Abstract
Sporadic Parkinson disease (sPD) or brainstem-predominant type of Lewy body (LB) disease, and dementia with Lewy bodies (DLB), the two most frequent α-synucleinopathies, are progressive multisystem neurodegenerative disorders with widespread occurrence of α-synuclein (AS) deposits in the central, peripheral, and autonomic nervous system. For both LB-related disorders, staging/classification systems based on semiquantitative assessment of the distribution and progression pattern of Lewy-related/AS pathology are used that are considered to be linked to clinical dysfunctions. In PD, a six-stage system (Braak) has been suggested to indicate a predictable sequence of lesions with ascending progression from medullary and olfactory nuclei to the cortex, the first two presymptomatic stages being related to incidental LB disease, stages 3 and 4 with motor symptoms, and the last two (cortical) stages may be frequently associated with cognitive impairment. DLB, according to consensus pathologic guidelines, by semiquantitative scoring of AS pathology (LB density and distribution) in specific brain regions, is distinguished into three phenotypes (brainstem, transitional/limbic, and diffuse neocortical), also considering concomitant Alzheimer-related pathology. Retrospective clinico-pathologic studies, although largely confirming the staging system, particularly for younger onset PD with long duration, have shown that between 6.3 and 43% of the cases did not follow the proposed caudo-rostral progression pattern of AS pathology. There was sparing of medullary nuclei in 7–8.3% of clinically manifested PD cases with AS inclusions in midbrain and cortex corresponding to Braak stages 4 and 5, whereas mild parkinsonian symptoms were already observed in stages 2 and 3. There is considerable clinical and pathologic overlap between PD (with or without dementia) and DLB, corresponding to Braak LB stages 5 and 6, both frequently associated with variable Alzheimer-type pathology. Dementia often does not correlate with progressed stages of LB pathology, but may also be related to concomitant Alzheimer lesions or mixed pathologies. There is no relationship between Braak LB stage and the clinical severity of PD, and the predictive validity of this concept is doubtful, since large unselected, retrospective autopsy series in 30–55% of elderly subjects with widespread AS/Lewy-related pathology (Braak stages 5 and 6) reported no definite neuropsychiatric symptoms, suggesting considerable cerebral compensatory mechanisms. Applying the original criteria to large dementia samples, 49% of positive cases were not classifiable. Therefore, modified criteria for the categorization of Lewy-related pathology were proposed for patients with a history of dementia. The causes and molecular basis of the not infrequent deviations from the current staging schemes of AS pathology in PD and DLB, its relation to the onset of classical parkinsonian symptoms and for the lack of definite clinical deficits despite widespread AS pathology in the nervous system remain to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lewy body (LB)-associated disorders, such as sporadic Parkinson disease (sPD) and dementia with Lewy bodies (DLB), are progressive neurodegenerative disorders, morphologically characterized by accumulation of abnormal filamentous protein inclusions with α-synuclein (AS) as their major component (for reference, see [77, 85]). These inclusions in neurons and neurites [LBs and Lewy neurites (LNs)], glia [25, 143], and presynaptic terminals [101] may involve the central, peripheral, and autonomic nervous systems [85], recently referred to as ‘classic parkinsonism’ [55]. Given the fundamental nature of the AS-containing lesions, these and other disorders, such as autonomic failure, LB dysphagia, and multiple system atrophy (MSA), have been summarized as α-synucleinopathies [56, 85, 158].
sPD or brainstem type of LB disease [96, 119, 120] is the most common neurodegenerative movement disorder in the elderly, with progressive degeneration of the dopaminergic nigrostriatal system and other neuronal networks, caused by loss of pigmented neurons in the substantia nigra compacta (SNc) and in a variety of subcortical nuclei including nondopaminergic ones [24]. There is widespread occurrence of LBs and LNs in the brain and—very early—in specific nuclei of the spinal cord [21, 93]. Lewy-related pathology (LRP) is not confined to the central nervous system, but also involves autonomic nuclei, sympathetic ganglia, cardiac and pelvic plexuses, nerves, as well as adrenal medulla, salivary glands, and skin [9, 16, 21, 23–26, 39, 66, 74, 80, 132, 134, 136, 148, 165, 173, 174, 176, 177], clearly indicating that PD is a true multisystem neurodegenerative disorder (for review, see [25, 26, 85]). This is in agreement with animal studies demonstrating that AS lesions restricted to the SN or dopamine pathways do not necessarily model human sporadic PD [122].
DLB, the second most frequent cause of dementia in the elderly after Alzheimer disease (AD), with the core neuropsychiatric features of fluctuating consciousness, visual hallucinations, and parkinsonism, is morphologically featured by a variable burden of α-synucleinopathy with (often widespread) cortical LBs and various degrees of AD-related pathology [119, 120].
The distribution pattern of AS/LRP within selectively vulnerable neuronal populations has been considered to be intimately linked to the neurological dysfunctions seen in both disorders. AS lesions in the brainstem have been mainly claimed to be responsible for extrapyramidal motor symptoms, whereas cognitive impairment has been attributed to the limbic and neocortical spread of LB lesions with or without concomitant Alzheimer-type pathology [10, 75, 100, 117, 118]. At present, PD and DLB are believed to represent phenotypes in a continuum within the spectrum of LB disorders, wherein the clinical manifestations predominantly depend on the anatomical distribution of LBs and the load of AS pathology [20, 74, 76, 97, 120]. Because of clinical, biochemical, and morphologic similarities and dissimilarities, the relations between the two disorders have been discussed controversially [1, 54, 85, 109, 128, 130, 151, 168].
Two morphological staging/classification systems are currently used for the assessment of the progressive regional distribution of AS pathology in PD and DLB [20, 119, 120], the applicability and clinical relevance of which has been critically discussed recently [7, 89, 91, 108, 142]. A third classification considers LRP predominantly involving the neocortex, referred to as diffuse type of LB disease [98], whereas for dementia samples a modification in the published criteria has been proposed that allows classification of a greater number of Lewy-positive cases [106]. However, it should be emphasized that the two morphologic staging systems of AS pathology should be considered in a different manner: (1) While at present the pathologic diagnosis of sPD does not seem to be questioned (except for one negative statement [171]), its value for the assessment of the progression of PD pathology and its relation to clinical symptomatology are under current discussion; (2) The (revised) consensus pathologic guidelines for DLB, due to still existing difficulties in the diagnosis of this disease, are not prepared to be related to the disease progression; (3) Both staging systems are not useful for the cases with predominant clinical dementia, although this point has recently been a matter of discussion [106, 140, 142]. With these reservations in mind, the essentials and validity of the two major grading classification systems for the pathologic assessment of LRP will be critically evaluated, based on personal studies and recent literature, although, admittedly, not all studies (most of them retrospective) are comparable.
