Abstract
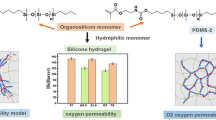
To clarify the influence of molecular weight of siloxane macromonomer on phase separation morphology, oxygen permeability, and mechanical properties, silicone hydrogels were prepared by copolymerizing mixtures of methacrylate-terminated siloxane macromonomer (MTSM), silicon-containing monomers tris(trimethylsiloxy)-3-methacryloxpropylsilane, and three hydrophilic monomers N,N-dimethylacrylamide, N-vinylpyrrolidone, and hydroxypropyl methacrylate. The number of Si–O–Si repeating units was equal in every silicone hydrogel while the molecular weight of MTSM ranged from 1000 to 10000 g/mol. The oxygen permeability coefficient (Dk), equilibrium water content (EWC), light transmittance, mechanical properties, and internal morphologies of obtained silicone hydrogels were characterized, and their relationships were discussed in detail. The results showed that the Dk value increased first and then decreased with the chain length of MTSM increasing. The EWC presented an increasing trend and the light transmittance decreased with the increase of MTSM chain length. The elongation increased while the modulus and the tensile strengths of the silicone hydrogels decreased along with the MTSM molecular weight increasing. The internal morphologies of the silicone hydrogels were observed by transmission electron microscope. The results indicated that the silicone hydrogels presented different phase separation structures depending on the molecular chain length of MTSM. The continuous silicone phase formed and made significant contributions to high Dk value as the molecular chain length of MTSM was moderate. Besides, a model was proposed to explain the effect mechanism of the MTSM chain length on the oxygen permeability and the EWC. This work provided a facile method to preparing the silicone hydrogel materials with desirable water absorption and high oxygen permeability by adjusting the molecular weight of macromonomers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogel has been widely used as a biomedical material due to its good biocompatibility [1, 2], robust mechanical property [3], impressive hydrophilic properties [4], and gas permeability [5, 6]. Among these properties, gas permeability is an important index that determines the wearing time and comfort of biomaterials such as contact lenses. Unfortunately, the highest oxygen permeability of conventional hydrogel is only about 40 barrer [7], which limits its application in many fields [8–10]. Silicone has been reported as a high gas-permeable material because of its loose structure [11]. The empty d-orbital of the Si atom in silicone polymers leads to good affinity with oxygen [12]. Many previous works focused on modifying hydrophilic hydrogel with gas-permeable silicone to obtain silicone hydrogels [13, 14], which were widely applied to contact lenses [15, 16], histological engineering materials [17–19], drug-delivery carriers [20, 21], etc.
In order to improve the oxygen permeability coefficient (Dk), silicon-containing monomers have been widely copolymerized with hydrophilic monomers to prepare silicone hydrogels. The copolymeric silicone hydrogels with little silicon-containing monomers including bis(trimethylsilyloxy)methylsilylpropylglycerolmethacrylate (SiMA) [12], tris(trimethylsiloxy)-3-methacryloxpropylsilane (TRIS) [22], and γ-methacryloxypropyltrimethoxysilane (KH-570) [23] have been extensively researched. Structurally diverse silicon-containing monomers attribute to different internal morphologies of silicone hydrogels, which has a significant impact on oxygen permeability. Zhao et al. [22] found that the silicone phase of SiMA and TRIS was of granular and fibrous textures, respectively. The latter contributed to the higher oxygen permeability. Although the little silicone-containing monomers can improve the Dk value, many studies revealed that the improvement of oxygen permeability was limited [22]. The Dk reported by Zhao et al. was only 29.6 (10−11 cm2/s) when the TRIS content was 30% (1 barrer = 1 × 10−11 cm3 (STP) cm/cm2 s−1 mmHg). The restricted enhanced ability should be attributed to the low molecular weight and the small molecular structure of monomers.
