Abstract
To investigate the influence of crosslinking density and free polysiloxane chain length on oxygen permeability and hydrophilicity of multicomponent silicone hydrogels, two kinds of silicone macromonomers, single methacrylate terminated silicone macromonomers (PDMS-1) and double methacrylate terminated silicone macromonomers (PDMS-2), were synthetized, respectively. Then, a series of multicomponent silicone hydrogels with different crosslinking densities and free polysiloxane chain lengths were prepared by copolymerization of obtained silicone macromonomers, silicone monomer tris(trimethylsiloxy)-3-methacryloxpropylsilane (TRIS), and hydrophilic monomers. The oxygen permeability coefficient (Dk), equilibrium water content (EWC), light transmittance, and mechanical properties of silicone hydrogels were characterized. The results indicated that the longer free polysiloxane chain was beneficial to the increase of Dk values and EWC of silicone hydrogels. With the increase of crosslinking density, the Dk values and EWC of the silicone hydrogels decreased. This work provided a new idea for the preparation of high-permeability silicone hydrogels in the future.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxygen permeability is an important performance of materials in the field of biomedicine, especially medical dressings [1] and contact lenses [2, 3]. It is generally measured by the oxygen permeability coefficient (P = Dk), which is the product of oxygen solubility (k) and oxygen diffusion coefficient (D) [4]. Hydrogels are hydrophilic polymers with the three-dimensional network structure that swell in water but do not dissolve [5]. Due to their good biocompatibility [6, 7], excellent hydrophilic properties [8], and gas permeability [9, 10], hydrogels have been widely used as medical dressings and contact lenses. However, the hydrophilic polymer network itself has poor oxygen permeability. Hydrogels mainly rely on water molecules swelled in the polymer network to carry and transfer oxygen [11]. Many studies have shown that the oxygen permeability coefficient (Dk) values of hydrogels are directly proportional to their equilibrium water contents (EWC) [12, 13]. Limited by the oxygen permeability of water molecules, the maximum Dk value of traditional hydrogels is still less than 40 Barrer [14]. On the other hand, excessively high EWC will reduce the mechanical properties of hydrogels. Thus, traditional hydrogels are difficult to meet the high oxygen permeability requirements of the biomedical field [15,16,17,18,19,20,21].
In order to improve the oxygen permeability of hydrogels, an effective strategy is to introduce polydimethylsiloxane (PDMS) chain segments into the polymer backbone to prepare silicone hydrogels. PDMS has good chain flexibility and loose molecular structure, which is conducive to oxygen diffusion [22]. Therefore, the Dk value of silicone polymer material is up to 600 Barrer [23], which was higher than that of traditional hydrogels. Coupled with its good elasticity and biocompatibility, PDMS has been widely used as a highly oxygen-permeable medical material [24]. The silicone hydrogels combine the excellent hydrophilicity of hydrogels and the high oxygen permeability of silicone materials, which meet the double requirements of high oxygen permeability and high hydrophilicity in biomedical field, especially for contact lenses.