Staging of Lewy-related pathology in sporadic PD
Based on semiquantitative assessment of AS-positive inclusions in 413 autopsies including 41 PD cases, 69 with AS inclusions, and 58 aged-matched controls, a hypothetic staging system of brain pathology indicating a predictable sequence with increasing severity throughout the brain and ascending progression has been proposed [19–21, 24, 26, 37]. AS/LRP was divided into six successive stages: The earliest lesions were seen in the lower medulla oblongata with a few LNs in the dorsal motor nucleus of the vagus nerve (DMV), in anterior olfactory structures, chiefly affecting the anterior olfactory nucleus embedded in the olfactory tract, and some AS-positive aggregates in preganglionic vagal axons [24] and in the enteric nervous system [26], with the nucleus basalis of Meynert (NBM) and midbrain regions being preserved (stage 1). More severe lesions in the DMV with extension to the caudal raphe nuclei and gigantocellular portions of the adjoining reticular formation, some LNs in the noradrenergic locus coeruleus (LC), and involvement of the enteric nervous system are seen in stage 2. These initial stages (observed in 7% of the cohort) were considered (pre)symptomatic and may explain early nonmotor (autonomic and olfactory) symptoms that preceed the somato-motor dysfunctions [36, 66, 150, 183]. In stage 3, the LC, the central nucleus of the amygdala, the tegmental pedunculo-pontine nucleus, and cholinergic nuclei of the basal forebrain including the NBM are the focus of cytoskeletal changes and neuronal depletions, the posterolateral and posteromedial parts of SNc showing pale bodies (precursors of LBs [33]) and LBs without neuronal loss with additional involvement of the hypothalamic nuclei, while the allocortex and neocortex are preserved. In stage 4, in addition to destruction of SNc, the principal and parabrachial brainstem nuclei, the anteromedial temporal limbic (transentorhinal) and mesocortex, and amygdala are affected. Stages 3 and 4 have been correlated with clinical motor symptoms (11% of their cohort). In stage 5, the lesions in the temporal mesocortex are more striking and from there progress to adjoining association fields of the temporal and prefrontal neocortex, while in stage 6, the lesions involve the neocortex, first affecting the high-order sensory association cortex and prefrontal areas, later progressing to the primary sensory and motor areas or involving the whole neocortex. The cases with severe LB pathology (stages 5 and 6), accounting for 6% of their cohort, frequently show cognitive decline that was suggested to relate to the severity of the neuropathologic stage [22–24]. The late stages 5 and 6 of LB pathology (involvement of neocortex) suggest overlap or transition between PD and DLB [82, 83, 85, 155]. Unfortunately, limited clinical informations in a number of autopsy cases, the lack of neuron counts, quantitative methods, and of immunohistopchemistry to identify neuronal types seriously undermine the validity of the Braak hypothesis of LB staging in PD [91]. More recently, the origininal LB staging scheme has been revised, proposing that AS pathology in PD begins in the olfactory bulb and within enteric cell plexuses [71]. This has lead to the so-called “dual-hit hypothesis,” whereby an unknown neurotoxic pathogen enters the brain either by the nasal route with anterograde progression to the amygdala and temporal lobe or after gastric entry by subsequent retrograde and transneuronal transport. Recent studies, however, have clearly shown that, in incidental LB disease (ILBD/preclinial PD), AS pathology simultaneously occurs in multiple regions of the central and peripheral nervous system, which argues against a uniform caudo-rostral progression of AS pathology [43, 89, 91].
Consensus pathologic guidelines for the diagnosis of DLB
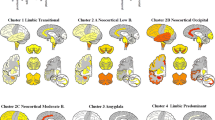
According to the consensus pathologic guidelines, LBs are scored semiquantitatively according to the severity and anatomical distribution, separating brainstem-predominant (PD), limbic/transitional, and diffuse neocortical types, depending on the anatomical distribution of the AS-positive structures [119]. The revised consensus guidelines [120] proposed semiquantitative assessment of LB density, based on AS immunohistochemistry in brainstem, limbic, and five cortical regions (Tables 1, 2). Another pattern concentrated on AS pathology in cortical areas [98]. Considering the significance and clinical impact of concomitant AD-related pathology frequently seen in aged subjects with and without dementia, the revised consensus criteria for DLB have recommended to take it into account seriously (Table 3). This protocol was simplified by excluding the frontal region because of the common occurrence of occasional LBs in these regions in PD in the absence of dementia [68]. However, the stage of AD-related pathology is usually not associated with the particular pattern of AS lesions [85, 186].
These guidelines did not provide definite diagnostic criteria, as it is sometimes mistakenly assumed, and were not included in the CERAD protocol, which is used for the semiquantitative evaluation of neuritic plaques and neurofibrillary tangles [123]. Moreover, this classification scheme has not been systematically applied to autopsy samples of dementia patients. One available study found that 49% of LB-positive demented autopsy cases were not classifiable; therefore, modification of the published McKeith criteria were proposed (Table 4). According to the Hisayama autopsy cohort study, 10.3% of the nondemented and 31.2% of the demented elderly subjects showed LB pathology. Applying the new DLB criteria [120] to 205 demented subjects, 5.4% were brainstem-predominant and 11.7% each were limbic and diffuse neocortical type. The likelihood of DLB modified by concomitant AD pathology was as follows: 13.2% showed low, 7.8% each intermediate and high likelihood. Both the latter groups were considered pathologic DLB [53].
Studies to evaluate the reliability of Lewy-related stagings
The proposed staging procedure for AS pathology in PD rested, in part, on the assumption that incidental LB pathology is the first step along a disease continuum [37], but that sPD, like most neurodegenerative disorders, is not a static but a dynamic biologic process and that so-called incidental lesions (LBs/LNs seen in subjects without PD related signs and symptoms) represent presymptomatic (subclinical) correlates of a pathologic condition ultimately leading to a manifest clinical disease [23, 41, 51, 59].
Incidental LB disease (ILBD) is based on the presence of LBs in the nervous system in subjects without clinically documented parkinsonism or cognitive impairment [51, 59], which was not considered when establishing the initial studies on the staging of LRP in PD [37]. Epidemiological studies indicated that autonomic symptoms, REM sleep behavioral disorder, and olfactory dysfunction may precede overt extrapyramidal motor symptoms by years [2, 3, 163]. These conditions are thought to result from LBs and LNs in the enteric plexuses [23, 24, 26, 178], affection of lower brainstem nuclei [17, 115] and the sympathetic cardiac nervous system [124]. It was suggested that ILBD is present in 10–15% of subjects aged 60–70 years, in 20% of those in their eighties, and in a third or more in those at age over 90 years [37]. Recent studies of ILBD cases have shown a distribution of LBs similar to that in PD, involving one or multiple brain areas, some also with sparse LBs in limbic or temporal cortex (average Braak stage 2.7 ± 0.3) in comparison to definite PD cases with more numerous LBs in all regions and significantly higher Braak PD stage (average 4.4 ± 0.3). When both groups were taken together, a significant inverse correlation existed between neuronal densities in the three anatomical regions studied (SN, striatum, and epicardial nerves) and the Braak PD stage [43]. Furthermore, there was decreased tyrosine hydroxylase (TH) immunoreactivity in both the striatum and sympathetic epicardial nerve fibers in cases with incidental LB pathology compared to normal controls but not in the same extent as in PD [13, 43]. On the other hand, incidental AS pathology may strike solely the LC and SN without affection of the medullary nuclei [12, 83, 139, 142]. Incidental LBs are not considered a normal aging phenomenon, whereas neurofibrillary tangles increase with age in the SN [52], and are associated with increasing neuritic AD stages [11]. These findings suggest that ILBD is likely a precursor to or preclinical form of PD, and the lack of symptoms is due to subthreshold AS pathology or—more correctly—subthreshold neuronal loss.
Some recent studies have largely confirmed the staging of LRP in sporadic PD, showing that all brains of subjects with clinical PD revealed AS-positive inclusions and neuronal losses in medullary and pontine nuclei and SN, and additional lesions in NBM (90–98%), limbic cortex (50–60%), cingulate area (32–46%), frontal cortex (29–31%), and amygdala (25%), corresponding to LB stages 4–6 [82, 83]. In a previous study, LBs in SN were found in 99.2% of clinico-pathologically confirmed PD cases [81]. Postmortems in 21 patients (17 demented, 4 nondemented) of the Sydney multicenter study of PD showed brainstem LRP in all, with limbic and/or neocortical LBs in 47% and AD pathology in 18% of demented cases [72]. LRP first appears in the ventrolateral part of SNc, spreads to the paranigral nucleus and then to the medial part, and finally to the dorsal part of SNc [179]. There is severe depletion of melanized neurons (45–66%) and of dopaminergic neurons immunoreactive for TH (60–85%) in the A9 group of SNc, particularly in the ventrolateral tier (91%) projecting to the striatum, followed by the ventromedial (71%) and dorsal parts (56%) [50]. The calbindin (CAB)-rich compartments show greater cell loss in the caudal and mediolateral region (98%) than the adjacent matrix. From there, it spreads to other nigrosomes and finally to the matrix along a caudorostral, lateromedial, and ventrodorsal progression [34]. These changes differ from age-related lesions in the dorsal tier of SNc that is involved only in late stages of PD [50, 65].
The degree of A9 SN cell loss and the resulting dopamine decrease in the striatum [15] as well as the reduction of TH and dopamine transporter (DAT) immunoreactivity in the putamen followed by caudate nucleus and nucleus accumbens show close correlation to the duration and severity of motor dysfunction [111]. Akinesia and rigidity are linked with SN neuronal loss, but the percentage of LB-bearing neurons in SN is not correlated with disease duration, and apparently stable over time, involving 39% of SN neurons on average. Such stability suggests that, during the course of disease, the destruction of LBs is equal to their production and that they are destroyed together with the afflicted neurons. With a mean survival time of LB-bearing neurons estimated at around 6.2 months, a neuronal loss of 71% would be reached after 20 years, fitting with the standard progression of the disease [62, 63].