Another strategy is introducing silicone compounds with long Si–O–Si chains such as polydimethylsiloxane (PDMS) into the polymer backbone. The high oxygen-permeable silicone hydrogels have been obtained by copolymerizing PDMS-containing macromonomers with hydrophilic monomers [24]. Previous researches indicated that the oxygen permeability of silicone hydrogels was related to the silicon monomer content. The higher Dk value of silicone hydrogels can be obtained by increasing the content of the PDMS-containing macromonomer. The Dk values of silicone hydrogels made by Lin et al. were 92 barrer with 80% PDMS and that increased to 110 barrer with 92.3% PDMS [8]. Lamberti et al. [25] found that the gas permeability could be regulated by PDMS composition and the value improved apparently when the ratio of oligomer to crosslinker increased. Yokota et al. [26] also reported that the oxygen permeability could be enhanced with the chain length of silicone macromers increasing. These studies all prove that more Si–O–Si repeating units in silicone hydrogels lead to the higher oxygen permeability. However, if the number of Si–O–Si repeating units is controlled constant, the effect of the molecular structure, especially the chain length of macromonomer, on the properties of silicone hydrogels is still unknown.
In this work, a series of silicone hydrogels were prepared to study the influence of siloxane chain length on the phase separation morphology, the oxygen permeability, and the mechanical properties. The Dk value and the phase separation texture were explored by a coulometric oxygen permeation apparatus and a transmission electron microscope (TEM), respectively. Furthermore, the relationships between the chain length of PDMS-containing macromonomers and the internal morphology, oxygen permeability, equilibrium water content (EWC), and mechanical properties of silicone hydrogels were discussed, and a model was proposed to explain the mechanism in detail. This work provided a facile method to preparing hydrogel materials with moderate water absorption and high oxygen permeability by adjusting the molecular weight of macromonomers.
Experimental section
Materials
Bis hydroxy-terminated polydimethylsiloxane (HO-PDMS-OH) was purchased from TECH Polymer Copolymer. The molecular weights were 1000 g/mol (1 K), 2000 g/mol (2 K), 4000 g/mol (4 K), 5000 g/mol (5 K), 8000 g/mol (8 K), and 10,000 g/mol (10 K), respectively. 2-Isocyanatoethyl methacrylate (IEM) was bought from GINRAY. Tris(trimethylsiloxy)-3-methacryloxypropylsilane (TRIS) was obtained from TCI. N,N-dimethylacrylamide (DMA) was purchased from Adams Reagent Copolymer. N-Vinylpyrrolidone (NVP), hydroxypropyl methacrylate (HPMA), ethylene glycol dimethacrylate (EGDMA), 2-hydroxy-2-methylbenzene acetone (D-1173), and dibutyltin dilaurate (DBTDL) were obtained from Aladdin Industrial Copolymer. Dichloromethane (DCM), petroleum ether (PE), and isopropanol (IPA) were brought from Sinopharm Chemical Reagent Copolymer. All of these materials were used without further purification.
Synthesis of methacrylate-terminated siloxane macromonomer
Methacrylate-terminated siloxane macromonomer (MTSM) was synthesized with HO-PDMS-OH and IEM according to Peng’s method [27]. The molar ratio of HO-PDMS-OH to IEM was 1:2. DCM and DBTDL were used as solvent and catalyst, respectively. The mixture was stirred at 35 °C for 24 h to ensure a complete reaction. In order to purify products, PE was added to the mixture as extraction agent to remove the catalyst and the unreacted monomers. Then, MTSM was obtained by evaporating under reduced pressure with a rotary evaporator. The structure of MTSM is shown in Fig. 1, which also includes all the structures of monomers, crosslinker, and photoinitiator used in this work.
