, Rambo RP, Lancaster WA, Dyer KN, Borbat PP, Poole FL 2nd, Adams MW, Freed JH et al (2014) Aggregation propensities of superoxide dismutase G93 hotspot mutants mirror ALS clinical phenotypes. Proc Natl Acad Sci USA 111:E4568–E4576. doi:10.1073/pnas.1308531111
Roberts BR, Lim NKH, McAllum EJ, Donnelly PS, Hare DJ, Doble PA, Turner BJ, Price KA, Lim SC, Paterson BM et al (2014) Oral treatment with Cu-II(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 34:8021–8031. doi:10.1523/jneurosci.4196-13.2014
Rotunno MS, Bosco DA (2013) An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front Cell Neurosci 7:253. doi:10.3389/fncel.2013.00253
Roudeau S, Chevreux S, Carmona A, Ortega R (2015) Reduced net charge and heterogeneity of pI isoforms in familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase. Electrophoresis 36:2482–2488. doi:10.1002/elps.201500187
Saccon RA, Bunton-Stasyshyn RKA, Fisher EMC, Fratta P (2013) Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain J Neurol 136:2342–2358. doi:10.1093/brain/awt097
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al (2012) Fiji: an open source platform for biological-image analysis. Nat Meth 9:676–682. doi:10.1038/nmeth.2019
Schneeberger A, Tierney L, Mandler M (2015) Active immunization therapies for Parkinson’s disease and multiple system atrophy. Mov Disord. doi:10.1002/mds.26377
Siebert M, Sidransky E, Westbroek W (2014) Glucocerebrosidase is shaking up the synucleinopathies. Brain J Neurol 137:1304–1322. doi:10.1093/brain/awu002
Soon CP, Donnelly PS, Turner BJ, Hung LW, Crouch PJ, Sherratt NA, Tan JL, Lim NK, Lam L, Bica L et al (2011) Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem 286:44035–44044. doi:10.1074/jbc.M111.274407
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. doi:10.1073/pnas.95.11.6469
Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. doi:10.1038/42166
Takahashi H, Snow BJ, Bhatt MH, Peppard R, Eisen A, Calne DB (1993) Evidence for a dopaminergic deficit in sporadic amyotrophic lateral sclerosis on positron emission scanning. Lancet 342:1016–1018
Toichi K, Yamanaka K, Furukawa Y (2013) Disulfide scrambling describes the oligomer formation of superoxide dismutase (SOD1) proteins in the familial form of amyotrophic lateral sclerosis. J Biol Chem 288:4970–4980. doi:10.1074/jbc.M112.414235
Valdmanis PN, Belzil VV, Lee J, Dion PA, St-Onge J, Hince P, Funalot B, Couratier P, Clavelou P, Camu W et al (2009) A mutation that creates a pseudoexon in SOD1 causes familial ALS. Ann Hum Genet 73:652–657
Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM (2014) Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 25:4010–4023. doi:10.1091/mbc.E14-02-0741
Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG et al (2013) Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 240:190–204. doi:10.1016/j.expneurol.2012.11.020
Wilcox RR (2012) Introduction to robust estimation and hypothesis testing. Elsevier, Oxford
Williams JR, Trias E, Beilby PR, Lopez NI, Labut EM, Bradford CS, Roberts BR, McAllum EJ, Crouch PJ, Rhoads TW et al (2016) Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD(G93A) mice co-expressing the copper-chaperone-for-SOD. Neurobiol Dis 89:1–9. doi:10.1016/j.nbd.2016.01.020
Acknowledgements
Tissues were received from the New South Wales Tissue Resource Centre at the University of Sydney, supported by the Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism [NIH (NIAAA) R24AA012725], from the Sydney Brain Bank, which is supported by Neuroscience Research Australia and the University of New South Wales, and from The London Neurodegenerative Diseases Brain Bank, which receives funding from the MRC and as part of the Brains for Dementia Research programme, jointly funded by Alzheimer’s Research UK and Alzheimer’s Society. The authors acknowledge the facilities as well as the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility (http://ammrf.org.au) node at the University of Sydney. The authors thank Michael Kuligowski (University of Sydney) for excellent technical assistance, Danielle Davies (Brain and Mind Centre) and Sydney Brain Bank for the antibody provision, and Laura Perrin-Verdugier (University of Bordeaux) for assistance with isoelectric focusing experiments.
Author information
Authors and Affiliations
Contributions
BGT, KMD, DJH, and KLD designed the study. BGT, KMD, and KLD applied for all human tissues. KLD raised funds for the study. BGT and KLD gained human research ethics approval. SJGL, PS, BS, CT, CV, CS, SAS, and GMH provided clinical information for all human tissue cases obtained. BGT, KMD, VC, RO, SR, AC, VW, DJH, and KLD designed and performed the experiments. SG and KDS assisted with the experiments. BGT, KMD, VC, RO, SR, AC, VW, HJB, DJH, and KLD analyzed the data. BGT, DJH, and KLD wrote drafts of the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by funds from the Australian Research Council, the National Health and Medical Research Council of Australia (NHMRC), Parkinson’s NSW (2015 and 2016 seed grants), and the University of Sydney (Biomedical Science, BRIG).
Conflict of interest
The authors declare no competing financial interests or conflicts of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trist, B.G., Davies, K.M., Cottam, V. et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol 134, 113–127 (2017). https://doi.org/10.1007/s00401-017-1726-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1726-6