The high oxygen-permeable silicone hydrogels are usually obtained by copolymerizing PDMS-containing macromonomers with hydrophilic monomers [25, 26]. Both water molecules swelled in the polymer network, and silicone components contribute to carry and transfer oxygen. The Dk values of silicone hydrogels are not only related to EWC, but mainly depend on the components of silicone monomers. Lai et al. [27] prepared a series of PDMS-based polyurethane prepolymers containing different arrangements of hard and soft segments, then the polyurethane prepolymers were used to prepare hydrogels with hydrophilic monomers 2-hydroxyethyl methacrylate (HEMA) and N,N-dimethyl acrylamide (DMA). It was found that oxygen permeability of the silicone hydrogels was much higher than those of hydrogels and decreased as the water contents increased. In several different silicone hydrogel systems, the oxygen permeability decreased first and then increased with the increase of the silicone contents. Yokota et al. [28] reported a series of crosslinked copolymers composed of acrylic monomers and methacryloyl capped PDMS macromonomers with different molecular weights (Mn), and the effect of PDMS chain lengths on the oxygen permeability coefficient of copolymers was explored. The results showed that the oxygen permeability coefficient of copolymers increased with the increase of the chain length of PDMS macromonomers. Tao et al. [29] prepared a kind of silicone hydrogels by copolymerizing mixtures of PDMS-based silicone macromonomer and hydrophilic monomers. The influence of Mn of silicone macromonomer on the phase separation morphology and Dk values of silicone hydrogels was investigated. It was found that the Dk values of silicone hydrogels increased first and then decreased with the increase of the chain length of the silicone macromonomer. The silicone hydrogels presented different phase separation structures depending on the molecular chain length of the PDMS macromonomer. The morphology of the silicone phase played an important role in oxygen permeation. The continuous silicone phase structures significantly contributed to high Dk values of silicone hydrogels. Jiang et al. [30] also found that the hydrogels presented two different phase separation structures depending on the types of the silicon-containing monomers. The silicone phase in bis(trimethylsilyloxy) methylsilylpropyl glycerol methacrylate (SiMA)containing hydrogel presented to be a granular texture, while the silicone phase in TRIS containing hydrogel formed a fibrous texture which resulted in a higher Dk value.
Previous researches had shown that the chemical structure, chain length, and content of silicone macromonomer determined the oxygen permeability of silicone hydrogels. The number of active terminal groups of silicone macromonomers will affect the crosslink density [31] and free polysiloxane chain length of polymer [32], which may affect the oxygen permeability of the silicone hydrogels. However, few relevant researches have been reported.
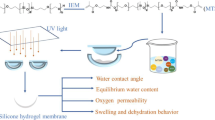
In this work, a series of multicomponent silicone hydrogels with different crosslinking densities and free polysiloxane chain lengths were prepared by copolymerization of PDMS-containing macromonomers and hydrophilic monomers, and the influences of crosslinking density and free polysiloxane chain length on the oxygen permeability, hydrophilicity, and mechanical properties of multicomponent silicone hydrogels were discussed in detail. Furthermore, a model was proposed to explain the mechanism of oxygen permeability.
Experimental section
Materials
Single hydroxyl terminated polydimethylsiloxane (HO-PDMS) and double hydroxyl terminated polydimethylsiloxane (HO-PDMS-OH) were purchased from TECH Polymer Copolymer (Shanghai, China), and their molecular weights both were 1000 g/mol. Isocyanoethyl methacrylate (IEM), dichloromethane (DCM), and tris(trimethylsiloxy)-3-methacryloxypropylsilane (TRIS) were obtained from TCI. N,N-Dimethylacrylamide (DMA) was purchased from Adams Reagent Copolymer. N-Vinylpyrrolidone (NVP), hydroxypropyl methacrylate (HPMA), ethylene glycol dimethacrylate (EGDMA), 2-hydroxy-2-methylbenzene acetone (D-1173), dibutyltin dilaurate (DBTDL), and petroleum ether (PE) were obtained from Aladdin Industrial Copolymer. All these materials were used without further purification.
Synthesis of methacrylate terminated silicone macromonomers
The single methacrylate terminated silicone macromonomers (PDMS-1) and double methacrylate terminated silicone macromonomers (PDMS-2) were synthesized by HO-PDMS and HO-PDMS-OH respectively according to Peng’s method [33], and their chemical structures are shown in Fig. 1. Firstly, HO-PDMS (10.0 g, 0.01 mol) and IEM (1.55 g, 0.01 mol) were added into a three-necked flask. Then, DCM as a solvent and DBTDL as catalyst were added to the reaction flask and mixed evenly. The mixture was stirred at 35 °C for 4 h to ensure a complete reaction. Then, the catalyst and the unreacted reactant were removed by PE extraction. Finally, the product methacrylate terminated silicone macromonomer PDMS-1 was obtained by vacuum evaporation. The product yield was about 99.3%. PDMS-2 was prepared using the same method; HO-PDMS-OH (10.0 g, 0.01 mol) and IEM (3.1 g, 0.02 mol) were added into a three-necked flask. The product yield was about 99.5%. The synthetic schemes of PDMS-1 and PDMS-2 are shown in Fig. S1 and Fig. S2 in supporting information, respectively. The 1H-NMR integrated spectra of PDMS-1 and PDMS-2 are shown in Fig. S3 and Fig. S4 in supporting information respectively, it confirmed that the number of active terminal groups in PDMS-2 was twice the number of active terminal groups in PDMS-1. The chemical structures of PDMS-1 and PDMS-2 were demonstrated by the appearance of new characteristic peaks of methacrylate group termination (δ = 5.52 ppm (s, C = CH2), δ = 6.05 ppm (s, C = CH2), (δ = 4.14 ppm (t, N-CH2)).
