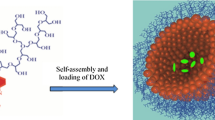
Abstract
Novel amphiphilic light-responsive block copolymer spiropyran-poly(2-methacryloyloxyethyl phosphorylcholine) (SP-PMPC) was reported and used as smart drug nanocarriers. SP-PMPC was easily synthesized via atom transfer radical polymerization (ATRP) of 2-methacryloyloxyethyl phosphorylcholine (MPC) using 2-bromo-2-methylpropanoate-ethyl-3′,3′-dimethyl-6-nitrospiro (2H-1-benzopyran-2,2′-indoline) (SP-Br) as initiator. SP-PMPC can self-assemble to micelles with relatively low critical micelle concentration (CMC) value (0.037 mg mL−1). Because of the reversible photochemical isomerization of hydrophobic spiropyran (SP) to hydrophilic merocyanine (MC), the self-assembly and disassembly of SP-PMPC micelles can be well controlled by an external light source, which was proved by ultraviolet-visible light (UV–vis) spectrometry, dynamic light scattering (DLS), and transmission electron microscopy (TEM). The hydrophobic anticancer drug doxorubicin (DOX) can be encapsulated into micelles. In vitro drug release studies showed that the release of DOX was accelerated in the presence of UV irradiation (λ = 365 nm) when compared to similar systems without UV irradiation treatment. The SP-PMPC micelles exhibited superior biocompatibility as measured by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay owing to the stealth phosphorylcholine outer shell. Moreover, the DOX-loaded SP-PMPC micelles under UV irradiation exhibited better anticancer activity than that of the nonirradiated ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past few decades, self-assembled polymeric micelles have emerged as promising nanocarriers for drug delivery due to their distinct advantages, such as high drug loading capacity [1–3], decreased side effects [4, 5], long circulation in the bloodstream [6, 7], passive targeting of tumor tissues via enhanced permeability and retention (EPR) effect [8]. and improved drug bioavailability [4, 9]. What is more, the chemical and physical properties of the micelles can be easily adjusted by choosing different monomers, polymerization degrees, and other parameters. Therefore, there is growing interest in designing polymeric micelles as prospective nanocarriers of active therapeutics.
The ideal drug nanocarriers can stably retain the encapsulated drug in the bloodstream, while releasing the drug rapidly upon reaching the site of action. In order to balance the conflicting requirements (retention and release), the use of drug nanocarriers with triggering mechanisms for drug release as a new strategy has attracted much attention. Therefore, various smart drug nanocarriers in response to external stimuli such as pH [10–12], redox [13, 14], temperature [15, 16], enzyme [17], and light [18–20] have been extensively studied. Among various chemical and physical stimuli, light is an especially attractive external stimulus since it provides a possibility for realizing remote and spatiotemporal drug release by easily tuning the light intensity, energy, and site of irradiation [21–24]. Furthermore, combination of light and interventional therapy, fixed-point drug release can be realized by taking advantage of optical fiber [25, 26]. After the first “proof-of-concept” example pioneered by Zhao’s group [27], various light-responsive polymeric micelles were reported [2, 28–31]. Such light-responsive micelles were extensively investigated as drug nanocarriers because of the feasibility of high controllability over the drug release profile using a specific wavelength of light. In order to design light-responsive micelles, a lot of light-responsive functional groups were introduced to construct light-responsive polymers. The photochromic spiropyran (SP) is one of the most well-known light-responsive molecules [32]. SP is a well-investigated photochromic molecule because of the spiropyran-merocyanine (SP-MC) chemistry. SP molecule is colorless, nonpolar, and hydrophobic. Under UV irradiation (365 nm), colorless SP can undergo a reversible isomerization to colored MC with greatly increased hydrophilicity associated with the structural conversion from neutral to zwitterionic (Scheme 1). Based on the unique light-responsive property, spiropyran-based materials were successfully applied in various areas, such as data storage, drug delivery, and light-actuated nanovalves [33–35].