It is unclear, however, whether the accumulation of pathologic AS in SN correlates with the dopaminergic deficit in the striatum as the major cause of motor symptoms in PD. According to recent studies, DAT immunoreactivity in the striatum is inversely correlated with the total AS burden in SN but not with LB counts alone. Nigral TH immunoreactivity did not correlate with AS immunoreactivity, since it can be preserved in neurons aggregating AS [99]. These data support the concept of synaptic dysfunction and/or axonal transport impairment caused by AS aggregation. A close topographic relationship between TH-negative neurons, LBs, and neuronal loss has been shown for the SN [126]. Reduced intensity of DAT mRNA in the remaining SN neurons is associated with reduced AS mRNA and DAT expression in SN, striatum, and amygdala, but not in SN in early stages of PD [31], whereas AS inclusions and neuritic changes in the neostriatum increase with disease progression [127]. Detection and staging of LBs alone, therefore, may not have clinical impact, whereas cytoplasmic and neuritic accumulation of pathologic AS and, in particular, neuronal loss in the involved brain areas may have more important implications for classification and staging the progression of the disease process. Unfortunately, no such quantitative investigations on neuron loss have been performed by Braak et al. [20-24].
The A10 group of dopaminergic neurons in the ventral tegmental area, nucleus parabrachialis, and parabrachialis pigmentosus (projecting to the striatal matrix), several thalamic nuclei [156], cortical and limbic areas (mesocortico-limbic system [14]) show less severe involvement (40–50% cell loss), the retrorubral A8 region containing only few dopaminergic but CAB-rich neurons, and the central periventricular gray show little or no degeneration [121]. Others reported greater cell loss in LC (area A6) than in SN in both PD and AD [187]. Morphometric studies showed a 35–41% reduction of pigmented SN cells, with severe loss of dopamine transporter (DAT)-immunoreactive neurons in older persons [113] and increase in the volume of these cells [28]. Some studies have estimated the neuronal loss as 4.3% per decade [28], while others have reported almost 10% [112]. Recent morphometric stereologic studies of the human SN revealed a significant loss of pigmented (−28.3%) and TH-positive (−36.2%) neurons in older compared to younger control subjects, with hypertrophy of cells in older controls, interpreted as a compensatory mechanism to allow normal motor function despite cell loss. PD showed a massive loss of SN neurons with significant atrophy of remaining cells (20% of controls), but most of the examined cases were in the end stage of the disease [153].
A prerequisite of the proposed staging system of LRP in PD is that the extent and severity of lesions increase as the disease progresses [23]. A study of 21 PD brains by six observers from five different institutions examining 11 different brain areas revealed highly significant inter- and intrarater reliability and supported the suitability of the staging procedure of AS pathology for application in routine neuropathology and brain banking [131]. However, the reliability of this staging system has recently been challenged [12, 82, 83, 89, 91, 140, 142]. In Braak’s cohort of 301 cases (including 176 clinical PD and 106 with incidental LBD and 163 age-matched controls), only 6.6% of PD brains diverged from the hypothetical staging scheme of AS pathology, with predominant involvement of olfactory structures and amygdala, and advanced concomitant AD-related neuritic pathology [24]. Among 71 cases of PD from the UK PD Society Tissue Bank at Imperial College, London, only 53% showed a distribution pattern of AS compatible with the caudo-rostral spreading suggested by Braak et al. [20] and 43% did not fit the predicted spread of AS inclusion pathology. The most frequently affected regions were SN and NBM (100 and 98.5%, respectively), followed by LC and DMV (97 and 92.9%, respectively). In 7%, the DMV was not affected, although AS inclusions were found in SN and/or cortical regions [89]. On the other hand, in a 68-year-old woman with late-onset, dopa-responsive parkinsonism of almost 13 years duration, autopsy revealed severe neuronal loss with many LBs in DMV but only moderate neuron depletion (60%) in SNc without any LBs and moderate cell loss with diffuse AS cytoplasmic staining in LC, suggesting unusual manifestation of LB disease in clinically definite PD [178]. In an autopsy series of 260 elderly subjects including 71 cases of autopsy-proven PD, 38 DLB, 116 AD, and 26 age-matched controls (with positive AS pathology in 51% of AD and 31% of controls), 30% of AD cases with multifocal AS pathology but without extrapyramidal symptoms (EPS) showed no involvement of the medullary nuclei [83]. In another autopsy series of 60 autopsy-confirmed cases (29 PD with dementia/PDD and 31 without dementia, mean age at death 82.5 years), some early PD symptoms were reported to occur already in rare cases with LB stage 2 (n = 2, 3.3%), e.g., autonomic and bladder dysfunctions, sleeping disorders, constipation, orthostatic hypotension, and depression, and more often in stage 3 (n = 9, 15.0%), in which most patients clinically revealed stiffness, asymmetric rigidity, and mild hypomimia but no tremor. Stage 4 (n = 30, 50%) and stage 5 (n = 19; 31.7%) showed typical PD-related motor features [12, 82]. Thirty-nine brains (65%) showed almost equal AS load in SN, NBM, LC, and both DMV and gigantocellular reticular formation, while 11 brains (18.3%) revealed considerable AS lesions in SN, LC, and NBM, but only mild involvement of DMV. In this material, the most frequently involved CNS regions were SN (96.7%), NBM (98%), and LC (96.7%), whereas the DMV was involved in 91.7%. Five brains (8.3%), despite definite involvement of SN, LC, and NBM in four and additional cingulate gyrus and limbic cortex involvement in one each, showed no AS lesions and almost no neuronal loss in DMV. The latter cases clinically showed rigid-akinetic and l-dopa-responsive PD with rest tremor in three and dementia in two cases (one each with frontal and limbic cortical involvement). Less frequently affected brain regions were the olfactory bulb (70%), CA 2/3 sector of hippocampus (39%), previously regarded as a means to differentiate diffuse DLB from PD [41] and cingulate gyrus and/or frontal cortex (31.6%) [12]. In general, DMV and SN were found to be equally susceptible nuclei, but structures even earlier affected by AS pathology include the spinal cord, DMV, olfactory bulb, and amygdala [16, 20, 37, 67, 93, 170]. The fact that between 6.3 and even 47% of all clinical and autopsy-confirmed PD cases obviously did not strictly follow the proposed staging system of AS inclusion pathology and that in 7–8.3% the DMV was not involved despite definite AS lesions in higher brainstem or even cortical regions [12, 89, 142] suggests that the proposed ascending pathway may not be the only possible route of disease progression and simultaneous involvement of subcortical and cortical regions appears to be possible. In very few cases of DLB with severe AD-pathology, the amygdala develops AS pathology and neuronal loss prior to the brainstem nuclei, while depletion of cardiac nerves is not necessarily seen [185]. AS/LRP beginning in the amygdala associated with AD [18, 67, 170] may show rostro-caudal spread to the entorhinal/transentorhinal cortex and midbrain/brainstem regions. However, the reasons for such deviations from the frequently but inconsistently observed caudo-rostral propagation and for sparing of medullar nuclei even in clinically manifested PD are not fully understood.
A recent study on the progression of pathology in longitudinally followed patients with PD verified three different clinicopathologic groups: (1) In a group of younger onset patients with long clinical duration and LB distributions consistent with the Braak staging, brainstem LBs dominated in those surviving up to 5 years; by 13 years, 50% of cases had a limbic distribution of LBs, and by 18 years, all had at least this pathologic subtype; (2) About 25% of cases had an early malignant, dementia-dominant syndrome and severe neocortical disease consistent with DLB; (3) The last group with later onset, shorter survival, and more complex disease course showed higher LB loads, suggesting that widespread LRP either occurs at the onset of clinical disease or rapidly infiltrates the brain with frequent concomitant lesions, in particular more plaque pathology, supporting a more aggressive and LB-linked phenotype [64]. These data suggest that the different phenotypes cannot be differentiated by pathology alone and are also not consistent with a unitary concept of the pathogenesis of LB disease [64, 108].
