Abstract
To obtain the proof of concept of a novel therapy for Alzheimer’s disease (AD), we conducted two prospective studies with hemodialysis patients who had amyloid β protein (Aβ) removed from their blood three times a week. One major pathological change in the brain associated with AD is Aβ deposition, mainly 40 amino acids Aβ1–40 and 42 amino acids Aβ1–42. Impaired Aβ clearance is proposed to be one cause of increased Aβ in the AD brain. Thus, we hypothesized that an extracorporeal removal system of Aβ from the blood may remove brain Aβ and be a useful therapeutic strategy for AD. In the first prospective study, plasma Aβ levels and the cognitive function of 30 hemodialysis patients (65–76 years old) were evaluated at baseline as well as 18 or 36 months after. Although plasma Aβ1–40 levels either decreased or remained unchanged, levels of Aβ1–42 either remained unchanged or increased at the second time point. Mini-Mental State Examination scores of most subjects increased or were maintained at the second time point. Aβ1–40 influx into the blood correlated with MMSE at the second time point. In the second prospective study, five patients (51–84 years old) with renal failure were evaluated before and after the initiation of hemodialysis. Plasma Aβ levels decreased, while cognitive function improved after initiating blood Aβ removal. Therefore, long-term hemodialysis, which effectively removes blood Aβ, might alter Aβ influx and help maintain cognitive function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the major pathological changes associated with Alzheimer’s disease (AD) is the deposition of amyloid β protein (Aβ) as senile plaques in the brain and an increase of Aβ peptides (Kuo et al. 1996; Selkoe 2001). There are several Aβ species in the brain and plasma that are approximately 4 kDa in weight, such as Aβ1–40 and Aβ1–42, which are comprised of 40 and 42 amino acids, respectively. Aβ1–42 is more toxic and aggregates more easily than Aβ1–40 (Hung et al. 2008), resulting in soluble Aβ oligomers which can cause synapse loss and affect long-term potentiation of hippocampal neurons (Walsh et al. 2002). One proposed mechanism underlying the increase of brain Aβ is caused by decreased Aβ clearance rather than an increase in Aβ production, particularly in sporadic AD cases. Aβ production in the brains of AD patients was reported to be similar to that of normal subjects, yet Aβ clearance from AD brains was approximately 30 % lower than in controls (Mawuenyega et al. 2010). In other words, it may be possible to treat AD by enhancing Aβ clearance from the brain. There are several known Aβ transporters, such as an Aβ influx pathway into the blood [e.g., LRP-1 or apo E (Donahue et al. 2006; Bell et al. 2007)], and RAGE (Silverberg et al. 2010), which is also known as an Aβ influx pathway into the brain. Perivascular elimination of Aβ in brain capillaries has been also proposed (e.g., Morris et al. 2014).
Peripheral administration of Aβ-binding substances, such as Aβ antibodies, non-immunogenic substances and albumin, can reduce Aβ burden in the brain. Further, therapeutic attempts with Aβ-binding substances in the blood resulted in the formation of Aβ complexes with Aβ-binding substances inside the body, sometimes retaining these complexes in plasma for long periods of time (DeMattos et al. 2001). Aβ antibodies generated by passive immunization or by active immunization using synthetic Aβ peptides reduced senile plaques and somewhat improved cognitive impairments in AD patients (Schenk et al. 1999; Hock et al. 2003). Further, non-immunogenic Aβ-binding substances, such as GM1-ganglioside or gelsolin, also decreased Aβ burden in the brain when peripherally injected into mouse models of AD (Matsuoka et al. 2003). Currently, a clinical trial in progress is treating AD patients using intravenous administration of albumin, an Aβ-binding substance (Boada et al. 2009). This Phase 2 trial of plasma exchange with albumin replacement removes the plasma of AD patients, which contains Aβ-albumin complexes, and introduces a new albumin solution; the results thus far suggest that this therapy improved AD cognitive function.
Based on these observations, removing Aβ from the blood is thought to act as peripheral drainage and an Aβ sink from the brain. We proposed that an extracorporeal blood Aβ removal system (E-BARS), which transfers Aβ out of the body, may be useful as an AD therapy (Kawaguchi et al. 2010) (Fig. 1). The rapid reduction of Aβ concentrations in the blood may act as a trigger to enhance Aβ excretion from the brain, resulting in cognitive improvement. Previously, we reported that hemodialyzers were able to remove Aβ1–40 and Aβ1–42, provoking a large influx of Aβ into the blood during hemodialysis sessions using a kinetic analysis (Kitaguchi et al. 2011, Kato et al. 2012). We also reported increased plasma Aβ concentrations, and impaired cognitive function along with the decline of renal function in renal failure patients without hemodialysis (Kato et al. 2012). However, regarding to hemodialysis patients in this cross-sectional study, plasma Aβ concentrations showed steady or slightly decreasing levels as duration of hemodialysis became longer. MMSE scores of hemodialysis patients did not decrease after longer duration of hemodialysis. Further, in terms of brain Aβ, postmortem brains of hemodialysis patients contained significantly fewer cored Aβ plaques than controls (Kitaguchi et al. presented at AAIC 2013, and, Sakai et al., a manuscript in preparation). These findings suggest that E-BARS may decrease brain Aβ through removal of blood Aβ out of the body with an extracorporeal system.