Preparation of silicone hydrogels
The multicomponent silicone hydrogels were prepared according to our previous study [29]. The silicone macromonomer, TRIS, and hydrophilic monomers (NVP, HPMA, and DMA) were added to the beaker and followed by D-1173 as a photoinitiator and EGDMA as a crosslinker. After stirring for 6 h at room temperature, the mixture was dripped into the molds and photopolymerized under ultraviolet light (365 nm) at 13 mW/cm2 for 40 min. Then, the multicomponent copolymer membranes were taken out and washed with ethanol to remove the unreacted monomers. Finally, the obtained membranes were hydrated in distilled water to form transparent silicone hydrogels. The copolymerization formulations of multicomponent silicone hydrogels are shown in Table 1. The photoinitiator accounts for 0.5% of the total mass of all monomers. The silicone hydrogel samples made by PDMS-1 and PDMS-2 were labeled as S1 and D2, respectively. The silicone hydrogel samples made by half PDMS-1 and half PDMS-2 were labeled as M1.5. Both silicone hydrogel samples S1.5 and S2 also were prepared by PDMS-1, while their crosslinking densities were consistent with that of samples M1.5 and D2, respectively, by adjusting the amount of crosslinking agent. As a typical representative, the FTIR comparison spectra of PDMS-1 and silicone hydrogel sample S1 are as shown in Fig. 2. For S1, the existence of characteristic peaks originated from silicon-containing monomers and hydrophilic monomers (Si-CH3 bending vibration at 1260 cm−1, Si–O–Si stretch vibration at 1100 cm−1, C–H stretch vibration at 2960 cm−1, C=O stretch vibration at 1720 cm−1). These characteristic peaks indicated that the silicone hydrogel was successfully prepared.
FTIR spectra of PDMS-1 and silicone hydrogel S1
Characterization
The 1H NMR spectra of silicone macromonomers were recorded using a 600-MHz NMR spectrometer (Avance III, Bruker, GER). The samples were dissolved in deuterated chloroform (CDCl3) and prepared into a 5-wt% solution to be tested. The hydrogen spectra of the silicone macromonomers were measured by using tetramethylsilane (TMS) as the internal standard.
The Fourier transform-infrared (FTIR) spectra of the silicone hydrogels samples were obtained using a Nicolet 5700 spectrometer (Thermo Fisher, Waltham, MA, USA). The scanning range of measurement was 500 ~ 4000 cm−1.
Measurement of equilibrium water content
The silicone hydrogel membranes were immersed in water for 24 h fully swelling. After wiping the water on the surface of swollen hydrogels with filter paper, the initial weight was measured as Ws. Then, the membranes were dried in a vacuum oven at 80 °C, 12 h, and their weight was measured as Wd. EWC was calculated by Eq. (1).
Oxygen permeability coefficient
The oxygen permeabilities of the silicone hydrogels were tested by a coulometric oxygen permeation apparatus (Labthink i-Oxtra 7600 Oxygen Transmission Rate Tester). The samples were covered with an aluminum foil mask, leaving a circular area of uncovered film. Nitrogen was fed into the Coulomb sensor, and the Dk values of the silicone hydrogels were obtained when they reached a steady state.