Biocompatibility is another very important issue for drug delivery systems. It is very important that the surface of the drug nanocarriers should be engineered to be biocompatible and bioinert. Poly(ethylene glycol) (PEG) is the most widely used biocompatible synthetic polymer. As an alternative to traditional PEG, bio-inspired zwitterions, including phosphorylcholine (PC), carboxybetaine (CB), and sulfobetaine (SB), are recently well established for construction of biocompatible nanoparticles for drug delivery [36]. Since Nakabayashi et al. synthesized a methacrylate monomer with a phospholipid polar group, 2-methacryloyloxyethyl phosphorylcholine (MPC) [37], PC-containing polymers with a wide variety of molecular architectures were developed and used in different areas [38]. A new concept was therefore proposed for designing biocompatible materials with cell membrane-mimic surfaces. Very recently, Ji and co-workers reported a series of zwitterionic phosphorylcholine modified micelles as drug nanocarriers, which showed outstanding biostability and biocompatibility [39, 40].
In this research, light-responsive micelles using SP initiated poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) were well designed. Since SP can undergo a reversible isomerization from hydrophobic to hydrophilic MC upon UV irradiation, the light-responsive self-assembly and disassembly of SP-PMPC were investigated. Moreover, the hydrophobic anticancer drug doxorubicin (DOX) was encapsulated into the micelles, and their performance as smart drug nanocarriers was investigated in terms of the in vitro light-responsive drug release. Finally, the cytotoxicity of DOX loaded SP-PMPC micelles with and without light irradiation was studied. All these results indicated that the novel amphiphilic light-responsive SP-PMPC could serve as promising drug nanocarriers for spatiotemporal drug delivery.
Experimental section
Materials
2-Methacryloyloxyethyl phosphorylcholine (MPC), 2-bromo-2-methylpropionyl bromide, and 2,2′-bipyridine (bpy) were purchased from Aldrich and used without further purification. Doxorubicin hydrochloride (DOX·HCl) was obtained from Zhejiang Hisun Pharmaceutical Co., Ltd.
Synthesis of spirobenzopyran-based ATRP initiator SP-Br
At first, 1′-(2-hydroxyethyl)-3′,3′-dimethyl-6-nitrospiro(2H-1-benzopyran-2,2′-indoline) (SP-OH) was prepared as previously described [41]. A clean, dry round-bottom flask containing 100 mL of dry methylene chloride was cooled to 0 °C in ice-water bath. The solvent was purged with N2 for 30 min before SP-OH (5.00 g) and triethylamine (8 mL) were added sequentially to the flask. 2-Bromo-2-methylpropionyl bromide (7 ) was then added dropwise into the flask. The flask was then removed from the ice-water bath, and the reaction was allowed to proceed for 24 h at room temperature. During this period, the reaction mixture changed to brown. The reaction mixture was washed with 200 mL of 5 % sodium bicarbonate three times. After the solvent was removed in vacuum, the crude product SP-Br was recrystallized from methanol twice to give 3.41 g yellowish powder in 48 % yield. 1H NMR (500 MHz CDCl3) δ:1.18 (s, 3H); 1.29 (s, 3H); 1.91(s, 6H); 3.48-3.60 (m, 2H); 4.3 (t, 2H); 6.00 (d, 1H); 6.73 (q, 2H); 6.93 (q, 2H); 7.11 (d, 1H); 7.21–7.27 (m, 1H); 8.01–8.04 (m, 2H).