Here, we conducted two prospective studies investigating plasma Aβ concentrations and the cognitive function of hemodialysis patients. The first study was comprised of 30 hemodialysis patients evaluated at baseline and at 18 months after (16 patients) or at 36 months after the baseline (14 patients); the second study was comprised of renal failure patients evaluated before and after the initiation of hemodialysis. The effect of smoking history was also investigated because smoking may affect brain blood flow and Aβ removal from the brain. Further, Aβ accumulation of 2 patients was measured by brain Aβ imaging 5 or 10 months after the initiation of hemodialysis.
Materials and methods
Subjects
Detailed information about study subjects is summarized in Table 1. All were non-diabetic, as diabetes mellitus is an AD risk factor. Sixteen hemodialysis patients (8 male and 8 female) between the ages of 65–76 (71.7 ± 3.5) years were recruited at Kawana Hospital and evaluated at baseline and 18 months after (the 2nd line) (Group A). Fourteen hemodialysis patients (6 males and 8 females) between the ages of 60–73 (68.6 ± 3.5) years old were recruited at Chiryu Clinic and evaluated at baseline and 36 months after treatment (also as the 2nd line) (Group B). Hemodialysis duration was 8.8 ± 6.0 years for Group A, and 18.3 ± 10.0 years for Group B at baseline. The patients continued to receive hemodialysis three times a week between the baseline and the 2nd line.
Five renal failure patients were evaluated during the periods before and after the initiation of hemodialysis (Group C in Table 1). Two females (51 and 56 years of age) and two males (65 and 84 years of age) were recruited at Fujita Health University, while one female (64 years of age) was recruited at Chita City Hospital.
Patient blood was sampled by the medical staff in each institution. The samples were processed just after sample collection, including centrifugation and freezing in each institution, by the researchers of Fujita Health University. For all subjects, plasma Aβ concentrations were measured in Fujita Health University. Cognitive function, as assessed by Mini-Mental State Examinations (MMSE), was measured in each institution by the researchers of Fujita Health University.
Dialysis conditions and blood sampling
The schematic outline of hemodialysis is described in Fig. 1. Briefly, blood (usually from the patient’s arm) was introduced into a dialyzer, passed through the dialyzer, and returned to the body. Blood flowed inside the dialyzer’s hollow fibers, while dialysates flowed outside the fibers. All subjects received hemodialysis three times a week, except No. 107, who received hemodialysis four times a week (Table 1). The following dialysis conditions are summarized in Table 1: Qb (blood flow rates into dialyzers); Qd (dialysate flow rates into dialyzers); duration of one hemodialysis (HD) session; hollow fiber materials in dialyzers such as polysulfones (PSf), cellulose triacetates (CTA), ethylene vinyl alcohol copolymer (EVAL) and polyether polymer alloy (PEPA) with polyvinyl pyrrolidone (PVP). As a standard procedure, a bolus injection of 3000 U heparin was administered at the beginning of each dialysis session, followed by a continuous infusion at a rate of 500 U/h. Blood of Group A and B was sampled with EDTA-2 K (bis-potassium ethylenediamine triacetate) at the inlet (pre) of the dialyzer before (0 h), during the middle (1 h), and at the end (4 h) of hemodialysis sessions, and at the outlet (post) of the dialyzer at the 1 h and 4 h time points. Plasma Aβ concentrations at the inlet of the dialyzer were considered to be equivalent to those in whole body circulation at the designated times. The collected samples were centrifuged in the shortest time after sampling. The obtained plasma was divided into aliquots of 200–300 μl in polypropylene tubes. The plasma aliquots were stored at −20 °C for several hours. Frozen plasma was then transferred into a deep freezer and stored at −80 °C. Freeze and thaw cycles were minimized.
Measurements of blood concentrations
Plasma Aβ concentrations were measured using the High Sensitive Human β Amyloid (1-40) and (1-42) ELISA Kit Wako II (WAKO Pure Chemical, Osaka, Japan). Aβ oligomers were measured using the Human Amyloid β Oligomers (82E1-specific) Assay Kit (IBL, Fujioka, Gunma, Japan). ApoE4 was measured in plasma by the ApoE4/Pan-ApoE ELISA kit (MBL, Nagoya, Japan) and the results are shown in Table 1.