Preparation of silicone hydrogels
To manufacture multicomponent silicone hydrogels, the monomers MTSM, TRIS, NVP, HPMA, and DMA were added sequentially to a beaker. Then, the photoinitiator D-1173, the crosslinker EGDMA, and the solvent IPA were added into the mixture and stirred for 6 h at room temperature. Afterwards, the mixture was transferred into the double stack polypropylene molds, followed by exposure under ultraviolet light (360 nm) at 13 mW/cm2 for 45 min to obtain multicomponent copolymer membranes. Finally, the obtained membranes were hydrated in distilled water to form transparent silicone hydrogels. The copolymerization formulations are listed in Table 1. The amounts of EGDMA and D-1173 were both 0.5 wt%. The silicone hydrogel samples made by MTSM with 1K, 2K, 4K, 5K, 8K, and 10K molecular weight are labeled as S-1K, S-2K, S-4K, S-5K, S-8K, and S-10K, respectively.
Characterization
Oxygen permeability coefficient
The Dk value of silicone hydrogels was measured by a coulometric oxygen permeation apparatus (Labthink i-Oxtra 7600 Oxygen Transmission Rate Tester). Film samples were masked with aluminum foil masks leaving a circular uncovered film area. Pure oxygen gas was flowing on one side of the film, and nitrogen gas was on the other side. Nitrogen gas was conducted to the coulometric sensor, and the value of Dk/t (t was the thickness of membrane) was obtained when the steady state was reached. To get the Dk value of oxygen permeability, Dk/t was multiplied by the thickness of the film.
Phase separation morphology
The phase separation morphology and the elemental composition were characterized by a transmission electron microscope (TEM) (JEM-2100) and a scanning electron microscope (SEM)/energy-dispersive spectrometer (EDS) (FEI Inspect F50). For preparing samples of TEM, silicone hydrogel membranes were immersed into the liquid nitrogen for 20 min, followed by grounding into powder rapidly. Then, the powder was dispersed in distilled water. TEM samples were prepared by putting a drop of the mixture onto the copper grids and drying under an infrared lamp. For preparing samples of SEM/EDS, the swollen membranes were dried in a vacuum drying oven at 90 °C for 12 h. Then, the membranes were glued to the conductive tapes and coated with gold.
Transmittance measurements
The transmittance spectra of silicone hydrogels were recorded by a UV-Vis spectrophotometer (UV-2550). Both dry and swollen membranes were cut into rectangle shape pieces. The cuvettes were filled with distilled water for swollen samples while empty for dry ones. The wavelength coverage was from 280 to 780 nm for all samples.
Water content
Free and bound water contents of silicone hydrogels were carried out on a differential scanning calorimetry (DSC) instrument (NETZSCH DSC 204F1). For preparing samples of DSC, water on the surface of saturated samples was wiped by absorbent tissue lightly. Then, a piece of membrane was put into a sealed aluminum crucible promptly. The samples were measured under nitrogen atmosphere ranging from −20 to 10 °C with the flow velocity of 40 mL/min and the heating rate of 0.5 K/min. For the EWC test, the membrane was hydrated in water for 24 h firstly. The weight of swollen membranes was measured as W s after absorbing the water on the surface lightly. Then, the membranes were dried with a vacuum oven at 90 °C overnight. The weight of dry membranes was measured as W d . The EWC was calculated by Eq. (1).
Mechanical properties
Mechanical properties were conducted on an electronic tensile testing machine (PC-XLW(L)). Samples were cut into the shape of circle with the diameter of 2 cm and the thickness of 0.3 mm. Both dry and swollen samples were measured with a crosshead speed of 50 mm/min at 25 °C.
Results and discussion
In this work, the molecular weight of methacrylate terminal groups is tiny and negligible compared with that of the PDMS. Moreover, the effect of the methacrylate terminal groups on the Dk value is also little enough to be ignored. Therefore, the same weight of MTSM in the formula can be considered as the same number of Si–O–Si repeating units. A series of silicone hydrogels with the same weight of every monomer were successfully prepared, to clarify the influence of the molecular weight of a siloxane macromonomer on the properties of silicone hydrogels. The specific results of the oxygen permeability, the water content, and the mechanical properties are shown in Tables 2 and 3.
