Abstract
Polyacrylamide hydrogel, a new biomaterial, has been used for injected breast augmentation in China since 1997. A series of 30 patients with various complications after injected polyacrylamide hydrogel visited the author’s department. Most of these patients had undergone injection of both breasts. The average age of the patients was 27.6 years, and the time of consultation for the complications was from 3 to 36 months postopertively. Nearly all the patients had breast lumps and other common complications including breast pain, disfigurement, and infection. Ultrasound examination showed diffuse, irregular, anechoic zones of mammary tissue. Pathologic results indicated inflammatory cell infiltration and fibrous capsular formation. An open suction technique and partial mastectomies via periareolar incisions were performed for the all patients. Most of their symptoms were relieved after removal of the polyacrylamide hydrogel. Only one patient had undergone immediate breast reconstruction with implants, whereas five patients had received breast implants secondarily via an axillary incision. The authors conclude that polyacrylamide hydrogel should be prohibited for injected breast augmentation before more scientific data are available about the long effect of the gel in breast tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For centuries, plastic surgeons have searched for an easy, painless, and safe biomaterial for injection breast augmentation. Gersuny [10] was the first to introduce petrolatum injection for the restoration of breast contour. Thereafter, several biomaterials such as paraffin, organogen, bioplaxm, and silicone gel were popularly used during the time of World War II in many countries [1,2,7]. Many late complications of injection breast augmentation arose, although the initial results were relative good. The complications included firmness, skin change, pain, siliconomas, and parafinomas, which resulted in great concern. The management of these problems presented a very challenging problem, and most of the patients had to be undergo subcutaneous mastectomies for removal of the large, hard granulomas.
Next, silicone implants were inserted into the breasts, and because sufficient subcutaneous tissue was lacking, a severe capsule formed, leading to an unsatisfactory result. Furthermore, some patients even lost their breasts [4]. Because of such disastrous complications, and outcomes, the injection biomaterials for breasts was forbidden in the Western countries and Asia [6,12,13].
In 1997, a new biomaterial, polyacrylamide hydrogel, imported from Eastern Europe, was approved by the China PDA for use in restoring contours. This alloplastic material is said to be a highly biocompatible, atoxic, stable gel. Although insufficient scientific data about the safety of polyacrylamide hydrogel is available, this biomaterial currently is extensively injected for facial depressions, lip enhancement, extremity deformities, and breast augmentation in local hospitals and private cosmetic clinics in China [3,9,14]. This injection material for breast augmentation still is controversial in China and seldom is used in university hospitals because of the rapidly increasing late complications and some unfavorable long-term results. We report complications after breast injection with polyacrylamide hydrogel and discuss its safety and management of the postoperative sequelae.
Patients
Our department has no clinical experience with the injection of polyacrylamide hydrogel for breast augmentation. The patients described in this report have come to our department with complications resulting from injections received in other small hospitals and cosmetic clinics.
Between May 1999 and November 2003, 30 women with late complications after receiving injected polyacrylamide hydrogel for breast augmentation in other hospitals and clinics were admitted to our hospital. Their ages ranged from 18 to 40 years (mean, 27.6 years).
The time since the breast injections was 3 to 36 months. The most common complication was breast lumps (93%), with about 67% patients feeling pain after injection of the polyacrylamide hydrogel. The pain became aggravated when the upper extremities moved. The other complications included firmness of the breast, infection, and breast disfigurement (Table 1). Before the operative procedure, all the patients underwent breast ultrasound check, and some patients received magnetic resonance imaging (MRI) to ascertain the distribution of injected polyacrylamide hydrogel. The results showed that the gel was involved in nearly all the breast tissue, and some extended even deep into the major pectoral muscle.
Most of the patients had received about 150 ml of polyacrylamide hydrogel in each breast. Some were even injected with as much as 200 ml. We could not determine how much polyacrylamide hydrogel had been injected in some cases because the physicians did not provide the patients with the information. It was impossible to determine the total amount of material used in the operations.
Surgical Technique
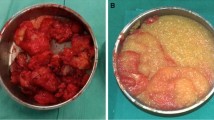
Patients with injected polyacrylamide hydrogel breasts present very difficult therapeutic problems. Although some reports suggest that polyacrylamide hydrogel in breasts can be removed completely by the cannula suction method, we found that nearly all the patients still had residual polyacrylamide hydrogel in breast tissue after repeated suction procedures in other hospitals. Furthermore, repeated blind suction techniques could injure surrounding normal tissue, and sometimes may increase the pain in the breast. From our clinical experience, we prefer to perform an open operation through a semiperiareolar incision. The dissection is performed between the subcutaneous tissue and the gland and then extends to the inferior margin of the pectoroalis fascia. The subgland space then is exposed, and we sometimes must open the gland or the pectoralis major muscle to expose the caviar-like pink jelly gel if the polyacrylamide hydrogel was injected into the gland or muscles (Fig. 1). Copious amounts of saline are used to irrigate the gel. Then massage is performed to make the polyacrylamide hydrogel more easy to remove
.