Aβ reduction rate for whole body circulation was defined as follows (Formula 1):
Aβ removal efficiency for the pre(inlet)/post(outlet) of the dialyzer was defined as follows (Formula 2):
Calculation of the Aβ influx index into the blood
When constant Aβ influx or production into the plasma during each hemodialysis session was postulated, the simulations fit the observed results based on a single-compartment model, at least during dialysis sessions (Kitaguchi et al. 2011). When there was no Aβ influx, plasma Aβ concentration could be calculated as C t(calc.) (Formula 3):
whose solution is:
where Ct is Aβ concentration in whole body circulation at time t, Co is Aβ concentration at the beginning of the dialysis, R is the removal efficiency of Aβ at pre/post of a dialyzer at time t, Qb is the blood flow rate, Hct is the hematocrit, and BW is the body weight of the patient.
The Aβ influx index into the blood from other organs/tissues, including the brain, at time t [Aβ Influx Index (t)] was defined by the subtraction of C t(calc.) from the observed Aβ concentration C t(obs.) (Formula 4):
Brain CT analysis
Brain computed tomography (CT) was performed at baseline for all patients of Group A and for 11 patients out of 14 of Group B. The scans were analyzed by an experienced neurologist to interpret the CT findings. Atrophy severity and other brain impairments were ranked from “−” (no lesion) to “5+” (most severe). These results are summarized in Table 2. All subjects had no strong atrophy or infarct, as measured by CT analysis.
PET imaging with Pittsburgh Compound B (PiB) as a probe
Three-dimensional static PET imaging for 50–70 min after intravenous injection of 555 ± 185 MBq PiB (N-methyl-11 C-2-(4-methylaminophenyl)-6-hydroxybenzothiazole) was carried out using a PET-CT camera, Biograph True V (Siemens). X-ray CT for attenuation correction was performed before PET imaging. PiB images were visually rated as PiB positive or PiB negative as described previously (Kaneko et al. 2014).
Neuropsychological examination
Cognitive function was measured by the Mini-Mental State Examination (MMSE) (Koumi et al. 2010). The maximum score was 30.
Smoking history
Patients were interviewed about their smoking history, including start age, age of smoking abstinence, and average number of cigarettes per day. Smoking dosage was defined as the product of years of smoking and the number of cigarettes per day.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) unless otherwise specified. Differences were determined using a Wilcoxon rank-sum test for non-parametric variables, a Wilcoxon signed-rank test for paired non-parametric variables and a Student’s t test for parametric continuous variables, unless otherwise specified, using the statistical package JMP11 (SAS Institute Inc., Cary, USA). Values of p < 0.05 were considered statistically significant.
Results
Change of plasma Aβ1–40 and Aβ1–42 concentrations from baseline to the 2nd line
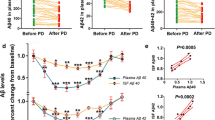
In Group A, plasma Aβ1–40 concentration levels at the 18-month evaluation (750.2 ± 129.3 pg/ml) significantly decreased from baseline (848.0 ± 123.3 pg/ml) (p = 0.0073) (Fig. 2a). In Group B, the plasma Aβ1-40 at the 36-month evaluation (628.7 ± 65.6 pg/ml) did not change from baseline (639.9 ± 146.4 pg/ml) (p = 0.78) (Fig. 2b). In contrast to Aβ1-40 levels, plasma Aβ1–42 levels remained unchanged in Group A (from 64.5 ± 13.7 pg/ml at baseline to 66.6 ± 12.9 pg/ml at the 18-month evaluation, p = 0.51) (Fig. 2c) and significantly increased in Group B (from 61.9 ± 10.7 pg/ml at baseline to 73.0 ± 12.7 pg/ml at the 36-month evaluation, p = 0.0025) (Fig. 2d). As a result, the ratio of plasma Aβ1–42/Aβ1-40 slightly increased for Group A (from 7.7 ± 1.6 to 9.1 ± 2.6, p = 0.013) (Fig. 2e) and Group B (from 10.1 ± 2.8 to 11.6 ± 1.8, p = 0.075) (Fig. 2f).
Change of plasma Aβ concentrations and the pre/post-Aβ removal efficiency from the baseline to the 2nd line. a Group A changes in plasma Aβ1–40; b Group B changes in plasma Aβ1–40; c Group A changes in plasma Aβ1–42; d Group B changes in plasma Aβ1–42; e Group A changes in the ratio of plasma Aβ1–42/Aβ1–40; f Group B changes in the ratio of plasma Aβ1–42/Aβ1–40; g Group A changes in pre/post-Aβ removal efficiency of Aβ1–40 at 1 h; h Group B changes in pre/post-Aβ removal efficiency of Aβ1–40 at 1 h; i Group A changes in pre/post-Aβ removal efficiency of Aβ1–42 at 1 h; j Group B changes in pre/post-Aβ removal efficiency of Aβ1–42 at 1 h
The resulting decrease/no change of Aβ1–40 levels and increase/no change of Aβ1–42 levels at the 2nd line evaluation were not directly attributed to changes in Aβ removal efficiency for the pre(inlet)/post(outlet) dialyzers, because the change in Aβ removal efficiencies demonstrated an opposite tendency compared to plasma Aβ change. During baseline, the Aβ1-40 removal efficiency at the 1 h time point of each dialysis session (65.3 ± 10.7 %) was significantly decreased compared to the 2nd line for Group A (57.9 ± 8.6 %, p = 0.0011) (Fig. 2g). For Group B, the Aβ1–40 removal efficiency at the 1 h time point of each dialysis session was 70.1 ± 6.9 % at the baseline, and 60.9 ± 16.5 % at the 2nd line, resulting in a non-significant decrease (p = 0.103) (Fig. 2h). For Aβ1-42 removal in both Groups A and B, the Aβ1–42 removal efficiency for the pre(inlet)/post(outlet) of the dialyzers at the 1 h time point of each dialysis session was maintained at the 2nd line, compared with baseline: (Group A: 48.7 ± 10.5 % at baseline and 46.3 ± 9.1 % at the 2nd line, p = 0.254; Fig. 2i) (Group B: 55.2 ± 4.1 % at baseline and 49.7 ± 10.6 % at the 2nd line; p = 0.129; Fig. 2j).