Oxygen permeability of silicone hydrogels
Figure 2 shows the relationship between the Dk value of silicone hydrogels and the molecular weight of MTSM according to Table 2. With the MTSM molecular weight increasing from 1K to 5K, the Dk values increased sharply from 83 barrer to the maximum value of 243 barrer. After that, the Dk values dropped to 129 and 136 barrer when the molecular weights of MTSM were 8K and 10K, respectively.
Oxygen permeation (P) through a medium is a two-step process. Firstly, the oxygen molecules dissolve in the medium and get equilibration according to the solution coefficient (S). Secondly, oxygen molecules diffuse throughout the medium according to the diffusion coefficient (D) [28]. The solution–diffusion model P = D × S was employed in many works to simulate oxygen permeation through silicone hydrogels. With the certain temperature and pressure, gas solubility is generally affected by the polarity and crystallinity of polymers [29]. In this work, the hydrophilic monomers and Si–O–Si chain contents for every sample were equal, thereby giving all silicone hydrogel samples the same polarity. Besides, the multicomponent silicone hydrogels obtained were noncrystalline. Accordingly, the solubility of oxygen in the samples can be considered equal, and the different Dk values of the silicone hydrogels are mainly caused by the diffusion coefficient. Diffusion is mostly dependent on the free volume [29] and the morphology structure of polymers [22]. Due to the same silicon and hydrophilic monomer content for the samples, the total free volume and its influence on diffusion were equivalent. Therefore, it can be presumed that the different diffusion of silicone hydrogels attributes to their morphology structures.
Internal morphology of silicone hydrogels
Figure 3 shows the internal morphologies of silicone hydrogels characterized by TEM. It can be clearly seen that silicone hydrogels presented phase separation structures where the silicone phase had a granular texture. The silicone phase in the images was presented as darker grains because silicon was the biggest atom with the highest electronic contrast in the multicomponent hydrogels. When the molecular weight of MTSM was 1K g/mol (Fig. 3a), only a few silicone granules were observed and the diameter was less than 50 nm. When the molecular weight of MTSM was 2K g/mol (Fig. 3b), the silicone granules turned darker and denser, but the diameter was similar with that of Fig. 3a. When the MTSM molecular weight reached 4K and 5K g/mol, as shown in Fig. 3c, d, respectively, the silicone grains became dense enough to form a continuous phase. However, with the MTSM molecular weight increasing to 8K and 10K g/mol, the silicone phases turned into deep black areas with large size, which led to the macroscopic phase separation, as shown in Fig. 3e, f.
In order to further verify the TEM result, elemental compositions of samples S-1K, S-4K, and S-10K as representatives were measured by EDS. The red dots represented carbon atom, green ones represented oxygen atom, and blue ones represented silicon atom. Almost the same amount of red dots shown in Fig. 3 (a’, c’, and f’) confirmed the same number of Si–O–Si repeating units in these silicone hydrogels. However, the distributions of silicon elements were obviously different. In sample S-1K, the silicon element was nearly homogeneously dispersed (Fig. 3 (a’)). With the MTSM molecular weight increasing, the silicon elements began to gather in sample S-4K (Fig. 3 (c’)) to form the microphase separation structure. When the molecular weight of MTSM was 10K g/mol (Fig. 3 (f’)), the silicon elements presented obviously aggregation, indicating the formation of macroscopic phase separation, which coincided with the TEM result.
Oxygen permeation is contributed by both the silicone phase and the hydrogel phase in silicone hydrogels. Oxygen permeation is mainly via the hydrogel phase when the silicone phase is dispersive. Since the oxygen transportation ability of the hydrogel phase was much worse than that of the silicon phase, the Dk value of silicone hydrogels was only 83 barrer in sample S-1K with a discontinuous silicone phase (Fig. 2). With the MTSM molecular weight increasing, the continuous silicone phase was gradually formed and resulted in a rapid increase of the Dk value. However, with the further increase of MTSM molecular weight, the macroscopic phase separation appeared in silicone hydrogels, which destroyed the continuous structure of the silicone phase and caused the decline of oxygen permeability.