Many patients were terribly concerned with their resultant appearance and expected to have a “good result.” Although we informed the patients of the possible problem, seldom did they a subcutaneous mastectomy. Because multiple lumps were distribute through the whole gland, and some even into the major pectrolis muscle, we concluded that it would be impossible to remove all the polyacrylamide hydrogel using the suction technique and partial mastectomy. Sometimes it is necessary to remove the involved portions of the muscle.
We performed immediate reconstruction with subpectoral placement of silicone mammary implants, inserted via axillary incisions in one patient. Five patients underwent breast augmentation with silicone protheses 6 months after the first removal of the polyacrylamide hydrogel. The remaining patients did not receive further breast augmatation for fear of further operations.
When immediate breast augmentation with silicone implants was performed, the subpectoral and suprapectoral spaces were kept separate, and each had a vacuum drain. Mild compressive dressing was applied for at least 7 days. All excised breast tissue was sent for histopathologic examination. All the patients were told to report back for follow-up examinations after 6 months, and suggestions were made for routine breast ultrasound assessment to identify whether traces of the polyacrylamide hydrogel remained.
Case Reports
Case 1
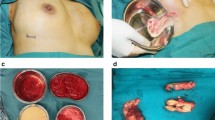
A 27-year-old woman had undergone breast polyacrylamide hydrogel injection 2 years previously because of breast atrophy after breast-feeding, The volume of polyacrylamide hydrogel was 150 ml for each breast. She came to our hospital with breast lumps and chest pain. During physical examination, masses of various sizes were palpable in the gland of the breast, and the breast was hard. The ultrasound result showed that multiple irregular anechoic areas were distributed throughout the whole gland in the left breast, that the normal gland structure had disappeared and replaced by numerous anechoic net structures of various sizes, and that the deeper muscle was not very clear (Fig. 2). In the right breast, the anechoic areas were located mainly in the subcuanteous tissue, with some deeper in the major pectoral muscle.
An open procedure was performed. We observed many thin fibrous capsules around the injected polyacrylamide hydrogel forming cystoid lumps in and between the gland and muscle. Some strips of muscle were detached because of the polyacrylamide hydrogel. The fibrous capsule was dissected, and the lumps were irrigated with normal saline. The polyacrylamide hydrogel was sucked out by the negative pressure machine. More than 100 ml of polyacrylamide hydrogel was removed, and we still found many lumps involving the gland and muscle. Some strips of muscle become paralyzed, with no reaction occurring after stimulation. We finally had to perform a subcutaneous mastectomy to remove the polyacrylamide hydrogel as completely as possible. Histological examination of excised tissue showed that polyacrylamide hydrogel had infiltrated the mammary gland and muscle, and that an inflammatory reaction had occurred, with numerous macrophage cells surrounding the capsule of polyacrylamide hydrogel (Figs. 3, 4, and 5).
Case 2
A 34-year-old woman had both of her breasts injected with polyacrylamide hydrogel (PAAG) because of mastatrophy and ptosis of the breasts after breast-feeding. The total volume of polyacrylamide hydrogel in both breasts was 350 ml, but the exact volume in each breast was not clear. She had received the injection in a local cosmetic clinic. Alhough she followed the advice of her doctors after the injections, she still felt multiple lumps in both breasts in addition firmness to breast after the operation. The 18-day postoperative examination indicated redness in the left breast, accompanied by pain and tenderness, which the patient felt after treatment with physical and antibacterial therapy.
The infection was cured, but multiple nodules were palpable, and unsatisfactory results were noted. The injected PAAG in the left breast was partially removed by the suction technique with a fine cannula in February 2003. After the operation, the patient still felt the nodules. Therefore, 1 month later, 120 ml of the gel was drawn from the right breast. However, because the gel was injected again into the same breast by the physician, the volume of gel in the left breast was not clear after the cannula suction technique.
The patient visited our hospital with multiple lumps in both breasts and felt chest pain. During the physical examination, multiple nodules could be felt in both breasts, with the patient reporting an uncomfortably hard feeling and slight ptosis.