Synthesis of SP-PMPC using SP-Br initiator
Typical protocols for the controlled polymerization of MPC using SP-Br initiator was shown as follows. SP-Br (100 mg, 0.2 mmol) and MPC (885 mg, 3 mmol) were dissolved in 7 mL of methanol and 1 mL of tetrahydrofuran (THF) mixture. The bpy ligand (62.48 mg, 0.4 mmol) and the CuBr catalyst (28.8 mg, 0.2 mmol) were added to this degassed solution at 25 °C. Polymerization occurred immediately. After 12 h, the solution was exposed to air. The resulting SP-PMPC polymers were precipitated into THF two times, then re-dissolved in methanol, and then passed through a silica gel column to remove the spent ATRP catalyst. After solvent evaporation, the remaining polymer was dried in a vacuum oven at 40 °C for at least 12 h. The yield was 66 %. 1H NMR is shown in Fig. 1. The degree of polymerization was 13, which was calculated from 1H NMR.
Preparation of SP-PMPC micelles
The blank SP-PMPC micelles were prepared by a typical cosolvent approach. Briefly, 10 mg of SP-PMPC polymer was dissolved in 2 mL of methanol at room temperature and stirred for at least 2 h. After that, 2 mL of distilled water was added dropwise under stirring with a magnetic bar. The resulting mixture was kept stirring for another 2 h and then dialyzed against deionized water for 48 h (MWCO 1000).
Determination of CMC
The CMC was determined using pyrene as the hydrophobic fluorescent probe. Pyrene was dissolved in acetone (2 mg mL−1) and then diluted to 100 μM to serve as a stock solution. Ten microliters of 100 μM pyrene solution was added to 1 mL of polymer solution in water (with different concentrations from 5 × 10−4 to 0.5 mg mL−1). The samples were sonicated for 5 min and kept for 24 h at room temperature prior to measurement. The fluorescence of each pyrene-loaded micellar solution was measured at an emission wavelength of 372 nm using a fluorometer. The CMC value was determined by calculating the ratio of the fluorescence intensities at wavelengths of 339 and 332 nm.
Preparation of DOX-loaded micelles
At first, 2 mg of DOX·HCl and 10 mg of SP-PMPC were dissolved in 2 mL of methanol. Twenty microliters of triethylamine was added to obtain hydrophobic DOX. Then, the mixture was added slowly to 2 mL of phosphate-buffered saline (PBS, pH 7.4). After being stirred for an additional 3 h, the solution was dialyzed against water for 24 h in dark to remove free DOX (MWCO 1000). In order to determine the total loading of DOX, the DOX-loaded micellar solution was lyophilized and then dissolved in methanol. The fluorometric measurement was used to determine the loading amount. Drug loading content (DLC) was calculated according to the follow equation.
In vitro drug release
The release of DOX from SP-PMPC micelles was studied with and without light irradiation at 37 °C. Briefly, 5-mL solution of DOX-loaded micelles was irradiated under UV light at 365 nm for 20 min. The solution was placed in a dialysis bag with a molecular weight cutoff of 1000 Da. The dialysis bag was then immersed in 95 mL of the release medium (buffer) at 37 °C in dark with constant shaking (100 rpm). Meanwhile, the DOX-loaded micellar solution without UV light irradiation was used as a control. At each predetermined time interval, 2 mL of the release medium outside the dialysis bag was taken out, and the same volume of each sample was replaced by the same volume of fresh medium. The DOX concentration in each withdrawn solution was determined using UV–vis spectra at 485 nm against the predetermined calibration curve.
In vitro on-off release of coumarin 102
The florescent molecule coumarin 102 was loaded into the micelles using the same procedure as DOX-loaded micelles. At first, 2 mg of coumarin 102 and 10 mg of SP-PMPC were dissolved in 2 mL of methanol. Then, the mixture was added slowly to 2 mL of PBS (pH 7.4). After being stirred for an additional 3 h, the solution was dialyzed against water for 24 h in dark to remove free coumarin 102 (MWCO 1000).
The on-off release of coumarin 102 was carried out as follows. The coumarin 102 loaded micellar solution was alternatively irradiated or not irradiated with UV light (365 nm) for every 10 min. The fluorescence emission spectra of coumarin 102 were recorded every 5 min from 450 to 700 nm with the excitation wavelength of 420 nm.