Light transmittance
Light transmittance is another approach to reflect the internal morphology of silicone hydrogels. Figure 4 shows the light transmittance of the silicone hydrogel samples both in dry and swollen states. It can be seen in Fig. 4a that samples S-1K and S-2K were optically clear with transmittance both above 90% in dry state. Samples S-4K and S-5K remained transparent, and the transmittance values were around 80%. However, the transparency of samples S-8K and S-10K decreased to below 70%. The transmittance of swollen silicone hydrogels also presented a downward trend, as shown in Fig. 4b.
The light transmittance is related to the size of separated phase in the multicomponent materials [30]. The diameter of the silicone granule in samples S-1K and S-2K were much less than the wavelength of visible light, which is about 400 nm [31], so these two samples were transparent within the range of wavelengths from 280 to 780 nm. Although the size of the single silicone phase granule in samples S-4K and S-5K is also less than 400 nm, the interconnection of granules resulted in a larger silicone phase and a decline of transmittance. The single silicone bulk in samples S-8K and S-10K was big enough to cause light scattering; as a consequence, several silicone bulks brought about opaque and the lowest transmittance values. The decrease of transmittance indicated the gradually obvious phase separation, and this result corresponded with TEM and EDS data.
The mechanism of oxygen transferring through silicone hydrogels
Based on the internal morphology structures characterized by TEM, EDS, and light transmittance, a model is proposed as shown in Fig. 5 to explain the effect of the MTSM molecular weight on the oxygen permeability of silicone hydrogels. The phase separation structure of multicomponent copolymer materials is influenced by the compatibility of polymerized monomers. Short MTSM molecular chains and hydrophilic monomers are well compatible to form random copolymers. Under this situation, only a few short silicon chains assemble to small uniformly distributed silicone phase granules. There are no continuous paths for penetrant gas molecules, as shown in Fig. 5a, resulting in the low oxygen permeability of silicone hydrogels. With the molecular weight of MTSM increasing, the worse compatibility between hydrophilic monomers and MTSM facilitates more silicone chains to assemble together. The silicone hydrogels will obtain a significant oxygen permeability improvement once the silicone phase is continuous, because it provides a nonblocking channel for oxygen transportation, as shown in Fig. 5b. As the molecular weight of MTSM further increases, the obtained silicone hydrogels turn into segmented copolymers with two separated phases. Although the separated silicone phases appear to be large bulks and offer more free volume for oxygen, there are no continuous channel to expedite the diffusion and the oxygen permeation returns back to a lower value, as indicated in Fig. 5c.
Water content of silicone hydrogels
Except for the morphology structure, the water content of silicone hydrogels also has an impact on the oxygen permeability, although the difference of water content in the samples is delicate and it is not the determining factor for the final Dk value. The equilibrium water content along with free and bound water contents was measured to investigate their respective relations with the oxygen permeability, and the results are shown in Fig. 6. Figure 6a presents the melting curves of water in swollen silicone hydrogels characterized by DSC. It can be clearly seen that every sample had both sharp and broad water melting peaks overlapping together, except for sample S-1K. The sharp peaks at about −0.3 °C ascribed to bound water, and the broad peaks around 0.4 °C attributed to free water. These results coincide with that reported by Ping et al. [32]. Figure 6b shows the EWC as well as the free and bound water contents of silicone hydrogels. The EWC was calculated by Eq. (1). The free and bound water contents were calculated by fitting peaks in Fig. 6a with a Gaussian peak type. With the MTSM chain length increasing, the EWC of silicone hydrogels increased. At the same time, the percentage of free water in these silicone hydrogels decreased while the percentage of bound water increased. The EWC of sample S-1K was not enough for DSC to detect two peaks.