α-Synuclein/Lewy-related pathology in the aged human nervous system
LRP in the central and peripheral autonomic system in a neurologically unremarkable elderly human population is not an infrequent finding [6, 16, 51, 82, 83, 138, 140–142, 181], which was detected only when systematically studying all parts of the nervous system. Earlier studies showed LBs in the brains of 50 elderly persons without extrapyramidal symptoms [51], AS pathology in SNc in 10–12% of neurologically unimpaired elderly persons [138], and in midbrain and limbic cortex in 31% of asymptomatic aged controls with a mean age of 82.0 years. These were referred to as ILBD, while multifocal AS pathology in10% of them corresponded to Braak LB stage 4 [83]. Among 241 autopsy cases without neurologic disease (average age 78.7 years) from the Mayo Clinic Tissue Registry (Rochester, MN, USA), 36 cases (15%) with incidental LBs were found [54]. Another retrograde clinico-pathologic study of AS pathology in 1,241 consecutive elderly patients distinguished LB as stage 0 (87.3%), stage 1 (incidental LBs, 12%), stage 2 (LBs without attributable clinical symptoms, 3.8%), stage 3 (PD without dementia, 8.1%), stage 4 (DLB transitional form, 2.1%), and stage 5 (diffuse neocortical DLB, 1.9%) [155], while in a single neurologically unimpaired subject, widespread and abundant AS pathology was detected [141], which was suggested to predict neither extrapyramidal symptoms nor dementia. Among 98 elderly autopsy cases without PD-associated symptoms, AS pathology was found in the brain, spinal cord, and peripheral autonomic system in 17.3% [16]. Among 208 autopsy cases from the MRC CFAS brain donor cohort aged 65+ years (almost 75% over age 80; 50% of those demented), 36.5% showed LBs. Only 51% of these conformed to the Braak LB staging, while 17% had AS lesions in a higher region, which was absent in a lower region. Further 29% showed amygdala-predominant AS pathology. Six brains presented with predominant neocortical AS with minor involvement of amygdala and SN. There was no relationship between amygdala AS pathology and neuritic AD changes [186]. This population-based study showed that 80% of those with AS pathology conformed either to a DLB consensus/Braak stage pattern (51%) or to amygdala-predominant AS-cases (29%). The latter was therefore present in 60% of a DLB/Braak stage conforming pattern. The remaining 20% did not fit into either system of AS progression, a previously unreported group. A small group in which AS pathology was predominantnly neocortical with minimal amygdala involvement and absent transentorhinal/cingulate AS lesions (8%) corresponds to the diffuse cortical form of DLB [98, 184]. The MRC CFAS study confirms that AS frequently coexists with AD-type pathology, but suggests that there is no consistent hierarchy in the progression of the two disease processes [139]. Colocalization of AS and tau in the olfactory bulb in AD cases with amygdala LBs suggests that neurodegeneration in these two anatomically connected regions is linked, whereas a subset of AD cases may have extremely severe limbic system neuritic tau pathology that makes them uniquely vulnerable to AS pathology [54].
In a retrospective clinicopathologic study of 426 demented subjects, SN LBs were identified in 22%, considerable SN degeneration in 63 cases (15%), three of whom had never exhibited parkinsonian signs, suggesting that in demented patients there is an inconsistent relationship between the expression of clinical parkinsonism and severe SN degeneration with LBs identified at autopsy [5].
In the hitherto largest retrospective autopsy series of 1,720 individuals aged at death >40 years, from Kuopio, Finland, the frequency of AS lesions was 14% [142]. Eighty-three percent of them showed a distribution pattern of AS lesions comparable to the two current staging systems of PD and DLB, but 55% of subjects with widespread AS pathology (Braak PD stages 5 and 6) lacked clinical signs of dementia (MMSE > 26) or EPS. Similarly, among those subjects who fulfilled the McKeith criteria for DLB and displayed only mild concomitant AD-related pathology, only 48% were demented and 54% displayed EPS. When only demented subjects were included in the analysis, the correlation between AS pathology and dementia was 85%. Seventeen percent of all cases showed deviations from the suggested caudo-rostral disease progression. One subject with EPS was found already in Braak PD stage 2, whereas none of the cases in stage 4 displayed EPS, and more importantly, no EPS had been reported in 55% of subjects who exhibited widespread AS pathology (Braak PD stages 5 and 6), as compared to the 14% previously reported [20]. These and other results suggest that the risk of EPS increases with disease progression of LRP though not to the same extent as previously believed. On the other hand, in large autopsy samples, between 30 and 55% of elderly subjects with widespread AS pathology were neuropsychiatrically intact lacking clinical symptoms or were not classifiable [83, 106, 142]. Among 178 subjects with widespread AS pathology (Braak LB stages 5 and 6), 53% without AD-related pathology were cognitively unimpaired [4].
Initial decline in cognition was postulated to occur already during stages 3 and 4, i.e., around the same time when initial manifestations of somatosensory and motor dysfunction appear. Among 88 subjects, dementia was observed in stage 3 (36%), stage 4 (67%), stage 5 (94%), and in 100% in stage 6, indicating that increasing cognitive decline (decreasing MMSE scores) correlated with increasing AS stages [22], which was not confirmed by others [32, 86, 142, 182]. In the hitherto largest autopsy study of AS pathology in humans, the percentage of demented persons increased from 0 to 50% between LB stages 3 and 6. However, when subjects with either EPS or dementia were included, 91 and 94%, respectively, were assigned to PD stages 5 and 6, indicating that only when subjects with clinical symptoms were included in the analysis, the correlation between stage/severity of AS pathology and EPS and/or dementia was well in line with previous reports [142]. In a personal autopsy series of 330 elderly patients with clinical parkinsonism (37.6% demented), only 1.6% of the PDD cases (MMSE < 20) showed LB Braak stages 3–5, which were found in the majority of nondemented PD cases, while 35.5% of Parkinson-dementia (PDD) cases revealed LB stages 4 or 5 with superimposed severe Alzheimer-type pathology (neuritic Braak stages 5 and 6). More than half of them showed a strong relationship between AS and tau pathology, particularly in the limbic system. DLB with low-grade or high-grade Alzheimer lesions were seen in 40% of patients with PDD, but almost one-third of diffuse DLB cases, i.e., those with mild AD lesions restricted to amyloid plaques or tau pathology in the limbic system, did not show considerable cognitive impairment [86].
Since 49% of LB-positive cases in a large dementia autopsy sample using current criteria for categorizing DLB were not classifiable, a modification by reducing the number of examined regions, allowing more variability in LB pathology severity scores within specific regions, and introducing an amygdala-predominant category was proposed [104]. Application of the modified criteria to the referral-based (n = 208) and an unrelated community-based (n = 226) cohort permitted correct classification in 97 and 96% of LB-positive cases, respectively (Table 4).
Clinical relevance of Lewy-related pathology in the nervous system
The clinical relevance of cortical AS pathology for both motor and cognitive impairment is a matter of intense debate. Akinesia and rigidity were negatively correlated with the density of neurons in SN [15, 61], but were not correlated with the density of AS accumulation as shown there [61], and no relationship between the LB stage and both clinical severity of PD (Hoehn and Yahr score) and age at death were found [27]. Similar reflections against staging and in favor of heterogeneity of PD have been published by others [108]. Some authors have emphasized their key causative role [10, 75, 100, 118], whereas others have reported abundant LB cortical lesions in nondemented patients with PD [32] and in neuropsychiatrically unimpaired elderly subjects [83, 141]. These data clearly indicate that detailed regional assessment of AS pathology at autopsy, the inter- and intrarater reliability of which has recently been reviewed [7, 8], cannot reliably predict the clinical status observed intra vitam [141]. This challenges the clinical relevance of AS deposition in both PD and DLB as the sole indicator of disease progression as used in the Braak staging scheme, whereas neuronal loss and/or glial changes may be more relevant parameters, since there may be no strong correlation between AS deposits and neuronal cell loss.
The neuropathology of PD with and without dementia and DLB shows both similarities and differences. Morphology and immunohistochemistry of cortical and subcortical LBs and the ascending spreading pattern of AS pathology do not significantly differ between both subtypes, the late cortical stages 5 and 6 of LRP suggesting a transition between PD and DLB. The SN and other subcortical nuclei in DLB show variable neuronal loss often indistinguishable from sPD, except for an occasionally more severe loss in the ventro(dorso)lateral tier compared to predominant cell loss in the medioventral parts of SNc in PD and more frequent involvement by AS deposits of the limbic system, in particular, the CA 2/3 subarea of the cornu Ammonis in DLB than in PD/PDD (79 vs. 36% [86]). A major morphologic difference is the significantly more frequent and severe load with diffuse amyloid plaques in the striatum in DLB, irrespective of the severity of cortical AD-type lesions, while nondemented PD cases are virtually free of Aβ pathology as is the globus pallidus [84, 88, 90]. There are neither correlations between LB density in any brain area and neuritic Braak AD stages or frequency of neuritic plaques [88], nor between LBs in cortex and SN, suggesting that DLB should not be considered a severe form of PD. On the other hand, striatal Aβ pathology was more often seen in demented subjects independently of tau and/or AS status [4].