Hemolytic assays
The blank SP-PMPC micellar solutions were prepared in sterilized Tris Buffered Saline (TBS) (10 mM Tris, 142 mM NaCl, pH 7.4) and stored in aliquots at −20 °C. Serial 2-fold dilutions of a copolymer stock solution were made with sterilized TBS, which yielded copolymer solutions for hemolysis assays. Human red blood cell (RBC) suspension (100 μL) was washed with sterilized TBS (12 mL), harvested via centrifugation at 700 rcf, and resuspended into 20 mL TBS to yield the RBC stock suspension (0.5 % blood cells). RBC stock suspension (160 μL) and diluted copolymer solution (40 μL) were added into each centrifuge cup. After incubation at 37 °C for 60 min with shaking at 250 rpm, the centrifuge cups were subsequently centrifuged at 956 rcf for 5 min and the supernatant (30 μL) was transferred into a well of a 96-well microplate and diluted with TBS (100 μL). Absorbance at 415 nm of a solution was measured using microplate reader. Controls included untreated RBC suspension and RBC suspension (160 μL) treated with Triton X-100 (50 %, 40 μL) to provide reference for 0 and 100 % hemolysis, respectively. Each hemolysis assay trial was carried out in triplicate and the reported results are the averages of two independent trials.
Cytotoxicity assay
Cytotoxicity assay was performed using the traditional 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Human umbilical vein endothelial cells (HUVEC) and human epithelial carcinoma (HeLa) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10 % fetal bovine serum (FBS).
In order to measure the cytotoxicity of blank SP-PMPC micelles, HUVEC or HeLa cells were seeded in 96-well plates at 1 × 104 per well in 200 μL of culture medium for 24 h. The culture medium was replaced with fresh one. SP-PMPC micellar solution with the final concentration from 10 to 1000 μg mL−1 was then added. After 48 h, the cell samples were treated with 20 μL of MTT (5 mg mL−1) for 4 h, which was followed by the addition of 150 μL DMSO to dissolve the formazan crystals. The absorbance was measured at a wavelength of 570 nm. Data are expressed as average ± SD (n = 3).
The cytotoxicity of DOX-loaded micelles against HeLa cells was also examined in vitro by the MTT assay. After HeLa cells were incubated for 24 h in 96-well plates at an initial seeding density of 1 × 104 per well, the culture medium was removed and replaced with 200 μL of medium containing serial dilutions of micelles. The cells were irradiated under UV light at 365 nm for 30 min after incubation with DOX-loaded micelles for 3 h and cultured for another 48 h. Meanwhile, cells without UV irradiation were also set as a control. The cytotoxicity of blank HPAE-PC micelles against HeLa cells with or without UV light irradiation was also investigated as controls. The cell samples were treated with 20 μL of MTT (5 mg mL−1) for 4 h, which was followed by the addition of 150 μL DMSO to dissolve the formazan crystals. The absorbance was measured at a wavelength of 570 nm. Data are expressed as average ± SD (n = 3).
Characterizations
1H NMR spectra was recorded on a Bruker AV400 spectrometer operating at 400 MHz. Dynamic light scattering (DLS) measurements were carried out at 25 °C using a Zetasizer Nano-ZS from Malvern Instruments equipped with a He-Ne laser at wavelength of 633 nm. Transmission electron microscopy (TEM) was performed using a JEOL-2010 operated at an accelerating voltage of 200 kV. The samples were prepared by dropping 0.1 mg mL−1 micellar solution on the copper grid. UV–vis spectra were carried out on a UV–vis Shimadzu UV-2550 spectrometer. Fluorescence spectra were carried out from Hitachi F-4500 fluorescence spectrometer with a band width of 10 nm. UV irradiation was conducted using a 365 nm UV light lamp (Spectroline, model no. EN-280 L, USA).