Akon et al. [33] reported that the oxygen diffusion coefficient in bound water is lower than that of free water, while the solubility of oxygen in bound water is higher than that of free water. The free water content plays a more important role on both hydrogel phase and silicone phase. For hydrogel phase, higher free water content can increase the diffusion coefficient, because free water carries more oxygen molecule through the medium [34]. But for silicone phase, higher free water plays a negative effect because it reduces the Si affinity sites available to oxygen sorption and restrains the oxygen permeability in the silicone phase [12]. In the silicone hydrogels, the diffusion coefficient of the silicone phase is much larger than that of the hydrogel phase, and the inhibiting effect of the free water on the silicone phase is greater than the enhanced effect on the hydrogel phase. Accordingly, although the free water content presented a decreasing trend, the Dk value increased with the chain length of MTSM increasing from 1K to 5K.
The free and bound water contents are also related to the morphology of silicone hydrogels, as shown in Fig. 5d, e of the proposed model. Figure 5d is a partially enlarged drawing of Fig. 5a. Figure 5e is a partially enlarged drawing of Fig. 5b, c. In Fig. 5d, the high hydrophobic and short flexible Si–O–Si chains copolymerized with the hydrophilic chains loosen the obtained silicone hydrogel network. For one thing, the loose network disperses the hydrophilic groups so as to reduce the interaction force between hydrophilic groups and water molecules. For the other, the loose network is favorable for water to transfer freely. As a result, the EWC of silicone hydrogels is low and the free water content is more than that of bound water, as shown in Fig. 6b. With the MTSM chain length increasing, the siloxane chains and the hydrophilic monomers respectively gather together and form separated phases. The pure separated hydrogel phase is more compact and the gathering hydrophilic groups have stronger interaction force with water molecules, leading to the higher EWC and bound water content, as shown in Figs. 5e and 6b.
Mechanical properties of silicone hydrogels
During the characterization procedure, it was found that the mechanical properties of multicomponent silicone hydrogels varied with the Si–O–Si chain length, and the proposed model provides a good explanation for the mechanism of mechanical properties as well. Figure 7a shows the relationship between the elongation of silicone hydrogels and the MTSM molecular weight. Both in dry and swollen states, the elongation of silicone hydrogels increased along with MTSM molecular weight increasing. The elongation at break was 231% in swollen state and 115% in dry state of sample S-1K. The values rose to 398% (swollen state) and 329% (dry state) when the molecular weight of MTSM increased to 10K g/mol. Silicone has higher mobility and better elastic property than hydrogel [35, 36]. When the random copolymers are obtained, the elongations are relatively short because the Si–O–Si chains are intermittent and the mechanical properties mainly depend on hydrogel. When the silicone chain length is long enough to form segmented copolymers, the mobility of silicone phases leads to increased elongation.
The modulus of both dry and swollen silicone hydrogels decreased with MTSM molecular weight increasing, as shown in Fig. 7b. The modulus of sample S-1K in dry and swollen states was 189 and 57 MPa, respectively. The values decreased to 39 MPa (dry state) and 12 MPa (swollen state) as the molecular weight increased to 10K g/mol. The descent amplitude of dry samples was larger than that of swollen ones. In Fig. 7c, the tensile strengths of silicone hydrogels also decreased as the MTSM molecular weight increased. According to previous research, the hydrogel phase is the hard segment and the silicone phase is the soft segment in multicomponent silicone hydrogels [8, 37]. Therefore, when the silicone phase is discontinuous, the tensile strength and Young’s modulus mainly depend on the hydrogel phase and result in higher values. With the MTSM molecular weight increasing, the long Si–O–Si chains gradually form a continuous silicone phase and primarily determine the tensile strength and Young’s modulus to downtrend.
Conclusions
In this work, silicone hydrogels were prepared by copolymerizing two silicon-containing monomers MTSM and TRIS with three hydrophilic monomers DMA, NVP, and HPMA. The Dk, EWC, light transmittance, mechanical properties, and internal morphologies of obtained silicone hydrogels were measured, and their relationships were discussed in detail. The results showed that the Dk value increased first and then decreased with the chain length of MTSM increasing. The EWC presented an increasing trend, and light transmittance decreased with the increase in MTSM chain length. The elongation increased while the modulus and the tensile strengths of silicone hydrogels decreased along with MTSM molecular weight increasing. The TEM, EDS, and transmittance results revealed that the phase separation structure gradually formed as the MTSM chain length increased, and the morphology of the silicone phase played an important role in oxygen permeation and the EWC. Besides, a model was proposed to explain the effect mechanism of the MTSM chain length on the oxygen permeability and the EWC. These results will help us to design silicone hydrogels with better properties and wider application in contact lenses.