Transmittance measurements
The transmittance spectra of multicomponent silicone hydrogels were measured by an Ultraviolet–visible photometer (UV-2650). The swollen membranes were cut into rectangle-shapes pieces and then were affixed to the slide for measurement. The wavelength range of measurement was 300 ~ 800 nm.
Mechanical properties
The mechanical properties of multicomponent silicone hydrogels were tested on the electronic tensile testing machine (PC-XLW(L)) at room temperature. The samples were taken out after swelling to a saturated state, and the surface water was wiped off with filter paper. The samples were cut into rectangular strips of 2 cm in length, 1 cm in width, and 0.15 mm in thickness. The samples were stretched at a rate of 25 mm/min.
Results and discussion
In order to ascertain the effect of crosslinking density and free polysiloxane chain length on the properties of the materials, three kinds of silicone hydrogels S1, M1.5, and D2 with different crosslinking densities and free polysiloxane chain lengths were prepared. For the three kinds of silicone hydrogels, all components were identical except for silicone macromonomers, as shown in Table 1. The silicone macromonomer PDMS-2 has two polymerizable double bonds, which can act as a crosslinking agent in the polymerization system. As the content of PDMS-2 increases, the crosslinking densities of the silicone hydrogels will increase. After polymerization, the free polysiloxane chain length of PDMS-2 is shorter. Thus, both the crosslinking density and free polysiloxane chain length of the silicone hydrogel may affect the properties of the material.
In order to further study the effect of crosslinking density on the properties of hydrogels, two other silicone hydrogels, S1.5 and S2, were also prepared by PDMS-1. The terminal groups of PDMS-1, PDMS-2, and EGDMA adopted in this work were all based on methacrylate groups. By changing the amount of crosslinking agent EGDMA, the numbers of terminal groups of S1.5 and S2 were consistent with those of M1.5 and D2, respectively, so that the crosslinking density of S1.5 and S2 was the same as that of M1.5 and D2, respectively. The compositions are also shown in Table 1. The Dk values, EWC, transmittance, and mechanical properties of the silicone hydrogels were analyzed, and all results are shown in Table 2.
Oxygen permeabilities of silicone hydrogels
The oxygen permeabilities of silicone hydrogels were measured by the coulometric method, and their Dk values are shown in Fig. 3. In the case of identical composition, the sample S1 prepared by single methacrylate terminated silicone macromonomer (PDMS-1) had the highest Dk value, up to 153 Barrer, while the sample D2 prepared by double methacrylate terminated silicone macromonomer (PDMS-2) had the lowest Dk value, 112 Barrer. The Dk value of M1.5 obtained by mixing PDMS-1 and PDMS-2 equally was 121 Barrer, which was slightly higher than that of D2.
Oxygen permeation through hydrogel materials usually goes through a process of first dissolution and then diffusion. Therefore, the oxygen permeability of silicone hydrogels depends on the dissolution coefficient and diffusion coefficient of oxygen in the materials [34]. The oxygen solubility in polymer materials depends on the polarity of polymers [23]. In this work, the siloxane units and the hydrophilic monomers were identical in the three kinds of silicone hydrogels, so they had similar polarity. Hence, the dissolution coefficients of these three kinds of hydrogel materials were the same, and their oxygen permeation values were mainly determined by diffusion coefficient. Seitz et al. [28] found that the oxygen permeability of the material increased nonlinearly with the increase of the silicon-containing polymer content, which was related to the spatial arrangement of the silicone phase region and their fluidity. The molecular gap formed by the fluffy chain structure between silicon-containing polymers can provide a transport channel for oxygen molecules, increase the free volume, help to improve the diffusion rate of oxygen, and further improve the oxygen permeability of materials. Since the molecular weight and chemical structure of two silicone macromonomers, PDMS-1 and PDMS-2, were alike, the spatial arrangement of the silicone phase region in the hydrogels should be similar. Therefore, it can be speculated that the difference in oxygen permeation between silicone hydrogels was mainly caused by the fluidity of the silicone phase region. The silicone macromonomer PDMS-1 had only one polymerizable double bond. After polymerization, only one end was fixed and the other end could move freely. The free polysiloxane chain segment mobility increased the fluidity of the silicone phase region, which improved the diffusion rate of oxygen and further raised the oxygen permeability of the silicone hydrogels. PDMS-2 had two polymerizable double bonds. After polymerization, the free polysiloxane chain length of PDMS-2 was shorter than PDMS-1, which limited the mobility of silicone segments and was not conducive to the diffusion of oxygen. Therefore, the Dk value of silicone hydrogel S1 was higher than that of D2.