Comparison of cognitive function at baseline and the 2nd line
MMSE score averages for both Group A and B were mostly maintained during the evaluation period (Group A: 27.1 ± 3.0 at baseline, 27.4 ± 2.0 at the 2nd line; Group B: 27.4 ± 1.7 at baseline to 28.4 ± 1.9 at the 2nd line) (Fig. 3a, b). MMSE scores of 21 patients out of 30 subjects increased or maintained at the 2nd line evaluation when the scores were analyzed individually (p = 0.043) (Fig. 3c).
Cognitive functions (MMSE) of hemodialysis patients. a, b, c show change of MMSE scores from baseline to the 2nd line; a for Group A; b for Group B; c the individual change of MMSE. d Correlation between the degree of white matter ischemia at baseline and MMSE scores at the 2nd line; −, n = 9; ±, n = 1; 1+, n = 12; 2+, n = 4
Using analysis of brain CT scans, there were no correlations between MMSE scores for either Group A or B at baseline to several measurements, including brain atrophy (frontal, temporal, and parietal lobes as well as hippocampus), ventricular enlargement, white matter ischemia, infarct lesion, and cerebroartery calcification (Table 2). However, patients whose MMSE scores decreased by 4 or 5 points (No. 107 and N02, respectively; Table 2) exhibited a score of 2+ regarding cerebroartery calcification as well as several cortical atrophies. Further, MMSE scores at the 2nd line correlated with white matter ischemia scores at baseline (Fig. 3d).
These findings suggest that hemodialysis, when performed three times a week to effectively remove blood Aβ, may maintain or slightly improve cognitive function.
Calculation of Aβ influx into the blood
The purpose of E-BARS is to accelerate the transport of Aβ out of the brain and into the blood by removing blood Aβ extracorporeally. Therefore, Aβ influx into the blood at the 1 h and 4 h time points during each dialysis session was estimated using the Aβ Influx Index (as defined by Formula 4). The results are summarized in Table 3. The Aβ1–40 Influx Index, which reflected Aβ1–40 influx into the blood, decreased at the 2nd line compared with baseline (Group A: p = 0.0196 for 1 h; Group B: p = 0.0197 for 1 h, p = 0.0483 for 4 h). For Group A, the difference for the Aβ1-40 Influx Index at the 4 h time point was not significant (p = 0.133). In contrast to Aβ1–40, the Aβ1–42 Influx Index was maintained between baseline and the 2nd line for both groups (Table 3).
Cognitive assessments at the 2nd line correlated with the Aβ1–40 Influx Index at the 4 h time point of each dialysis session. MMSE scores at 2nd line were strongly and positively correlated with the Aβ1–40 Influx Index at the 4 h time point of each dialysis session at baseline [INF(Aβ40 at 4 h BL)] (p = 0.0099; Fig. 4a). MMSE scores at 2nd line were negatively correlated with increases in the Aβ1–40 Influx Index at the 4 h time point from baseline to the 2nd line [ΔINF(Aβ40 at 4 h)2nd L-BL] (p = 0.0002; Fig. 4b).
The effect of smoking history on cognitive function
There were no significant differences in cognitive function between patients who had a previous smoking history (Group Yes) and those without a smoking history (Group No). At baseline, MMSE scores were 27.5 ± 2.0 for Group Yes and 26.9 ± 2.8 for Group No; at the 2nd line, MMSE scores were 28.0 ± 2.0 for Group Yes and 27.7 ± 2.0 for Group No.
Frontal/temporal atrophy and temporal/parietal atrophy were more severe in Group Yes than Group No, as detected by brain CT scans (p = 0.0465 and p = 0.0062, respectively, by χ 2 test) (Fig. 5).