Results and discussion
Synthesis of SP-PMPC
The synthetic route of SP-PMPC is shown in Scheme 2. The functional SP-containing ATRP initiator was firstly synthesized and proved by 1H NMR. The amphiphilic polymer SP-PMPC was prepared via ATRP using SP-Br as initiator. The successful synthesis of SP-PMPC was confirmed by 1H NMR as shown in Fig. 1 which clearly showed the characteristic signals of spiropyran (6.7–7.3 and 8.0–8.2 ppm) and phosphorylcholine (3.6–3.8 and 4.0–4.4 ppm). The degrees of polymerization (DP) of the MPC block was calculated to be 13 by integrating the signals of the aromatic groups of the SP-Br initiator at 8.1 ppm and those of the MPC block in the range of 4.0 to 4.4 ppm. The monomer conversion of MPC was 86.7 %. In addition, the molecular weight of SP-PMPC can also be determined to be 4200 g/mol by 1H NMR.
Self-assembly and stimuli-responsiveness of SP-PMPC
Similar to other polymeric amphiphiles, SP-PMPC might also be able to self-assemble into nanosized micelles because of its amphiphilic structure. The self-assembly behavior of SP-PMPC was well investigated by DLS and TEM. The intensity-average hydrodynamic diameter (D h) of the micelles was 37.2 nm with a polydispersity index (PDI) of 0.286 measured by DLS. The morphology of the micelles was investigated using TEM. The TEM image indicated that the micelles were presumably spherical with mean diameter of about 15.5 nm (Fig. 2), which was much smaller than the D h measured by DLS. It is most likely due to the shrinkage of hydrophilic shells upon drying the samples. The CMC of the SP-PMPC micelles was determined using pyrene as the hydrophobic fluorescent probe. The estimated CMC value was 44.87 mg L−1 (Fig. 3). It should be noted that the CMC value was much smaller than those of the low molecular weight surfactants and was comparable with those of other polymeric micelles [42]. The stability of drug nanocarriers in plasma is very important to avoid unfavorable leakage of drugs. The SP-PMPC micelles were very stable in plasma. Neglectable changes of hydrodynamic diameter were observed after 24-h incubation of SP-PMPC in plasma (Fig. 4).
As a result of the reversible photochemical isomerization of SP segment, the UV-responsive property of SP-PMPC was evaluated in aqueous solution by UV–vis spectra. After the solution was irradiated with UV light (365 nm), the characteristic peak of the hydrophilic MC form at 515 nm appeared (Fig. 5a). During the UV light irradiation process, the absorption peak at 515 nm increased dramatically, which indicated the isomerization of hydrophobic SP to hydrophilic MC form. The solution color also changed from colorless to light pink upon UV irradiation (Fig. 6). The photochemical isomerization of SP to MC was reversible. The characteristic peak of the MC form at 515 nm gradually decreased under irradiation with visible light (620 nm), which suggested the isomerization of merocyanine to spiropyran (Fig. 5b). What is more, the reversible isomerization could be repeated several times. As shown in Fig. 6, the photochemical isomerization of SP was indicated by the absorbance change at 515 nm due to spiropyran molecules switching between open (UV light, 365 nm) and closed (visible light, 620 nm) states. The slightly decrease of the amplitude with the number of the cycles indicated “fatigue” effects caused by photodegradation of the SP dyes [43].
Because of structural isomerization of hydrophobic SP to hydrophilic MC, the amphiphilic SP-PMPC was converted to all-hydrophilic MC-PMPC, which might result in the disassembly of the micelles. The reversible self-assembly and disassembly of SP-PMPC micelles were investigated by DLS and TEM. As discussed above, the D h of SP-PMPC micelles was 37.2 nm as measured by DLS. However, after UV irradiation for 10 min, the D h decreased significantly to 4.7 nm, which indicated disassembly of the micelles into unimers (Fig. 7). If the solution was again irradiated with visible light (620 nm), the D h was changed to 40.6 nm, which was similar to that of the initial micelles. The light-responsive self-assembly and disassembly was confirmed by TEM as well. As shown in Fig. 8, the SP-PMPC micelles were dissociated and no regular aggregates were observed after UV light irradiation (365 nm). Spherical micelles were formed again after visible light irradiation (Fig. 8b), which was in accordance with the DLS results (Fig. 7).