Whereas LB densities, in general, cannot separate DLB from PD/PDD, the severity and duration of dementia appears to be related to both increased hippocampal LB numbers and neuritic plaque grade. A screening algorithm suggesting that LB density thresholds in parahippocampus may distinguish demented from nondemented PD cases independent of other pathologies [69] awaits further confirmation. However, individuals can show significant cognitive disturbance with no or minimal cortical LBs and, conversely, widespread cortical LB pathology without essential cognitive decline [22, 32]. In the Finnish autopsy cohort, 56% of those subjects where limbic/diffuse neocortical AS pathology combined with mild to moderate AD-related changes remained cognitively intact. However, when only demented subjects with severe AD-related pathology (neuritic Braak stages 5 and 6) were examined, 85% were assigned to a high likelihood category of DLB and all of them were demented [142]. Hence, in cognitively impaired subjects, there is a good correlation between dementia and particular pathologies.
While some recent clinico-pathologic studies confirmed that the current staging/ categorization systems can readily be applied to most of the subjects when assessing regional distribution and progression patterns of AS pathology, in a certain percentage of cases, this caudo-rostral propagation pattern suggested for PD cannot be definitely confirmed. In such cases, the pattern of relevant lesions may have been modified by other coexisting pathologies or genetic factors [94, 103, 104, 170]. Various clinical, biochemical, and morphological overlaps between PD, DLB, and AD including colocalization of tau and AS epitopes in LBs suggest that the process of LB formation is triggered, at least in part, by AD pathology [78, 85, 116]. Deposition of tau can be demonstrated in a proportion of LBs, being greatest in neurons vulnerable to both LB and NFT formation, such as in LC, NBM, and amygdala [45, 79]. This suggests that AS and tau may be related to several pathologic processes (bystander effect), which explains the frequent overlap between synucleinopathies and tauopathies [55, 105]. The collision of two or more processes may occur in the same brain region or even in single cells in the human brain, e.g., in LRRK2 mutations [58] and in animal models of PD [47], with association of phospho-tau and AS in both NFTs and LBs [159] and in vitro promotion of tau aggregation by AS and vice versa [57]. Others have suggested that amyloid rather than tau enhances AS pathology in human brain and transgenic mice [104, 145]. These interactions highlight the interface between these and other misfolded proteins [105, 162]. They may represent molecular mechanisms in overlapping pathology of PD/DLB and AD [114], together with recent biochemical data on tau and β-amyloid [40] that challenge the view of PD and DLB as distinct entities, whereas increasing evidence supports PDD as being distinct from AD [48].
The recent MRC CFAS study found no evidence for preponderance of amygdala-predominant AS pathology over the prototypical DLB/Braak hierarchy in terms of an association with AD [186]. According to these authors, this does not support the proposed hypothetical link between AS in the amygdala and AD pathology [67, 170]. This was explained by the fact that the studies from which these hypotheses emerged included only clinically referred—i.e., selected cases—, whereas another recent study demonstrating colocalization of tau and AS in the olfactory bulb of AD with amygdala LBs distinguished them from AD cases without those [54]. In amygdala and hippocampus that are vulnerable to tau and AS pathology, severe TDP-43 pathology was demonstrated in AD and DLB but not in PD, while frontal cortex and basal ganglia were negative. TDP-43 was partially superimposed with AS, although neither LBs and NFTs showed TDP-43 immunoreactivity, suggesting that TDP-43 may be related in some way to AD and LB pathology [73].
On the other hand, in retrospective studies of unselected autopsy cohorts, up to 50% of cases displaying abundant AS pathology had been reported to be clinically intact without neuropsychiatric deficits. It has been suggested that the key lesions may develop a considerable time prior to the appearance of clinical symptoms [50], but the duration and progression of this “preclinical” phase is still under discussion. According to functional neuroimaging studies, this preclinical periods range from 4.6 to 6.6 years, with an annual decline of striatal dopamine uptake of 8–10% and of DAT of 10–13%, while others suggested that the preclinical phase of the illness may last between 5 and 20 years [70]. Higher nigrostriatal dopamine loss has been suggested in early than late onset PD [160]. Reduction of striatal dopamine by 57–80% [152] and DAT loss of 56% cause motor symptoms [133]. Thus, about 50% of dopaminergic striatal innervation appears to be sufficient for normal motor function [15, 49]. Striatal dopamine release was 60% reduced in patients with PD, whereas in the frontal cortex it was within normal range, indicating that it remained preserved even in severe stages of the disease [144]. Decreased DAT ultimately results in increased dopamine turnover preposing towards the occurrence of motor complications as PD progresses [161]. However, recent studies in both movement disorders and dementing processes demonstrated that some subjects may tolerate substantial amounts of both AS and tau pathology without definite clinical manifestation, suggesting considerable compensatory mechanisms even of the aging brain [35, 95, 129, 146, 147]. It should be emphasized, however, that the biological significance and clinical impact of abnormal AS aggregations are not yet clear. Like other fibrillary proteinaceous inclusions, such as NFTs or Pick bodies, they may represent end products of reactions to hitherto unknown neuronal degenerative processes [135]. The question whether LBs and other AS aggregates are harmful and interfere with normal cell function due to production of oxidative stress, mitochondrial energy deficiency, and other noxious factors or are cytoprotective still remains unresolved [29, 117, 125, 149, 157, 164]. Biophysical studies have suggested that the protofibrillary rather than the fibrillary form of AS is cytotoxic [30, 167, 172], whereas the LBs and LNs composed of fibrillary AS, which are typically observed at autopsy, may be the structural manifestation of a cytoprotective response designed to confine and to eliminate cytotoxic proteins [102, 135, 137, 166]. There is no correlation between the density of LB formation and neuronal cell loss [60], and the comparatively low number of neurons containing LBs in any brain region would not be expected to result from altered synaptic function. Nevertheless, significant intracellular protein aggregation, such as LB formation, are pathologic processes, reflecting changes in the cellular environment that may finally contribute to dysfunction of the involved cells. Recent studies showed development of LBs in grafted neurons in subjects with PD, suggesting host-to-graft propagation [107]. Therefore, the presence or absence of abnormal immunostaining for AS in neurons cannot be interpreted as evidence that the cell shows or is free of PD-related dysfunction for which pathologic AS is responsible.
Conclusions
Existing definitions and classification systems should be based on an understanding of the underlying pathologic process, which, in the case of synucleinopathies, is still incomplete. Therefore, in view of the caveats discussed above and the inter- and intraindividual biological variabilities, the neuropathologic staging procedures are imperfect instruments [110, 169], but may serve as a framework for current or future prospective clinico-pathologic (autopsy-controlled) studies trying to define the morphological substrates and the possible pathophysiologic correlates of early and progressed sPD. Although recent studies indicate that LB pathology is indeed progressive, the assessment of regional distribution patterns of AS/LRP may either evaluate a stage of degeneration or conversely monitor the level of functional neuroprotection, concluding that the topographic mapping of AS/LB deposition may be of debatable value, because it is unclear whether LB pathology is the primary (or solely relevant) pathology in PD and related disorders [142]. The clinical and pathophysiologic significance of AS/LRP in any anatomic pattern is still unclear. The following possibilities can be considered: (1) the presence of LBs is merely pathognomonic of a synucleinopathy process in the brain that can also affect clinically still asymptomatic subjects with ILBD; (2) LB quantification in and by itself does not present a reliable surrogate marker for PD and DLB, or of the clinical intermediate between the two, ‘PDD’; (3) LB formation may rather reflect one of several response patterns of the human brain to dysregulated AS metabolism; and (4) actual cell loss or the associated synaptic dysfunction could represent a more suitable surrogate marker. Such dysregulated AS metabolism could include an increase in non-fibrillar types of AS or in post-translationally modified variants that could be otherwise invisible at the microscopic level [158]. If it is the synucleinopathy that characterizes and drives sPD (and DLB) [180], the question arises about the degree of lesion density that has to be reached to induce clinical symptoms. These may rather be related to neuronal loss in the vulnerable CNS areas than to LB density alone. The presence of abnormal AS aggregations, which have been observed in PD to develop first in axons as LNs [134, 175], may finally damage the parent nerve cell by interfering with axonal transport [23, 24, 38, 44, 46, 92, 154]. It should be emphasized that both the staging/classification schemes of AS pathology [20, 120] apparently have no relevance for cases with predominant dementia, although the impact of LRP on cognitive impairment needs further elucidation. Keeping all these pros and cons in mind, one has to conclude that if robust correlations between clinical course and morphologic changes will be confirmed by future prospective clinico-pathologic studies, the neuropathologic staging/classification systems must be revised accordingly. In accordance with another recent critical reappraisal of the Braak staging scheme for PD [27] it is concluded that the relationship between patterns of AS immunostaining in the human brain and the disease entity (entities) now recognized as sPD (and DLB) remains to be reevaluated. The resolution of these questions will require much more study, including additional assessments of the patterns of synucleinopathy in the human brain, and, most importantly, how these patterns relate to validated biomarkers for progression of PD. At a more basic level, we need a better understanding of the neurobiology of synuclein. In particular, studies on the pathologic significance of AS and LBs and its relations to neuronal loss, most suitably in a prospective clinico-pathologic setting, are needed.