Correlation between smoking history and brain atrophy of combined Groups A and B at baseline. a frontal/temporal atrophy (p = 0.0465, χ 2-test); b temporal/parietal atrophy (p = 0.0062, χ 2 test). FL frontal lobe, TL temporal lobe, PL parietal lobe. No Group No; Yes Group Yes based on smoking history
No differences were observed between Group Yes and Group No in plasma concentration of Aβ1–40 and Aβ1–42, removal efficiencies of pre/post-dialyzers, reduction rates of Aβ1–40 and Aβ1–42, and Aβ Influx Indices (data not shown). Smoking history did not correlate with Aβ values.
Prospective study of renal failure patients during periods preceding and following the initiation of hemodialysis (Group C)
To investigate whether the initiation of hemodialysis affected plasma Aβ concentration levels and cognitive function, five renal failure patients were recruited and evaluated before and after the initiation of hemodialysis (i.e., from renal failure without hemodialysis to after hemodialysis initiation). The longest observation period was 721 days, from Day −133 to Day +588, with Day 0 defined as initiation of hemodialysis. Plasma Aβ1–40, Aβ1–42, and plasma Aβ oligomers decreased after hemodialysis initiation; these lowered levels were maintained throughout hemodialysis (Fig. 6a–c). Cognitive function also improved after hemodialysis initiation (Fig. 6d).
Brain Aβ accumulation was measured with PiB/PET in two patients, a 64-year-old woman on the 125th day after hemodialysis initiation and a 65-year-old man on the 320th day after initiation. Both patients showed little Aβ accumulation in the brains (PiB negative, data not shown).
Discussion
In our prospective study, end-stage renal failure patients received hemodialysis three times a week for either 18 or 36 months as renal replacement therapy, which also means removal of blood Aβ three times a week. Although plasma Aβ1–40 levels either decreased or did not change, Aβ1–42 levels either were unchanged or significantly increased at the 2nd line. As a result, the ratio of plasma Aβ1–42/Aβ1–40 slightly increased (Fig. 2). Further, cognitive function was maintained or slightly improved (Fig. 3a–c). Initiation of hemodialysis lowered high Aβ concentrations in the blood of renal failure patients who had not received hemodialysis (Fig. 6). These data indicate that long-term hemodialysis, which removes blood Aβ, may affect blood Aβs concentration levels as well as Aβ influx into the blood from certain tissues.
From the point of view of the total mass balance of Aβs, the influx of Aβs into the blood during hemodialysis sessions are roughly estimated by comparison of total Aβs removed by a dialyzer and the total blood Aβs changed in systemic circulation during a hemodialysis session. For the combined Group A and B at the baseline, the average Aβ removal efficiencies for the pre/post of dialyzers at the 1 h point of dialysis sessions were as follows; 67.3 ± 8.7 % for Aβ1–40 and 51.3 ± 8.8 % for Aβ1–42. These Aβ removal efficiencies at 1 h point are used for the mass balance estimation because we already revealed that Aβ removal efficiencies at the pre/post of dialyzers are not significantly changed at 1 h and 4 h of a hemodialysis session (Kato et al. 2012). Total Aβs removed by a dialyzer in one hemodialysis session are calculated as the products of (Aβ removal efficiencies) × (plasma Aβ concentrations at pre-dialyzer at the designated time) × (1-hematocrit/100) × Qb × (the designated time period), where Qb (blood flow rate), 200 ml/min; average hematocrit, 35 %; total session time, 240 min. The average Aβ concentration at 0 h and 1 h is used for Aβ removed by a dialyzer from 0 to 1 h (0–1 h) and the average concentration at 1 h and 4 h is for Aβ removed from 1 to 4 h (1–4 h). The total Aβ removed during whole 4 h sessions is the sum of 0–1 and 1–4 h. Then, the blood Aβs which were decreased in systemic circulation during a dialysis session are estimated as the product of (whole blood volume) × (1-hematocrit/100) × (plasma Aβ concentrations at pre-dialyzer at the designated time, which is regarded as Aβ concentrations in systemic circulation), where whole blood is 4000 ml. The results are summarized in Table 4. Total Aβ1–40 removed by a dialyzer during a hemodialysis session is calculated as 10.3 μg and the blood Aβ1–40 decreased in systemic circulation is 1.0 μg. Therefore, the total Aβ1–40 influx into the blood (including the production in the blood) during a hemodialysis session is estimated at 9.2 μg as the subtraction of the two values above. Similarly, total Aβ1–42 removed by a dialyzer is estimated at 0.78 μg and the blood Aβ1–42 decreased in systemic circulation is 0.06 μg, resulting in 0.72 μg as the total Aβ1-42 influx into the blood during a hemodialysis session. Meanwhile, the Aβ concentration in cerebrospinal fluid (CSF) of AD is reported as 7.4–42.7 ng/ml for Aβ1–40 and 0.12–0.67 ng/ml for Aβ1–42 (Schoonenboom et al. 2005). Because the total amount of CSF is reported as 150 ml (Megson et al. 1996), the total amount of Aβs in CSF at certain moment, regardless of time-dependent production of Aβs in the brain, is estimated at 6.4 μg for Aβ1–40 and 0.10 μg for Aβ1–42, which are comparable with this roughly estimated total Aβs influx into the blood during one hemodialysis session of 4 h, 9.2 μg for Aβ1–40 and 0.7 μg for Aβ1–42, as described above. However, this estimation does not indicate the origin of Aβ influx (preferably from the brain). It should be revealed by Aβ imaging of the brain or biochemical study which includes the measurement of brain-originated Aβ in the blood.