In vitro light-responsive drug release
In order to evaluate the potential of the light-responsive micelles as smart drug nanocarriers, loading and release of anticancer drug DOX from SP-PMPC micelles were investigated. DOX was loaded into the micelles by a cosolvent technique as mentioned in “Experimental section.” The drug loading content (DLC) was 5.73 % as measured by fluorescent spectra. The drug release of the DOX-loaded SP-PMPC micelles was investigated with and without light irradiation at pH 7.4 by dialysis. The cumulative release percentages of DOX released from the micelles versus time were presented in Fig. 9. It is interesting to note that the DOX release was accelerated significantly in the presence of UV irradiation than without UV irradiation. In the presence of UV irradiation, about 50 % of DOX was released from SP-PMPC micelles after 24 h. However, only less than 20 % of DOX was released without UV irradiation within the same period. The fast DOX release from the SP-PMPC micelles under UV irradiation was most likely due to the disassembly of the SP-PMPC micelles because of the photochemical isomerization of SP units. Furthermore, the on-off controlled release can be realized through this drug delivery system. The fluorescent molecule coumarin 102 was used to study the on-off controlled release. As shown in Fig. 10, the release of coumarin 102 was very fast when the UV light was on. However, the release of coumarin 102 was very slow without UV irradiation.
In vitro cytotoxicity assay
The biocompatibility is a basic requirement for an ideal drug delivery system. The hemocompatibility of blank SP-PMPC micelles was evaluated via hemolytic assays against human erythrocytes. As shown in Fig. 11, the micelles with PMPC shell showed very low hemolytic toxicity even if the concentration was as high as 1 mg mL−1. It is especially advantageous for intravenous administration. What is more, the cytotoxicity of SP-PMPC micelles was evaluated to investigate their potential as drug nanocarriers. The in vitro cytotoxicity of blank SP-PMPC micelles was investigated by MTT assay using HUVEC cells and HeLa cells as respective models of normal and cancer cells. Figure 12 showed the relative cell viability of the two kinds of cells after incubating with various concentrations of nanocarriers for 48 h. The viability of HUVEC cells and HeLa cells after treating SP-PMPC micelles was nearly 100 %, even at a high concentration of 1 mg mL−1, which indicated that SP-PMPC micelles have no acute or intrinsic cytotoxicity to both cell lines. The cell viability was a little higher than 100 % when the concentration was 25 μg mL−1 as shown in Fig. 12. In such a low concentration, the material was completely nontoxic. The cell viability (104 %) was very close but a little higher than 100 %, which was also observed in published literatures [44, 45].
The cytotoxicity of DOX-loaded SP-PMPC micelles with and without UV light irradiation was also investigated using HeLa cells by MTT assay. As shown in Fig. 13, the DOX-loaded SP-PMPC micelles pretreated with UV irradiation during incubation exhibited higher cytotoxicity than nonirradiated ones. The 50 % cellular growth inhibition (IC50) value of the DOX-loaded micelles with UV irradiation was about 1.47 μg mL−1, which was much lower than the one without UV irradiation (6.3 μg mL−1). It might be ascribed to the faster DOX release because of the disassembly of the micelles under UV irradiation. Meanwhile, in order to eliminate the possibility that the increased anticancer activity of DOX-loaded micelles after UV irradiation comes from the cell death under UV light, the in vitro cytotoxicity of blank SP-PMPC micelles with or without UV irradiation was also investigated using HeLa cells. As expected, the blank micelles with or without UV irradiation did not exhibit obvious cytotoxicity to HeLa cells. Therefore, the excellent anticancer activity of DOX-loaded SP-PMPC micelles under UV irradiation was not caused by the UV light (Fig. 14). At present, UV light was widely used as a trigger in intracellular studies in vitro. However, compared to longer wavelength lights, it is still a big challenge to use the UV light as a trigger in vivo due to its low tissue penetration, which greatly limits its applicability in clinical therapy [46].