It was worth noting that PDMS-2, as a bifunctional compound, could act as a crosslinking agent and change the crosslinking densities of silicone hydrogels, which maybe affect their Dk values. The silicone hydrogel D2 had the highest crosslinking density, followed by M1.5, and S1 had the lowest crosslinking density. To verify the influence of crosslinking density on oxygen permeability of silicone hydrogels, two other silicone hydrogels S1.5 and S2 were produced by PDMS-1 macromonomer, and their crosslinking densities were consistent with those of M1.5 and D2 respectively. The Dk values of silicone hydrogels M1.5 and D2 are also shown in Fig. 3. Comparing the Dk values of M1.5 and S1.5, D2 and S2, respectively, it was found that the oxygen permeabilities of the silicone hydrogels obtained by single methacrylate terminated silicone macromonomer was higher than that obtained by double methacrylate terminated silicone macromonomer when the crosslinking densities were the same. These results indicated that the free polysiloxane chain length of silicone chain segments played an important role in the oxygen permeability of silicone hydrogels. PDMS-1 with a longer free polysiloxane chain length was more beneficial to improve the oxygen permeability of the silicone hydrogels.
To further explain the influence of the free polysiloxane chain length and crosslinking density on the oxygen permeabilities of silicone hydrogels, a model was proposed and is shown in Fig. 4. As shown in Fig. 4a, the single methacrylate terminated silicone macromonomer chain has better mobility and freer segment movement, so free polysiloxane chain is easier to gather together. Once a continuous channel is formed, oxygen permeability of the silicone hydrogels will be significantly improved. Multifunctional compounds can be used as crosslinking agents. PDMS-2 has bifunctional groups, which is not only a monomer but also a crosslinking agent. When the content of PDMS-2 increases, the crosslinking density of the silicone hydrogels will increase and free polysiloxane chain length of silicone hydrogels will decrease. The silicone chains are easy to cross connect and grow, while not easy to gather together. Therefore, it is difficult to form a perfect channel, which is disadvantageous for the transfer of oxygen, as shown in Fig. 4 b and c.
Equilibrium water contents of silicone hydrogels
Hydrophilicity is an important index affecting the application performance of silicone hydrogels. Especially in the field of contact lenses, higher equilibrium water content generally increases the wearing comfort. In addition, the water molecules swollen in the hydrogels will also contribute to the oxygen permeability. The EWC of silicone hydrogels prepared from silicone macromonomers is shown in Fig. 5. Under the condition of the same amount of crosslinking agent EGDMA, the silicone hydrogel S1 prepared by a single methacrylate terminated silicone macromonomer had the highest EWC, and the EWC of the samples M1.5 and D2 gradually decreased. The EWC of S1 reached 40%, while that of D2 was only 27%, which may be attributed to by different crosslinking densities. With the increase of the crosslinking density of silicone hydrogels, the number of crosslinking sites in their spatial network structures increased, and the number of water molecules that can be accommodated decreased, thus the EWC reduced.