Regarding the unchanged/increasing tendency of blood Aβ1–42 in this prospective study (Groups A and B), Aβ1–42 is well known to deposit in the brain as senile plaques and is revealed to decrease in CSF of AD compared with cognitively normal subjects by the study of Alzheimer’s Disease Neuroimaging Initiative (ADNI) (Shaw et al. 2009). Wiltfang reported that the concentration of Aβ1–42 in CSF of AD is lower than non-demented disease controls whereas Aβ1–40 shows no apparent difference (Wiltfang et al. 2002). This decrease of Aβ1–42 in CSF of AD is thought to be caused by deposition of Aβ1-42 as senile plaques. The subjects in the present study were not AD patients, but hemodialysis patients who received Aβ removal by dialyzers three times a week. The changes of blood Aβ concentrations in the present prospective study (Groups A and B) may be strongly affected by Aβ removal and the influx into the blood. Meanwhile, both Aβ1–40 and Aβ1–42 in the blood of cognitive normal subjects increased during a 5-year observation period (Lopez et al. 2008). Therefore, the results of our study, blood Aβ1–40 was decreased/unchanged and blood Aβ1-42 was unchanged/increased, are not directly related to decrease of CSF Aβ1–42 in AD. Our results of prospective change of blood Aβs might be caused by the influx into the blood from certain organs/tissues triggered by blood Aβ removal, not by deposition of Aβs.
Then, we analyzed the relation between Aβ influx and the change of Aβ in the blood from the baseline to the 2nd line. Although Aβ1-40 removal efficiencies at the 2nd line decreased or were maintained (i.e., removed less Aβ), the plasma Aβ1–40 concentrations for whole body circulation were maintained or slightly decreased at the 2nd line. This can be explained by decreased Aβ1–40 influx into the blood (Aβ Influx Index in Table 3). In other words, the decreasing or unchanging plasma Aβ1–40 levels in whole body circulation may be attributed to the decrease of Aβ1–40 influx at the 2nd line, although the Aβ1–40 removal efficiency of the dialyzers (i.e., Aβ removal devices) did not increase at the 2nd line (Figs. 2g, h, 7). The decrease in Aβ1–40 accumulation (i.e., decrease or exhaust of Aβ1–40 influx source) in the brain, including cerebral vessels, may be one possible reason why Aβ1–40 influx decreased at the 2nd line. This speculation is consistent with histopathological findings that demonstrated almost no cerebral amyloid angiopathy, which mainly consists of Aβ1–40, in hemodialysis patients (Sakai et al., in preparation). Moreover, cognitive function at the 2nd line strongly correlated with Aβ1–40 influx into the blood (Aβ Influx Index) at the 4 h time point (Fig. 4a). Further, cognitive function at the 2nd line strongly, but negatively, correlated with differences in the Aβ1–40 Influx Index between the 2nd line and baseline (Fig. 4b). It might relate to the decrease of Aβ1–40 accumulation described above. These relationships between Aβ1–40 Influx Index and cognitive function at the 2nd line are only correlation and do not indicate any cause and effect at present.
In contrast to Aβ1–40, the Aβ1–42 Influx Index (Table 3) and Aβ1–42 removal efficiency at the pre/post-dialyzers (Fig. 2i, j) did not reveal any clear relationship with MMSE change between baseline and the 2nd line. However, plasma Aβ1–42 levels maintained unchanged or increased from baseline to the 2nd line. This could be explained by a continuous influx of Aβ1–42 from a large source or pool of Aβ1–42, such as the brain, during the observation period (Fig. 7). This speculation is also consistent with histopathological findings that demonstrated fewer, but not no, senile plaques, which mainly consists of Aβ1–42, in hemodialysis patients compared to age-matched non-hemodialysis subjects (Sakai et al., in preparation).
In the point of view of the cognitive functions shown in Figs. 3c and 6d, removal of uremic toxins may somewhat contribute to the improvement of MMSE (Yaffe et al. 2010). However, the cognitive impairment caused by uremia is generally worst just before the initiation of hemodialysis and is improved in one or a few weeks after the initiation of hemodialysis. Some of the subjects of Group C showed the improvement of cognitive functions just after the initiation of hemodialysis as indicated in Fig. 6d. This kind of improvement might be attributed to the removal of uremic toxins. Contrary to the improvement in rather short period after the initiation of hemodialysis, the improvement of cognitive functions during the period of 18 and 36 months after 2–36 years duration of hemodialysis (Table 1; Fig. 3c) might not be caused by the removal of uremic toxins. Further, hemodialysis removes not only blood Aβ but also many kinds of small molecules and proteins smaller than albumin. Therefore, the cause of this improvement of cognitive functions observed in Group A, B and C is not clear at present.