Reference:
Bulpitt P, Aeschlimann D (1999) New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res 47(2):152–169
De Groot CJ, Van Luyn MJA, Van Dijk-Wolthuis WNE, et al. (2001) In vitro biocompatibility of biodegradable dextran-based hydrogels tested with human fibroblasts. Biomaterials 22(11):1197–1203
Raeburn J, Zamith CA, Adams DJ (2013) The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem Soc Rev 42(12):5143–5156
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Deliver Rev 64:18–23
Liu L, Chakma A, Feng X (2008) Gas permeation through water-swollen hydrogel membranes. J Membrane Sci 310(1–2):66–75
Liu Y, Yu S, Wu H, Li Y, Wang S, Tian Z, Jiang Z (2014) High permeability hydrogel membranes of chitosan/poly ether-block-amide blends for CO2 separation. J Membrane Sci 469:198–208
Wang Y (2014) Synthesis and characterization of silicone hydrogel contact lens materials. Applied Chemical Industry 02:316–318
Lin C, Yeh Y, Lin W, Yang M (2014) Novel silicone hydrogel based on PDMS and PEGMA for contact lens application. Colloids Surf B: Biointerfaces 123:986–994
Nicolson PC, Vogt J (2001) Soft contact lens polymers: an evolution. Biomaterials 22(24):3273–3283
Papas EB (2014) The significance of oxygen during contact lens wear. Contact Lens and Anterior Eye 37(6):394–404
Erdodi G, Kennedy JP (2005) Water-swollen highly oxygen permeable membranes: analytical technique and syntheses. J Polym Sci A Polym Chem 43(16):3491–3501
Sun D, Zhou J (2012) Effect of water content on microstructures and oxygen permeation in PSiMA–IPN–PMPC hydrogel: a molecular simulation study. Chem Eng Sci 78:236–245
Sugimoto H, Nishino G, Tsuzuki N, Daimatsu K, Inomata K, Nakanishi E (2012) Preparation of high oxygen permeable transparent hybrid copolymers with silicone macro-monomers. Colloid Polym Sci 290(2):173–181
Pozuelo J, Compañ V, González-Méijome JM, González M, Mollá S (2014) Oxygen and ionic transport in hydrogel and silicone-hydrogel contact lens materials: an experimental and theoretical study. J Membrane Sci 452:62–72
Chu MX, Miyajima K, Takahashi D, Arakawa T, Sano K, Sawada S, Kudo H, Iwasaki Y, Akiyoshi K, Mochizuki M (2011) Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 83(3):960–965
Alvord L, Court J, Davis T, et al. (1998) Oxygen permeability of a new type of high Dk soft contact lens material. Optometry Vision Sci 75(1):30–36
Qiao Q, Wang X, Sun J, Zhao R, Liu Z, Wang Y, Sun B, Yan Y, Qi K (2005) Management for postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthet Plast Surg 29(3):156–161
Morales-Hurtado M, Zeng X, Gonzalez-Rodriguez P, Ten Elshof JE, van der Heide E (2015) A new water absorbable mechanical epidermal skin equivalent: the combination of hydrophobic PDMS and hydrophilic PVA hydrogel. J Mech Behav Biomed Mater 46:305–317
Hamid ZAA, Lim KW (2016) Evaluation of UV-crosslinked poly(ethylene glycol) diacrylate/poly(dimethylsiloxane) dimethacrylate hydrogel: properties for tissue engineering application. Procedia Chemistry 19:410–418
Naskar J, Palui G, Banerjee A (2009) Tetrapeptide-based hydrogels: for encapsulation and slow release of an anticancer drug at physiological pH. J Phys Chem B 113(35):11787–11792
Rosa Dos Santos J, Alvarez-Lorenzo C, Silva M, Balsa L, Couceiro J, Torres-Labandeira J, Concheiro A (2009) Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials 30(7):1348–1355