References
Aarsland D, Ballard CG, Halliday G (2004) Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol 17:137–145
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57:456–462
Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Launer LJ, Curb JD, White LR (2007) Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 22:1581–1586
Aho L, Parkkinen L, Pirttila T, Alafuzoff I (2008) Systematic appraisal using immunohistochemistry of brain pathology in aged and demented subjects. Dement Geriatr Cogn Disord 25:423–432
Ala TA, Yang KH, Sung JH, Frey WH (2000) Inconsistency between severe substantia nigra degeneration with Lewy bodies and clinical parkinsonism in dementia patients: a cliniconeuropathological study. Acta Neuropathol 99:511–516
Alafuzoff I, Parkkinen L (2003) α-Synuclein in ageing and Alzheimer’s disease. In: Iqbal K, Winblad B (eds) Alzheimer’s disease and related disorders: research advances. Ana Aslan International Academy of Aging, Bucharest, pp 183–191
Alafuzoff I, Parkkinen L, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Morris J, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2008) Assessment of α-synuclein pathology: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol 67:125–143
Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H (2008) Inter-laboratory comparison of neuropathological assessments of β-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol 115:533–546
Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H (2005) Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol 15:29–34
Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW (2002) Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 59:102–112
Attems J, Quass M, Jellinger KA (2007) Tau and α-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol 113:53–62
Attems J, Jellinger KA (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol Appl Neurobiol (in press). doi:10.1111/j.1365-2990.2008.00937.x
Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG (2008) Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 115:445–451
Berger B, Gaspar P, Verney C (1991) Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci 14:21–27
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295
Boeve BF, Dickson DW, Olson EJ, Shepard JW, Silber MH, Ferman TJ, Ahlskog JE, Benarroch EE (2007) Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med 8:60–64
Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J, Jellinger K (1994) Amygdala pathology in Parkinson’s disease. Acta Neuropathol 88:493–500
Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J (1996) Pattern of brain destruction in Parkinson’s and Alzheimer’s diseases. J Neural Transm 103:455–490
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64:1404–1410
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Braak H, Muller CM, Rub U, Ackermann H, Bratzke H, de Vos RA, Del Tredici K (2006) Pathology associated with sporadic Parkinson’s disease—where does it end? J Neural Transm Suppl (70):89–97
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113:421–429
Braak H, Del Tredici K (2008) Nervous system pathology in sporadic Parkinson disease. Neurology 70:1916–1925
Burke RE, Dauer WT, Vonsattel JPG (2008) The Braak staging scheme for Parkinson’s disease: a reappraisal. Ann Neurol (submitted)
Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B (2002) Ageing of substantia nigra in humans: cell loss may be compensated by hypertrophy. Neuropathol Appl Neurobiol 28:283–291
Carvey PM, Punati A, Newman MB (2006) Progressive dopamine neuron loss in Parkinson’s disease: the multiple hit hypothesis. Cell Transplant 15:239–250
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298
Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI (1999) Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 409:38–56
Colosimo C, Hughes AJ, Kilford L, Lees AJ (2003) Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:852–856
Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN (1992) Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol 83:525–529
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain 122(Pt 8):1421–1436
Davis DG, Schmitt FA, Wekstein DR, Markesbery WR (1999) Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58:376–388
de Lau LM, Koudstaal PJ, Hofman A, Breteler MM (2006) Subjective complaints precede Parkinson disease: the rotterdam study. Arch Neurol 63:362–365
Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Del Tredici K, Braak H (2008) A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol 115:379–384
den Hartog Jager WA, Bethlem J (1960) The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 23:283–290
Deramecourt V, Bombois S, Maurage CA, Ghestem A, Drobecq H, Vanmechelen E, Lebert F, Pasquier F, Delacourte A (2006) Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol 65:278–288
Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13:179–189
Dickson DW, Schmidt ML, Lee VM, Zhao ML, Yen SH, Trojanowski JQ (1994) Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol 87:269–276
Dickson DW, Fujishiro H, Delledonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115:437–444
Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ (2002) Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol 52:205–210
Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ (2002) Concurrence of α-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol 104:7–11
Duda JE (2004) Pathology and neurotransmitter abnormalities of dementia with Lewy bodies. Dement Geriatr Cogn Disord 17(Suppl 1):3–14
Duka T, Rusnak M, Drolet RE, Duka V, Wersinger C, Goudreau JL, Sidhu A (2006) α-Synuclein induces hyperphosphorylation of tau in the MPTP model of parkinsonism. FASEB J 20:2302–2312
Farlow MR, Cummings J (2008) A modern hypothesis: The distinct pathologies of dementia associated with Parkinson’s disease versus Alzheimer’s disease. Dement Geriatr Cogn Disord 25:301–308
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Fearnley JM, Lees AJ (1994) Pathology of Parkinson’s disease. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 545–554
Forno LS (1969) Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc 17:557–575
Frigerio R, Apaydin H, Klos KJ, Josephs KA, Parisi JE, Boeve B, Dickson DW, Ahlskog JE (2008) Substantia nigra Lewy bodies, neurofibrillary tangles, and aging (abstract). In: 60th Annual Meeting of the American Academy of Neurology, Chicago, April 12–19, 2008:P06.109.