Comparing the samples D2 and S2 with the same crosslinking density, their EWCs were identical, despite being prepared from different silicone macromonomers. And the difference of EWC between samples M1.5 and S1.5 was also very small. It indicated that the EWC of the silicone hydrogels was affected mainly by their crosslinking densities, in the case of the same silicone contents. Besides, although the samples D2 and S2 have the same EWC, the Dk value of sample S2 was obviously higher than that of sample D2. And the Dk value of sample S1.5 was also obviously higher than that of sample M1.5. It can be inferred that the oxygen permeability depended mainly on the free polysiloxane chain length of the silicone hydrogels. Those results showed that the single methacrylate terminated silicone macromonomer was beneficial to improve the oxygen permeability. Meanwhile, it can improve the EWC of silicone hydrogels.
Transmittance of silicone hydrogels
Figure 6 shows the light transmittances of the silicone hydrogels with different crosslinking densities and free polysiloxane chain lengths in a swelling state. The silicone hydrogels S1, M1.5, and D2 had high light transmittance, which were about 95%, 96%, and 98% (500–600 nm), respectively. The lower light transmittance of sample S1 compared with sample D2 may be attributed to the free polysiloxane chain length of the single methacrylate terminated silicone macromonomer chain segments. In silicone hydrogels, good chain segment mobility was beneficial to the aggregation of silicone chains and the formation of the continuous silicone phases. But it was also easy to form a larger phase separation structure, which was unfavorable to the transmission of light. Compared with S1, the light transmittance of S1.5 and S2 is decreased, only about 93% and 91%, respectively. It should be related to their high crosslinking density. High crosslinking density was detrimental to light transmission. This result proved the rationality of our proposed model.
Mechanical properties of silicone hydrogels
Figure 7 shows the mechanical properties of the silicone hydrogels. High elongation at break reflects the good tensile property of the material, and the lower elastic modulus reflects the better flexibility of the material. For the samples S1, M1.5, and D2 prepared with different silicone macromonomers, the elongations at break of the silicone hydrogels increased with the increase of the free polysiloxane chain length. The elongation at break of silicone hydrogel sample S1 prepared by single methacrylate terminated silicone macromonomer was 180%, while that of silicone hydrogel sample D2 prepared by double methacrylate terminated silicone macromonomer decreased to 70%. On the contrary, the elastic moduli of the silicone hydrogels decreased with the increase of the free polysiloxane chain length. The elastic moduli of samples S1, M1.5, and D2 were about 2.0 MPa, 2.5 MPa, and 3.2 MPa, respectively. This was probably attributed to the crosslinking density and microstructure of polymer networks. As the number of terminal groups of silicone macromonomers increased, the crosslinking densities of the silicone hydrogels increased, which enhanced the interaction between the polymer segments and restricted the movement of the silicone chain segments. Thus, the moduli of silicone hydrogels were increased and the elongations were decreased.
Comparing the samples S2 and D2 with the same crosslinking density, it was found that the elongation of the sample S2 was slightly higher and the modulus was slightly lower than that of the sample D2. According to previous studies, hydrogels are hard segments and silicone phases are soft segments in multicomponent silicone hydrogels [26]. In sample S2, only one end of the silicone macromolecular was fixed, and the silicone chain segments had better mobility. Due to the hydrophobic interaction, the silicone chain segments tended to aggregate together, and the interaction between silicone chain segments was strengthened, resulting in the increase of elongation and the decrease of modulus. The results indicated that the crosslinking density and free polysiloxane chain length also had an effect on the mechanical properties of the silicone hydrogels, and PDMS-1 with longer free polysiloxane chain length was more beneficial to the increase of elongation and the decrease of modulus of silicone hydrogels.
Conclusions
In this work, the high oxygen permeability silicone hydrogels were prepared by silicone macromonomers with different numbers of terminal functional groups. The oxygen permeabilities, EWC, light transmittance, and mechanical properties of the silicone hydrogels were measured. The relationship between free polysiloxane chain length and the properties of the silicone hydrogels was analyzed in detail. The results showed that the silicone hydrogel S1 with the lowest crosslink density and the longest free polysiloxane chain length, which had the highest oxygen permeability and EWC. With the increase of free polysiloxane chain length, the Dk values and EWC of silicone hydrogels both increased. With the increase of crosslinking density, the Dk values and EWC of the silicone hydrogels decreased. The light transmittance test proved that the longer free polysiloxane chain length of silicone hydrogels was easy to aggregate, which reduced the light transmittance. The elongations decreased and the moduli increased with the increase of the free polysiloxane chain length of the silicone hydrogels. Therefore, the longer free polysiloxane chain was beneficial to the increase of oxygen permeability, EWC, and mechanical properties of silicone hydrogels. In addition, a model was presented to explain the mechanism of oxygen permeability in detail. This work provided a new idea for the efficient preparation of silicone hydrogels with high oxygen permeability.