Meanwhile, higher concentrations of plasma Aβ1–42 and a greater plasma Aβ1–42/Aβ1–40 ratio have been reported to be good predictive biomarkers for non-converters from healthy controls to AD during the observation period (Rembach et al. 2014). Therefore, the somewhat increase of plasma Aβ1–42/Aβ1–40 ratio in our study (Fig. 2e, f) may also relate to the slight improvement of cognitive function (Fig. 3c).
Regarding the difference between Group A and B, both groups exhibited similar tendencies of plasma levels of Aβ1–40 and Aβ1–42, but with some differences during the period between baseline and the 2nd evaluation. Further, the length of hemodialysis at baseline for Group B was significantly longer than the length of hemodialysis for Group A (18.3 vs. 8.8 years, respectively; p = 0.0076 by Wilcoxon/Kruskal–Wallis test) (Table 1). The plasma levels of Aβ1–40 and Aβ1-42 of Group B might be regarded as the later stage of Group A.
In AD patients who smoked, levels of soluble and insoluble Aβ1–40 and Aβ1–42 were significantly decreased in the frontal cortex, while levels of Aβ1-40 were significantly decreased in temporal cortex and hippocampus, compared to non-smokers with AD (Hellstroem-Lindahl et al. 2004). In our study, the subjects did not have AD. There were no obvious differences in baseline or the 2nd line blood Aβs levels between subjects with and without a smoking history. MMSE scores also did not differ based on smoking history. However, brain atrophies (i.e., frontal/temporal atrophy and temporal/parietal atrophy) were more severe in patients with a smoking history compared to those without a smoking history (Fig. 5). This suggests that while smoking may have enhanced atrophy of some cortex regions, it did not explicitly impair cognitive function of hemodialysis patients.
Brain CT scans taken at baseline revealed that the only two patients whose MMSE scores decreased 4 and 5 points at the 2nd line exhibited a score of 2+ for cerebroartery calcification (Fig. 3c; Table 2). Further, only white matter ischemia, as assessed by CT analysis, correlated with MMSE scores at the 2nd line (Fig. 3d). This suggests that cognitive impairment due to ischemic and vascular impairments was not improved by hemodialysis, although possible cognitive impairment caused by brain atrophy may have been somehow rescued by hemodialysis.
As a preliminary study, we conducted brain Aβ imaging using PiB/PET on two patients, a 64-year-old woman on the 125th day and a 65-year-old man on the 320th day after hemodialysis initiation. While the results were negative of Aβ accumulation for both cases, we were unable to conduct PiB/PET measurements prior to hemodialysis to generate a baseline. Therefore, we can make no concrete conclusions about these data at present.
Conclusion
As a model of therapeutic system for AD, hemodialysis patients whose blood Aβ was removed three times a week were investigated prospectively. Our prospective study of 5 renal failure patients before and after the initiation of hemodialysis revealed that plasma Aβ concentrations were decreased and cognitive function was improved after hemodialysis initiation. Our another prospective study of 30 hemodialysis patients, who received Aβ removal three times a week (by hemodialysis during the period between baseline and 18 or 36 months after) demonstrated that plasma Aβ1–40 levels decreased or remained unchanged, while Aβ1–42 levels were unchanged or significantly increased at the 2nd line. Based on the Aβ removal efficiencies of the dialyzers and analysis of Aβ influx into the blood, these data suggest that blood Aβ1–40 was removed, resulting in a decrease of Aβ1–40 influx sources. In addition, Aβ1–42 was also removed, but the influx into the blood still continued after 36 months. Importantly, cognitive function of most subjects, as assessed by MMSE scores, increased or was maintained at the 2nd lines. Therefore, a blood Aβ removal system is worth further investigation to develop this model for clinical application for therapy or prevention of AD.