Fujimi K, Sasaki K, Noda K, Wakisaka Y, Tanizaki Y, Matsui Y, Sekita A, Iida M, Kiyohara Y, Kanba S, Iwaki T (2008) Clinicopathological outline of dementia with lewy bodies applying the revised criteria: the Hisayama study. Brain Pathol 18:317–325
Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW (2008) Co-localization of tau and α-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol (in press). doi:10.1007/s00401-00008-00383-00401
Galpern WR, Lang AE (2006) Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol 59:449–458
Galvin JE, Lee VM, Trojanowski JQ (2001) Synucleinopathies: clinical and pathological implications. Arch Neurol 58:186–190
Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM (2003) Initiation and synergistic fibrillization of tau and α-synuclein. Science 300:636–640
Giasson BI, Covy JP, Bonini NM, Hurtig HI, Farrer MJ, Trojanowski JQ, Van Deerlin VM (2006) Biochemical and pathological characterization of Lrrk2. Ann Neurol 59:315–322
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Gomez-Tortosa E, Newell K, Irizarry MC, Albert M, Growdon JH, Hyman BT (1999) Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology 53:1284–1291
Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, Piette F, Hauw JJ, Duyckaerts C (2006) Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63:584–588
Greffard S, Verny M, Bonnet A, Seilhean D, Hauw J, Duyckaerts C (2008) The proportion of neurons containing Lewy bodies remains stable with time in the substantia nigra. A proposed model of neuronal death in relation to Lewy bodies (abstract). Clin Neuropathol 27:179
Greffard S, Verny M, Bonnet AM, Seilhean D, Hauw JJ, Duyckaerts C (2008) A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol Aging (in press)
Halliday G, Hely M, Reid W, Morris J (2008) The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol 115:409–415
Halliday GM, McRitchie DA, Cartwright H, Pamphlett R, Hely MA, Morris JGL (1996) Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J Clin Neurosci 3:52–60
Halliday GM, Del Tredici K, Braak H (2006) Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm Suppl (70):99–103
Hamilton RL (2000) Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 10:378–384
Harding AJ, Halliday GM (2001) Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol 102:355–363
Harding AJ, Stimson E, Henderson JM, Halliday GM (2002) Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 125:2431–2445
Hawkes CH, Deeb J (2006) Predicting Parkinson’s disease: worthwhile but are we there yet? Pract Neurol 6:272–277
Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33:599–614
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23:837–844
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H (2007) Concurrence of TDP-43, tau and α-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294
Hishikawa N, Hashizume Y, Yoshida M, Sobue G (2003) Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol 105:341–350
Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE (2000) α-Synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 54:1916–1921
Ince PG, Perry EK, Morris CM (1998) Dementia with Lewy bodies. A distinct non-Alzheimer dementia syndrome? Brain Pathol 8:299–324
Ince PG, Clark B, Holton J, Revesz T, Wharton SB (2008) Disorders of movement and system degenerations. In: Love S, Louis DN, Ellison DW (eds) Greenfield’s neuropathology, 8th edn. Hodder Arnold, London, pp 889–1030
Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T, Kosaka K (2003) Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol 105:265–270
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and α-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, Satoh A, Seto M, Tsujihata M, Takahashi H (1999) Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology 52:1269–1271
Jellinger K (1987) Overview of morphological changes in Parkinson’s disease. Adv Neurol 45:1–18
Jellinger KA (2003) α-Synuclein pathology in Parkinson and Alzheimer disease brain: incidence and topographic distribution—a pilot study. Acta Neuropathol 106:191–201
Jellinger KA (2004) Lewy body-related α-synucleinopathy in the aged human brain. J Neural Transm 111:1219–1235
Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112:253–260
Jellinger KA (2007) Lewy body disorders. In: Youdim MBH, Riederer P, Mandel SA, Battistin L, Lajtha A (eds) Degenerative diseases of the nervous system. Springer Science, New York, pp 267–343
Jellinger KA (2007) Morphological substrates of Parkinsonism with and without dementia. A retrospective clinico-pathological study. J Neural Transm Suppl (72):91–104
Jellinger KA (2008) Striatal β-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol 67:484
Jellinger KA, Attems J (2008) Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 115:427–436
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of α-synuclein staging. Neuropathol Appl Neurobiol 34:284–295
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2008) Striatal β-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol 67:155–161
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2008c) Controversies over the staging of α-synuclein pathology in Parkinson’s disease. Acta Neuropathol, 30 April 2008 [Epub ahead of print]. doi:10.1007/s00401-00008-00381-00403
Katsuse O, Iseki E, Marui W, Kosaka K (2003) Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies. J Neurol Sci 211:29–35
Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW (2006) α-Synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 66:1100–1102
Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, Hoge JA, Cohen AD, Ikonomovic MD, Saxton JA, Snitz BE, Pollen DA, Moonis M, Lippa CF, Swearer JM, Johnson KA, Rentz DM, Fischman AJ, Aizenstein HJ, DeKosky ST (2007) Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci 27:6174–6184
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Kosaka K, Mehraein P (1979) Dementia-Parkinsonism syndrome with numerous Lewy bodies and senile plaques in cerebral cortex. Arch Psychiatr Nervenkr 226:241–250
Kosaka K, Yoshimura M, Ikeda K, Budka H (1984) Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree - a new disease? Clin Neuropathol 3:185–192
Kosaka K (1995) Diffuse Lewy body disease. Rinsho Shinkeigaku 35:1455–1456
Kovacs GG, Milenkovic I, Preusser M, Budka H (2008) Nigral burden of α-synuclein correlates with the striatal dopamine deficit (abstract). Clin Neuropathol 27:177–178
Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2003) Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106:83–88
Kramer ML, Schulz-Schaeffer WJ (2007) Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27:1405–1410
Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of Lewy bodies: dissimilar incorporation of α-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253
Lang AE (2007) The progression of Parkinson disease: a hypothesis. Neurology 68:948–952
Lashley T, Holton JL, Gray E, Kirkham K, O’Sullivan SS, Hilbig A, Wood NW, Lees AJ, Revesz T (2008) Cortical α-synuclein load is associated with amyloid-β plaque burden in a subset of Parkinson’s disease patients. Acta Neuropathol 115:417–425
Lee VM, Giasson BI, Trojanowski JQ (2004) More than just two peas in a pod: common amyloidogenic properties of tau and α-synuclein in neurodegenerative diseases. Trends Neurosci 27:129–134
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ (2008) Empiric refinement of the pathologic assessment of lewy-related pathology in the dementia patient. Brain Pathol 18:220–224
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14(5):501–503
Linazasoro G (2007) Classical Parkinson disease versus Parkinson complex—reflections against staging and in favour of heterogeneity. Eur J Neurol 14:721–728
Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK (2007) DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology 68:812–819
Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D Jr, Price DL (2007) The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol 66:251–257
Ma SY, Roytta M, Rinne JO, Collan Y, Rinne UK (1997) Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson’s disease using disector counts. J Neurol Sci 151:83–87
Ma SY, Roytta M, Collan Y, Rinne JO (1999) Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol Appl Neurobiol 25:394–399
Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ (1999) Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol 409:25–37
Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R (2006) Interaction between Aβ peptide and α synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res 31:1153–1162
Marion MH, Qurashi M, Marshall G, Foster O (2008) Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? J Neurol 255:192–196
Marui W, Iseki E, Nakai T, Miura S, Kato M, Ueda K, Kosaka K (2002) Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci 195:153–159
Marras C, Lang A (2008) Changing concepts in Parkinson disease: moving beyond the decade of the brain. Neurology 70:1996–2003
Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M (2000) α-Synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol 100:285–290
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47:1113–1124
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
McRitchie DA, Cartwright HR, Halliday GM (1997) Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson’s disease. Exp Neurol 144:202–213
Meredith GE, Sonsalla PK, Chesselet MF (2008) Animal models of Parkinson’s disease progression. Acta Neuropathol 115:385–398
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Mitsui J, Saito Y, Momose T, Shimizu J, Arai N, Shibahara J, Ugawa Y, Kanazawa I, Tsuji S, Murayama S (2006) Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson disease. J Neurol Sci 243:101–104
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87
Mori F, Nishie M, Kakita A, Yoshimoto M, Takahashi H, Wakabayashi K (2006) Relationship among α-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. J Neuropathol Exp Neurol 65:808–815
Mori F, Tanji K, Zhang H, Kakita A, Takahashi H, Wakabayashi K (2008) α-Synuclein pathology in the neostriatum in Parkinson’s disease. Acta Neuropathol 115:453–459
Mori H (2005) Pathological substrate of dementia in Parkinson’s disease—its relation to DLB and DLBD. Parkinsonism Relat Disord 11(Suppl 1):S41–45
Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, Berg L (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46:707–719