Conclusions
A novel light-responsive drug delivery system was developed using SP-PMPC micelles. Well-defined amphiphilic copolymer SP-PMPC was synthesized by ATRP using functional SP initiator as evidenced by 1H NMR. SP-PMPC copolymer can self-assemble to micelles with D h of 37.2 nm. When exposed to UV light, the micelles were disassembled owing to the photochemical isomerization of hydrophobic SP to hydrophilic MC. This structural change of the micelles was reversible upon exposure to visible light. The potential of the SP-PMPC micelles as smart drug nanocarriers was investigated using DOX as a model hydrophobic therapeutic. The in vitro drug release evaluation showed that the release of DOX was much faster in the presence of UV irradiation than that without UV irradiation. The SP-PMPC micelles showed excellent biocompatibility because of the stealth phosphorylcholine outer shell. Furthermore, the DOX-loaded SP-PMPC micelles under UV irradiation exhibited better anticancer activity than that of the nonirradiated ones. These results indicated that the novel amphiphilic light-responsive polymers could be served as promising drug nanocarriers for controlled drug delivery.
References
Tamboli V, Mishra GP, Mitra AK (2013) Novel pentablock copolymer (PLA-PCL-PEG-PCL-PLA)-based nanoparticles for controlled drug delivery: effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid Polym Sci 291:1235–1245
Jin Q, Cai TJ, Han HJ, Wang HB, Wang Y, Ji J (2014) Light and pH dual-degradable triblock copolymer micelles for controlled intracellular drug release. Macromol Rapid Commun 35:1372–1378
Schulz A, Jaksch S, Schubel R, Wegener E, Di Z, Han Y, Meister A, Kressler J, Kabanov AV, Luxenhofer R, Papadakis CM, Jordan R (2014) Drug-induced morphology switch in drug delivery systems based on poly(2-oxazoline)s. ACS Nano 8:2686–2696
Alvarez-Lorenzo C, Concheiro A (2014) Smart drug delivery systems: from fundamentals to the clinic. Chem Commun 50:7743–7765
Zhao LZ, Wu CL, Wang F, Ying AG, Xu CD, Liu SF (2014) Fabrication of biofunctional complex micelles with tunable structure for application in controlled drug release. Colloid Polym Sci 292:1675–1683
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 11:991–1003
Kataoka K, Harada A, Nagasaki Y (2012) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 64:37–48
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46:6387–6392
Gupta S, Tyagi R, Parmar VS, Sharma SK, Haag R (2012) Polyether based amphiphiles for delivery of active components. Polymer 53:3053–3078
Moon JR, Kim MW, Kim D, Jeong JH, Kim JH (2011) Synthesis and self-assembly behavior of novel polyaspartamide derivatives for anti-tumor drug delivery. Colloid Polym Sci 289:63–71
Panda JJ, Chauhan VS (2014) Short peptide based self-assembled nanostructures: implications in drug delivery and tissue engineering. Polym Chem 5:4418–4436
Filippov SK, Chytil P, Konarev PV, Dyakonova M, Papadakis CM, Zhigunov A, Plestil J, Stepanek P, Etrych T, Ulbrich K, Svergun DI (2012) Macromolecular HPMA-based nanoparticles with cholesterol for solid-tumor targeting: detailed study of the inner structure of a highly efficient drug delivery system. Biomacromolecules 13:2594–2604
Yan Y, Wang Y, Heath JK, Nice EC, Caruso F (2011) Cellular association and cargo release of redox-responsive polymer capsules mediated by exofacial thiols. Adv Mater 23:3916–3921