References
Baran A, Zaleski T, Kulikowski E, Wieczorek J (2015) Hydrophysical and biological properties of sandy substrata enriched with hydrogel. Pol J Environ Stud 24(6):2355–2362
dos Santos JF, Alvarez-Lorenzo C, Silva M, Balsa L, Couceiro J, Torres-Labandeira JJ, Concheiro A (2009) Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials 30(7):1348–1355
Rico-Sergado L, Perez-Canales JL, Perez-Santonja JJ, Ciguenza-Sancho S (2015) Severe keratomalacia after 12 months of continuous hydrogel contact lens wear in a psychiatric patient. Cont Lens Anterior Eye 38(2):138–141
Hill RM (2000) Dk/t: closed eye equivalents. Int Cont Lens Clinic 27(3):101–102
Jalil A, Uludag H (2004) Hydrogels of thermoreversible comb-polymers exhibit increased resistance for dissolution. Materialwiss Werkstofftech 35(12):972–979
Cabral JD, Roxburgh M, Shi Z, Liu L, McConnell M, Williams G, Evans N, Hanton LR, Simpson J, Moratti SC, Robinson BH, Wormald PJ, Robinson S (2014) Synthesis, physiochemical characterization, and biocompatibility of a chitosan/dextran-based hydrogel for postsurgical adhesion prevention. J Mater Sci Mater Med 25(12):2743–2756
Hu X, Gong X (2016) A new route to fabricate biocompatible hydrogels with controlled drug delivery behavior. J Colloid Interface Sci 470:62–70
Busatto CA, Labie H, Lapeyre V, Auzely-Velty R, Perro A, Casis N, Luna J, Estenoz DA, Ravaine V (2017) Oil-in-microgel strategy for enzymatic-triggered release of hydrophobic drugs. J Colloid Interface Sci 493:356–364
Liu L, Chakma A, Feng X (2008) Gas permeation through water-swollen hydrogel membranes. J Membr Sci 310(1–2):66–75
Liu Y, Yu S, Wu H, Li Y, Wang S, Tian Z, Jiang Z (2014) High permeability hydrogel membranes of chitosan/poly ether-block-amide blends for CO2 separation. J Membr Sci 469:198–208
Yin L, Ding J, Zhang J, He C, Tang C, Yin C (2010) Polymer integrity related absorption mechanism of superporous hydrogel containing interpenetrating polymer networks for oral delivery of insulin. Biomaterials 31(12):3347–3356
Morgan PB, Efron N (1998) The oxygen performance of contemporary hydrogel contact lenses. Cont Lens Anterior Eye 21(1):2–6
Zhang X, Wang L, Tao H, Sun Y, Yang H, Lin B (2019) The influences of poly (ethylene glycol) chain length on hydrophilicity, oxygen permeability, and mechanical properties of multicomponent silicone hydrogels. Colloid Polym Sci 297(9):1233–1243
Young MD, Benjamin WJ (2003) Calibrated oxygen permeability of 35 conventional hydrogel materials and correlation with water content. Eye Cont Lens 29(2):126–133
Holden BA, Mertz GW (1984) Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Invest Ophthalmol Vis Sci 25(10):1161–1167
Dorishetty P, Dutta NK, Choudhury NR (2020) Bioprintable tough hydrogels for tissue engineering applications. Adv Colloid Interface Sci 281:102163
Balakrishnan B, Banerjee R (2011) Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev 11(8):4453–4474
Radhakrishnan J, Subramanian A, Krishnan U, Sethuraman S (2017) Injectable and 3D bioprinted polysaccharide hydrogels: from cartilage to osteochondral tissue engineering. Biomacromol 18(1):1–26