We chose the periareolar incision and dissection extended to the subcutaneous tissue, mammary gland, and major pectoral musle where the lumps were distributed. Some muscle strips became paralyzed and did not contract after stimulation. A thick fibrous capsule was formed around the PAAG. The lumps were scraped with a scoop, and then irrigated with a large volume of normal saline. About 120 ml of gel was drawn from the right breast. The gel was very hard to remove by a scoop from the left breast, and we had to remove nearly all of the gland along with involved musle tissue. After that, 260 ml of breast protheses were implanted into the subpectoral space via axillary incisions (Fig. 6 and 7).
Discussion
Polyacrylamide hydrogel is reported to be an atoxic, stable, biocompatible, watery gel consisting of approximately 2.5% cross-linked polyacrylamide and nonpyrogenic water [3, 11]. Polyacrylamide hydrogel has been used for soft tissue augmentation about 10 years in the former Soviet Union and Eastern Europe, where it is claimed to be safe. However, seldom have English articles in Western countries reported on breast augmentation with polyacrylamide hydrogel because injected biocompatible material is forbidden for breast augmentation. Although polyacrylamide hydrogel can be used to enhance the lip, the volume for lip reconstruction is very limited and much lower than that used in breast augmentation [14]. Because polyacrylamide hydrogel is very easy to inject through a stab incision to achieve good results immediately, and because the procedure can be performed in clinics for a high profit, it has become very popular in private clinics and small hospitals since it was approved by China’s FDA in 1997, In 1999 it was temporarily forbidden for clinical applications in China because a great controversial debate about polyacrylamide hydrogel application occurred among specialists of plastic surgery [15]. Because of the disastrous complications and difficult treatment after injected breast augmentation with liquid silicone, some physicians became greatly concerned as to whether polyacrylamide would degrade to monoacrylamide, which exhibits neurotoxic efforts and has permanent negative effects on breast tissue because polyacrylamide hydrogel stays in the body for a long time. Until recently, nearly all university hospitals had ceased performing polyacrylamide hydrogel injections because of increased complications after a few years of clinical application.
Although it has been suggested that physicians be trained by the manufacturer to decrease complications, we found complications rising rapidly although the use of polyacrylamide hydrogel does not have a long history in China. As compared with the average consultation time of nearly 9 year for complications after silicon-injected breasts, we believe that the sequelae of breast augmentation with injected polyacrylamide hydrogel will reach a peak in a few years. There have been reports linking silicone injection to autoimmune disease or so-called “human adjuvant disease” [8], but no such complications were reported for this polyacrylamide hydrogel. Although this biomaterial is said to be a noncarcinogenic and nonmutagenic substances, the changes in breast tissue associated with polyacrylamide hydrogel injections make it difficult to interpret clinical signs and examinations. Therefore, as with silicone injection, the possibility of early diagnosis of breast cancer is considerably reduced. To determine the actual rate of complications and the material’s safety, a serious randomization sample test is mandatory.
Some reports have maintained that polyacrylamide hydrogel could remain at the injection site without being degraded or displaced [8]. In our clinical observation, the polyacrylamide hydrogel was distributed throughout the gland and surrounding muscle although it was intended for injection into the subglandular space by experienced physicians. We are not sure that this was because of the technique used by inexperienced surgeons or because polyacrylamide hydrogel cannot be confined to the injection site, which sometimes occurs if the formed capsule is very thin. This issue should be investigated further in the future.
Some research also has reported that the injected gel could be drawn out easily by the suction method with a small stab incision [3], but according to our clinical experience, it is impossible to remove all the residual gel by this method. In addition, the blind suction may not be very precise and can hurt the surrounding normal breast tissue, which could develop into myofascitis and myositis. This would make the next operation very difficult.
In all our cases, we used a periareolar incision, which further exposed the gel directly and removed the involved breast tissue easily. The subcutaneous and glandular tissue then was dissected, and the injected gel was exposed by breaking off the fibrous capsules. The surrounding tissue then was irrigated with normal saline solution and antibiotics. We were able to suck most of the gel and remove the abnormal breast tissue. Two of the patients received subcutaneous mastectomy. Most of the patients refused subcutaneous mastectomy after discovering the unsatisfactory results that follow the operation. We only partially removed abnormal gland ulartissue among these patients, and there may still be some gel remaining. One patient had immediate breast reconstruction with prostheses implanted into the subpectoral space via axillary incisions. The implants were covered entirely with the pectoralis major muscle and partially dissected serratus anterior fascia to avoid gel contamination. Five patients were augmented secondarily, and the remaining patients refused further reconstruction due to their fear of operation.