Zhao Z, Xie H, An S, Jiang Y (2014) The relationship between oxygen permeability and phase separation morphology of the multicomponent silicone hydrogels. J Phys Chem B 118(50):14640–14647
Yan-long W (2014) Synthesis and characterization of silicone hydrogel contact lens materials. Applied Chemical Industry 02:316–318
Compan V, Lopez-Alemany A, Riande E, Refojo MF (2004) Biological oxygen apparent transmissibility of hydrogel contact lenses with and without organosilicon moieties. Biomaterials 25(2):359–365
Lamberti A, Marasso SL, Cocuzza M (2014) PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv 4(106):61415–61419
Yokota M, Ajiro H, Akashi M (2013) Effect of copolymerizing fluorine-bearing monomers on the relationship among internal structure, gas permeability, and transparency in copolymer networks composed of methacrylates and siloxane macromers. J Appl Polym Sci 127(1):535–543
Peng S, Guo Q, Hughes TC, Hartley PG (2011) In situ synchrotron SAXS study of polymerizable microemulsions. Macromolecules 44(8):3007–3015
Kraus OZ (2012) Development of a microfluidic platform to investigate effect of dissolved gases on small blood vessel function. Dissertation, University of Toronto
Ghosal K, Freeman BD (1994) Gas separation using polymer membranes: an overview. Polym Adv Technol 5(11):673–697
Matsuyama H, Takida Y, Maki T, Teramoto M (2002) Preparation of porous membrane by combined use of thermally induced phase separation and immersion precipitation. Polymer 43(19):5243–5248
Erdodi G, Kennedy JP (2005) Ideal tetrafunctional amphiphilic PEG/PDMS conetworks by a dual-purpose extender/ crosslinker. II. Characterization and properties of water-swollen membranes. J Polym Sci Pol Chem 43(20):4965–4971
Ping ZH, Nguyen QT, Chen SM, Zhou JQ, Ding YD (2001) States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer 42(20):8461–8467
Higuchi A, Abe M, Komiyama J, Lijima T (1984) Gas permeation through hydrogels: I. Gel cellophane membranes. J Membrane Sci 21(2):113–121
Wang Y, Tan G, Zhang S, Guang Y (2008) Influence of water states in hydrogels on the transmissibility and permeability of oxygen in contact lens materials. Appl Surf Sci 255(2):604–606
Lee S, Iyore OD, Park S, Lee YG, Jandhyala S, Kang CG, Mordi G, Kim Y, Quevedo-Lopez M, Gnade BE, Wallace RM, Lee BH, Kim J (2014) Rigid substrate process to achieve high mobility in graphene field-effect transistors on a flexible substrate. Carbon 68:791–797
Wooh S, Vollmer D (2016) Silicon brushes: omniphobic surfaces with low sliding angles. Angew Chem 55(24):6822–6824
Gorman SP, Tunney MM, Keane PF, Van Bladel K, Bley B (1998) Characterization and assessment of a novel poly(ethylene oxide)/polyurethane composite hydrogel (Aquavene) as a ureteral stent biomaterial. J Biomed Mater Res 39(4):642–649
Acknowledgments
This work was financially supported by the Jiangsu Provincial Natural Science Foundation of China (BK20130617, BK20130619), the National Natural Science Foundation of China (Grant Nos. 21374016 and 21304018), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tao, H., Zhang, X., Sun, Y. et al. The influence of molecular weight of siloxane macromere on phase separation morphology, oxygen permeability, and mechanical properties in multicomponent silicone hydrogels. Colloid Polym Sci 295, 205–213 (2017). https://doi.org/10.1007/s00396-016-4001-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-4001-9