Lin B, Megley K, Viswanathan N, Krogstad D, Drews L, Kade M, Qian Y, Tirrell MV (2012) pH-responsive branched peptide amphiphile hydrogel designed for applications in regenerative medicine with potential as injectable tissue scaffolds. J Mater Chem 22(37):19447–19454
Jiang Y, Hou Y, Fang J, Liu W, Zhao Y, Huang T, Cui J, Yang Y, Zhou Z (2019) Preparation and characterization of PVA/SA/HA composite hydrogels for wound dressing. Int J Polym Anal Charact 24(2):132–141
Gupta A, Kowalczuk M, Heaselgrave W, Britland ST, Martin C, Radecka I (2019) The production and application of hydrogels for wound management: a review. Eur Polym J 111:134–151
Rudy A, Kuliasha C, Uruena J, Rex J, Schulze KD, Stewart D, Angelini T, Sawyer WG, Perry SS (2017) Lubricous hydrogel surface coatings on polydimethylsiloxane (PDMS). Tribol Lett 65(1):3
Nicolson PC, Vogt J (2001) Soft contact lens polymers: an evolution. Biomaterials 22(24):3273–3283
Lamberti A, Marasso SL, Cocuzza M (2014) PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv 4(106):61415–61419
Compan V, Lopez-Alemany A, Riande E, Refojo MF (2004) Biological oxygen apparent transmissibility of hydrogel contact lenses with and without organosilicon moieties. Biomaterials 25(2):359–365
Lin CH, Yeh YH, Lin WC, Yang MC (2014) Novel silicone hydrogel based on PDMS and PEGMA for contact lens application. Colloids Surf., B 123:986–994
Lai Y-C (1995) Novel polyurethane–silicone hydrogels. J Appl Polym Sci 56(3):301–310
Yokota M, Ajiro H, Akashi M (2013) Effect of copolymerizing fluorine-bearing monomers on the relationship among internal structure, gas permeability, and transparency in copolymer networks composed of methacrylates and siloxane macromers. J Appl Polym Sci 127(1):535–543
Tao H, Zhang X, Sun Y, Yang H, Lin B (2016) The influence of molecular weight of siloxane macromere on phase separation morphology, oxygen permeability, and mechanical properties in multicomponent silicone hydrogels. Colloid Polym Sci 295(1):205–213
Zhao Z, Xie H, An S, Jiang Y (2014) The relationship between oxygen permeability and phase separation morphology of the multicomponent silicone hydrogels. J Phys Chem B 118(50):14640–14647
Sugimoto H, Nishino G, Tsuzuki N, Daimatsu K, Inomata K, Nakanishi E (2011) Preparation of high oxygen permeable transparent hybrid copolymers with silicone macro-monomers. Colloid Polym Sci 290(2):173–181
Pozuelo J, Compañ V, González-Méijome JM, González M, Mollá S (2014) Oxygen and ionic transport in hydrogel and silicone-hydrogel contact lens materials: an experimental and theoretical study. J Membr Sci 452:62–72
Peng S, Guo Q, Hughes TC, Hartley PG (2011) In situ synchrotron SAXS study of polymerizable microemulsions. Macromole 44(8):3007–3015
Nagai K, Masuda T, Nakagawa T, Freeman BD, Pinnau I (2001) Poly 1-(trimethylsilyl)-1-propyne and related polymers: synthesis, properties and functions. Prog Polym Sci 26(5):721–798
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21374016, 21304018), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, S., Zhang, X., Sun, Y. et al. Study on the influence of crosslinking density and free polysiloxan chain length on oxygen permeability and hydrophilicity of multicomponent silicone hydrogels. Colloid Polym Sci 299, 1327–1335 (2021). https://doi.org/10.1007/s00396-021-04850-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04850-5