The most common complication after polyacrylamide hydrogel injection is breast induration and lumps, which coincides with other reports [3, 15]. The lumps are distributed into glandular muscle tissue, and exist in various sizes and concentrations. Ultrasound and magnetic resonance imaging can identify their distribution and assists in the operation. After removal of polyacrylamide hydrogel, induration of the breast can be relieved immediately.
Mastalgia is the second common symptom that manifests the myositis or myofasitis of pectoral major muscle. Repeated suction injury, polyacrylamide hydrogel irritation to breast tissue and chest musles, and infection are the factors causing mastalgia. The pain sometimes becomes aggravated when the extremities move. The other sequelae include infection and skin change of the breast.
After polyacrylamide hydrogel injection, a cellular membrane of macrophages and foreign body giant cells were present in the pathohistologic examination [5]. The pathologic results also indicated that the gel caused a foreign body reaction in which even the normal structure of muscle and gland were destroyed. It needs to be emphasized that the gel may affect the surrounding tissue according to the pathohistologic result. In several patients, we found a thick, firm capsule in surrounding tissue similar to capsule developed after long-term silicone prostheses implantations. The results demonstrate that polyacrylamide hydrogel may give rise to severe foreign body reactions.
As a whole, although polyacrylamide hydrogel is more biocompatiable than liquid silicone, we should not neglect the increasing complications. Before it is applied extensively for breast augmentation, a double-blinded randomized clinical study controlled animal experiments and a large sample questionnaire survey for complications should be mandatory.
References
T Akiyama (1958) ArticleTitleBasic problem of medical use of dimethylpolysiloxane Jpn J Plast Reconstr Surg 1 244
K Boo-Chai (1969) ArticleTitleThe complications of augmentation mammaplasty by silicone injection Br J Plast Surg 22 281 Occurrence Handle5394228
MJ Chao WM Yin (2000) ArticleTitleApplication of hydrophilic polyacrylamide gel in augmentation mammaplasty (Chinese) J Pract Aesth Plast Surg 11 16
TH Chen (1995) ArticleTitleSilicone injection granulomas of the breast: Treatment by subcutaneous mastectomy and immediate subpectoral implant Br J Plast Surg 48 71 Occurrence Handle7743051
LH Christensen VB Breiting A Aasted A Jorgensen I Kebuladze (2003) ArticleTitleLong-term effects of polyacrylamide hydrogel on human breast tissue Plast Reconstr Surg 111 1883 Occurrence Handle12711948
W Col Robert MC Parsons R Col Harlan Mc Thering (1977) ArticleTitleManagement of the silicone-injected breast Br J Plast Surg 60 534
H Conway D Goulian SuffixJr (1963) ArticleTitleExperience with an injectable silastic RTV as a subscutaneous prothetic material Plast Reconstr Surg 32 294 Occurrence Handle14067038
KW Dunn PN Hall CTX Khoo (1992) ArticleTitleBreast implant materials: Sense and safety Br J Plast Surg 45 315 Occurrence Handle1294075
AV Filatov SN Mirolinbov (1998) ArticleTitleSoft tissue contour plastic repair of the maxillofacial area using a biologically compatible polyacrylamide gel Stomatologia Mosk 77 45
R Gersuny (1911) Ueber eine Subcutane Prothese D. Appleton & Co. New York
DJ King RR Noss (1989) ArticleTitleToxicity of polyacrylamide and acrylamide monomer Rev Environ Health 8 3 Occurrence Handle2485925
EH Kopf et al. (1976) ArticleTitleComplications of silicone injections Rocky Mountain M J 75 77
InstitutionalAuthorNameNaoyuki Ohtake, Yasumi Koganei, Masatsugu Itoh, et al. (1989) ArticleTitlePostoperative sequel of augmentation mammaplasty by injection method in Japan Aesth Plast Surg 13 67
I Niechajev (2000) ArticleTitleLip enhancement: Surgical alternatives and histologic aspects Plast Reconstr Surg 105 1173 Occurrence Handle10724279
InstitutionalAuthorNameNing-xin Cheng, Yuan-lu Wang, Jin-huang Wang, Xiao-man Zhang, Hong Zhong (2002) ArticleTitleComplications of breast Augmentation with injected hydrophilic polycrylamide gel Aesth Plast Surg 26 375
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, Q., Wang, X., Sun, J. et al. Management for Postoperative Complications of Breast Augmentation by Injected Polyacrylamide Hydrogel. Aesth Plast Surg 29, 156–161 (2005). https://doi.org/10.1007/s00266-004-0099-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-004-0099-0