References
Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV (2007) Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27:909–918
Boada M, Ortiz P, Anaya F, Hernández I, Muñoz J, Núñez L, Olazarán J, Roca I, Cuberas G, Tárraga L, Buendia M, Pla RP, Ferrer I, Páez A (2009) Amyloid-targeted therapeutics in Alzheimer’s disease: use of human albumin in plasma exchange as a novel approach for Abeta mobilization. Drug News Perspect 22:325–326
DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM (2001) Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 98:8850–8855
Donahue JE, Flaherty SL, Johanson CE, Duncan JA 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG (2006) RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol 4:405–415
Hellström-Lindahl E, Mousavi M, Ravid R, Nordberg A (2004) Reduced levels of Abeta 40 and Abeta 42 in brains of smoking controls and Alzheimer’s patients. Neurobiol Dis 15:351–360
Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM (2003) Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron 38:547–554
Hung LW, Ciccotosto GD, Giannakis E, Tew DJ, Perez K, Masters CL, Cappai R, Wade JD, Barnham KJ (2008) Amyloid-b peptide (Ab) neurotoxicity is modulated by the rate of peptide aggregation: Ab dimers and trimers correlate with neurotoxicity. J Neurosci 28:11950–11958
Kaneko N, Nakamura A, Washimi Y, Kato T, Sakurai T, Arahata Y, Bundo M, Takeda A, Niida S, Ito K, Toba K, Tanaka K, Yanagisawa K (2014) Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B Phys Biol Sci 90:353–364
Kato M, Kawaguchi K, Nakai S, Murakami K, Hori H, Ohashi A, Hiki Y, Ito S, Shimano Y, Suzuki N, Sugiyama S, Ogawa H, Kusimoto H, Mutoh T, Yuzawa Y, Kitaguchi N (2012) Potential therapeutic system for Alzheimer’s disease: removal of blood Abs by hemodialyzers and its effect on the cognitive functions of renal-failure patients. J Neural Transm 12:1533–1544
Kawaguchi K, Kitaguchi N, Nakai S, Murakami K, Asakura K, Mutoh T, Fujita Y, Sugiyama S (2010) Novel therapeutic approach for Alzheimer’s disease by removing amyloid-β protein from the brain with an extracorporeal removal system. J Artif Organs 13:31–37
Kitaguchi N, Kawaguchi K, Nakai S, Murakami K, Ito S, Hoshino H, Hori H, Ohashi A, Shimano Y, Suzuki N, Yuzawa Y, Mutoh T, Sugiyama S (2011) Reduction of Alzheimer’s Disease Amyloid-β in plasma by hemodialysis and its relation to cognitive functions. Blood Purif 32:57–62
Koumi H, Maeda A, Yamamoto A, Kato Y, Okamura K, Sonoda K, Ando E, Kishikawa Y (2010) The sensitivity and specificity of Japanese version of the Mini-Mental Sate Examination. Bull Facul Soc Welfare Hanazono Univ 18:91–95
Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE (1996) Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem 271:4077–4081
Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R, DeKosky ST (2008) Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 70:1664–1671
Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K (2003) Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral Administration of agents with an affinity to β-amyloid. J Neurosci 23:29–33
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330(6012):1774
Megson GM, Stevens DA, Hamilton JR, Dennig DW (1996) d-mannitol in cerebrospinal fluid of patients with AIDS and cryptococcal meningitis. J Clin Microbiol 34:218–221
Morris AWJ, Carare RO, Schreiber S, Hawkes CA (2014) The cerebrovascular basement membrane: role in the clearance of β-amyloid and cerebral amyloid angiopathy, Front. Aging Neurosci 6:1–9
Rembach A, Watt AD, Wilson WJ, Villemagne VL, Burnham SC, Ellis KA, Maruff P, Ames D, Rowe CC, Macaulay SL, Bush AI, Martins RN, Masters CL, Doecke JD, AIBL Research Group (2014) Plasma amyloid-β levels are significantly associated with a transition toward Alzheimer’s disease as measured by cognitive decline and change in neocortical amyloid burden. J Alzheimers Dis 40:95–104
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–177
Schoonenboom NS, Mulder C, Van Kamp GJ, Mehta SP, Scheltens P, Blankenstein MA, Mehta PD (2005) Amyloid beta 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol 58:139–142
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiolo Rev 81:741–766
Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Initiative Alzheimer’s Disease Neuroimaging (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65(4):403–413
Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE (2010) Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J Neuropathol Exp Neurol 69:98–108
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, Paul S, Schmidt B, Klafki HW, Maler M, Dyrks T, Bienert M, Beyermann M, Rüther E, Kornhuber J (2002) Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem 81:481–496
Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G (2010) Chronic kidney disease and cognitive function in older adults: findings from the Chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58:338–345
Acknowledgments
The authors sincerely thank Ms. Miwa Sakata for her technical assistance and Ms. Michiyo Hata for discussion regarding the cognitive function of Group B. This work was partly supported by KAKENHI (20509008, 23500531 and 26282126), Smoking Research Foundation, and Suzuken Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This study conformed to the Declaration of Helsinki Good Clinical Practice. This research was comprehensively reviewed and approved by the institutional review board (IRB) at Fujita Health University, which is responsible for the entire study. The study protocol approved by Fujita Health University was also approved by the IRB at each participating institution: Chiryu Clinic, Kawana Hospital of Seiju-Kai Group, and Chita City Hospital. All subjects provided written informed consent before participation.
Conflict of interest
Nobuya Kitaguchi and Kazunori Kawaguchi received a research grant from Asahi Kasei Medical Co., Ltd. Nobuya Kitaguchi owns stock in Asahi Kasei Corporation. The other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kitaguchi, N., Hasegawa, M., Ito, S. et al. A prospective study on blood Aβ levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer’s disease. J Neural Transm 122, 1593–1607 (2015). https://doi.org/10.1007/s00702-015-1431-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-015-1431-3