Mosimann UP, McKeith IG (2003) Dementia with Lewy bodies and Parkinson’s disease dementia—two synucleinopathies. Adv Clin Neurosci Rehabil 3:8–16
Müller CM, de Vos RA, Maurage CA, Thal DR, Tolnay M, Braak H (2005) Staging of sporadic Parkinson disease-related α-synuclein pathology: inter- and intra-rater reliability. J Neuropathol Exp Neurol 64:623–628
Murayama S (2004) Systemic pathology of Lewy-related Parkinson changes in aging. The experience of Tokyo Metropolitan Brain Bank for Aging Research. In: Yamamoto M (ed) Parkinson’s disease: advances in pathology and autonomic nervous system. Chugai Igaku, Tokyo, pp 34–45
Nurmi E, Ruottinen HM, Bergman J, Haaparanta M, Solin O, Sonninen P, Rinne JO (2001) Rate of progression in Parkinson’s disease: a 6-[18F]fluoro-l-dopa PET study. Mov Disord 16:608–615
Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, Tsuchiya K, Mori F, Wakabayashi K, Takahashi H (2005) Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol 109:583–588
Outeiro TF, McLean PJ, Hyman BT (2007) Protein aggregation disorders. In: Gilman S (ed) Neurobiology of disease. Elsevier Academic Press, Amsterdam, pp 111–123
Oyanagi K, Wakabayashi K, Ohama E, Takeda S, Horikawa Y, Morita T, Ikuta F (1990) Lewy bodies in the lower sacral parasympathetic neurons of a patient with Parkinson’s disease. Acta Neuropathol 80:558–559
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain (in press). doi:10.1093/brain/awm1318
Parkkinen L, Soininen H, Laakso M, Alafuzoff I (2001) α-Synuclein pathology is highly dependent on the case selection. Neuropathol Appl Neurobiol 27:314–325
Parkkinen L, Soininen H, Alafuzoff I (2003) Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 62:363–367
Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I (2005) α-Synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol 57:82–91
Parkkinen L, Pirttila T, Tervahauta M, Alafuzoff I (2005) Widespread and abundant α-synuclein pathology in a neurologically unimpaired subject. Neuropathology 25:304–314
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407
Piao YS, Mori F, Hayashi S, Tanji K, Yoshimoto M, Kakita A, Wakabayashi K, Takahashi H (2003) α-Synuclein pathology affecting Bergmann glia of the cerebellum in patients with α-synucleinopathies. Acta Neuropathol 105:403–409
Piccini P, Brooks DJ (2006) New developments of brain imaging for Parkinson’s disease and related disorders. Mov Disord 21:2035–2041
Pletnikova O, West N, Lee MK, Rudow GL, Skolasky RL, Dawson TM, Marsh L, Troncoso JC (2005) Aβ deposition is associated with enhanced cortical α-synuclein lesions in Lewy body diseases. Neurobiol Aging 26:1183–1192
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368
Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC (2001) Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 58:1395–1402
Probst A, Bloch A, Tolnay M (2008) New insights into the pathology of Parkinson’s disease: does the peripheral autonomic system become central? Eur J Neurol 15(Suppl):1–4
Przedborski S (2006) Etiology and pathogenesis of Parkinson’s disease. In: Jankovic JJ, Tolosa E (eds) Parkinson’s disease and movement disorders, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 77–92
Przuntek H, Muller T, Riederer P (2004) Diagnostic staging of Parkinson’s disease: conceptual aspects. J Neural Transm 111:201–216
Richard IH, Papka M, Rubio A, Kurlan R (2002) Parkinson’s disease and dementia with Lewy bodies: one disease or two? Mov Disord 17:1161–1165
Riederer P, Gerlach M, Muller T, Reichmann H (2007) Relating mode of action to clinical practice: dopaminergic agents in Parkinson’s disease. Parkinsonism Relat Disord 13:466–479
Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC (2008) Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol 115:461–470
Saha AR, Hill J, Utton MA, Asuni AA, Ackerley S, Grierson AJ, Miller CC, Davies AM, Buchman VL, Anderton BH, Hanger DP (2004) Parkinson’s disease α-synuclein mutations exhibit defective axonal transport in cultured neurons. J Cell Sci 117:1017–1024
Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S (2004) Lewy body-related α-synucleinopathy in aging. J Neuropathol Exp Neurol 63:742–749
Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C (2005) The primate thalamus is a key target for brain dopamine. J Neurosci 25:6076–6083
Schapira AH (2006) Etiology of Parkinson’s disease. Neurology 66:S10–23
Schlossmacher MG (2007) α-Synuclein and synucleinopathies. In: Growdon JH, Rossor MN (eds) The Dementias 2. Blue Books of Neurology. Butterworth-Heinemann Oxford, pp 184–213
Shao CY, Crary JF, Rao C, Sacktor TC, Mirra SS (2006) Atypical protein kinase C in neurodegenerative disease II: PKCiota/lambda in tauopathies and α-synucleinopathies. J Neuropathol Exp Neurol 65:327–335
Shih MC, Franco de Andrade LA, Amaro E Jr, Felicio AC, Ferraz HB, Wagner J, Hoexter MQ, Lin LF, Fu YK, Mari JJ, Tufik S, Bressan RA (2007) Higher nigrostriatal dopamine neuron loss in early than late onset Parkinson’s disease? A [99mTc]-TRODAT-1 SPECT study. Mov Disord 22:863–866
Sossi V, de la Fuente-Fernandez R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ (2007) Dopamine transporter relation to dopamine turnover in Parkinson’s disease: a positron emission tomography study. Ann Neurol 62:468–474
Soto C, Estrada LD (2008) Protein misfolding and neurodegeneration. Arch Neurol 65:184–189
Stiasny-Kolster K, Doerr Y, Moller JC, Hoffken H, Behr TM, Oertel WH, Mayer G (2005) Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for α-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 128:126–137
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci 30:244–250
Takeda S, Yamazaki K, Miyakawa T, Arai H (1993) Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol 86:397–398
Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM (2004) Aggresomes formed by α-synuclein and synphilin–1 are cytoprotective. J Biol Chem 279:4625–4631
Tofaris GK, Spillantini MG (2005) α-Synuclein dysfunction in Lewy body diseases. Mov Disord 20(Suppl 12):S37–S44
Tsuboi Y, Dickson DW (2005) Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinsonism Relat Disord 11(Suppl 1):S47–S51
Uchihara T, Paulus W (2008) Research into neurodegenerative disease: an entangled web of mice and men. Acta Neuropathol 115:1–4
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of α-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
Uitti RJ, Calne DB, Dickson DW, Wszolek ZK (2004) Is the neuropathological ‘gold standard’ diagnosis dead? Implications of clinicopathological findings in an autosomal dominant neurodegenerative disorder. Parkinsonism Relat Disord 10:461–463
Volles MJ, Lansbury PT Jr (2003) Zeroing in on the pathogenic form of α-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42:7871–7878
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221
Wakabayashi K, Takahashi H, Ohama E, Ikuta F (1990) Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79:581–583
Wakabayashi K, Takahashi H, Obata K, Ikuta F (1992) Immunocytochemical localization of synaptic vesicle-specific protein in Lewy body-containing neurons in Parkinson’s disease. Neurosci Lett 138:237–240
Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F (1993) Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol 60:609–612
Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 38(Suppl 2):2–7
Wakabayashi K, Toyoshima Y, Awamori K, Anezaki T, Yoshimoto M, Tsuji S, Takahashi H (1999) Restricted occurrence of Lewy bodies in the dorsal vagal nucleus in a patient with late-onset parkinsonism. J Neurol Sci 165:188–191
Wakabayashi K, Mori F, Takahashi H (2006) Progression patterns of neuronal loss and Lewy body pathology in the substantia nigra in Parkinson’s disease. Parkinsonism Relat Disord 12(1):92–98
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 27:494–506
Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T (2003) Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 106:374–382
Weisman D, Cho M, Taylor C, Adame A, Thal LJ, Hansen LA (2007) In dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology 69:356–359
Wolters E, Braak H (2006) Parkinson’s disease: premotor clinico-pathological correlations. J Neural Transm Suppl (70):309–319
Yamamoto R, Iseki E, Marui W, Togo T, Katsuse O, Kato M, Isojima D, Akatsu H, Kosaka K, Arai H (2005) Non-uniformity in the regional pattern of Lewy pathology in brains of dementia with Lewy bodies. Neuropathology 25:188–194
Yokota O, Tsuchiya K, Uchihara T, Ujike H, Terada S, Takahashi M, Kimura Y, Ishizu H, Akiyama H, Kuroda S (2007) Lewy body variant of Alzheimer’s disease or cerebral type lewy body disease? Two autopsy cases of presenile onset with minimal involvement of the brainstem. Neuropathology 27:21–35
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG (2008) Patterns and stages of α-synucleinopathy: relevance in a population-based cohort. Neurology 70:1042–1048
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–341
Fujishiro H, Ahn TB, Frigerio R, Uchikado H, Klos KJ, Josephs KA, DelleDonne A, Parisi JE, Ahlskog JE, Dickson DW (2008) Incidental Lewy bodies in various neurodegenerative disorders (abstr.). Mov Disord 23 (Suppl.1):S30
Kalaitzakis ME, Roncaroli F, Pearce RK, Gentleman S (2008) Non-steroetypical distribution of α-synuclein in the spinal cord and brain of a patient with dementia with Lewy bodies (DLB) (abstr.). Mov Disord 23 (Suppl.1):S247
Acknowledgments
The study was supported in part by the Society for the Support of Research in Experimental Neurology, Vienna, Austria. The author thanks G.H. Halliday for worthful comments and Mr. E. Mitter-Ferstl, PhD, for secretarial works.
Note added in proof
Among seven cases of autopsy proven DLB Kalaitzakis et al. [189] observed one case in which DMV and the whole spinal cord were devoid of AS pathology despite moderate AS deposition in rostral brainstem and cortex. The frequency, distribution, and severity of LBs in most neurodegenerative disorders (290 PSP, 13 Pick disease, 37 MSA, 49 FTLD, and 50 CBD) ranging from 8 to 12% was similar to that in 232 normal elderly controls, suggesting that in most cases this represents ILBD [188].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jellinger, K.A. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116, 1–16 (2008). https://doi.org/10.1007/s00401-008-0406-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0406-y