Hrubý M, Koňák C, Ulbrich K (2007) Poly(ethylene oxide)-coated polyamide nanoparticles degradable by glutathione. Colloid Polym Sci 285:569–574
Abulateefeh SR, Spain SG, Aylott JW, Chan WC, Garnett MC, Alexander C (2011) Thermoresponsive polymer colloids for drug delivery and cancer therapy. Macromol Biosci 11:1722–1734
Luxenhofer R, Han YC, Schulz A, Tong J, He ZJ, Kabanov AV, Jordan R (2012) Macromol Rapid Commun 13:1613–1631
Hu JM, Zhang GQ, Liu SY (2012) Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev 41:5933–5949
Liu G, Liu W, Dong CM (2013) UV- and NIR-responsive polymeric nanomedicines for on-demand drug delivery. Polym Chem 4:3431–3443
Jin Q, Cai TJ, Wang Y, Wang HB, Ji J (2014) Light-Responsive Polyion complex micelles with switchable surface charge for efficient protein delivery. ACS Macro Lett 3:679–683
Gohy JF, Zhao Y (2013) Photo-responsive block copolymer micelles: design and behavior. Chem Soc Rev 42:7117–7129
Zhao H, Sterner ES, Coughlin EB, Theato P (2012) o-Nitrobenzyl alcohol derivatives: opportunities in polymer and materials science. Macromolecules 45:1723–1736
Jin Q, Luy C, Ji J, Agarwal S (2012) Design and proof of reversible micelle-to-vesicle multistimuli-responsive morphological regulations. J Polym Sci A Polym Chem 50:451–457
Han DH, Tong X, Zhao Y (2011) Fast photodegradable block copolymer micelles for burst release. Macromolecules 44:437–439
Jin Q, Liu GY, Ji J (2010) Preparation of reversibly photo-cross-linked nanogels from pH-responsive block copolymers and use as nanoreactors for the synthesis of gold nanoparticles. Eur Polym J 46:2120–2128
Dolmans DEJGJ, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3:380–387
Li WY, Wang YX, Chen LN, Huang ZX, Hu QL, Ji J (2012) Light-regulated host–guest interaction as a new strategy for intracellular PEG-detachable polyplexes to facilitate nuclear entry. Chem Commun 48:10126–10128
Jiang JQ, Tong X, Zhao Y (2005) A new design for light-breakable polymer micelles. J Am Chem Soc 127:8290–8291
Zhao Y (2009) Photocontrollable block copolymer micelles: what can we control? J Mater Chem 19:4887–4895
Jin Q, Mitschang F, Agarwal S (2011) Biocompatible drug delivery system for photo-triggered controlled release of 5-fluorouracil. Biomacromolecules 12:3684–3691
Meng LL, Huang W, Wang DL, Huang XH, Zhu XY, Yan DY (2013) Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules 14:2601–2610
Jin Q, Wang Y, Cai TJ, Wang HB, Ji J (2014) Bioinspired photo-degradable amphiphilic hyperbranched poly(amino ester)s: facile synthesis and intracellular drug delivery. Polymer 55:4641–4650
Berkovic G, Krongauz V, Weiss V (2000) Spiropyrans and spirooxazines for memories and switches. Chem Rev 100:1741–1754
Lee H, Wu W, Oh JK, Mueller L, Sherwood G, Peteanu L, Kowalewski T, Matyjaszewski K (2007) Light-induced reversible formation of polymeric micelles. Angew Chem Int Ed 46:2453–2457
Jin Q, Liu GY, Ji J (2010) Micelles and reverse micelles with a photo and thermo double-responsive block copolymer. J Polym Sci A Polym Chem 48:2855–2861
Son S, Shin E, Kim B (2014) Light-responsive micelles of spiropyran initiated hyperbranched p[olyglycerol for smart drug delivery. Biomacromolecules 15:628–634
Jin Q, Chen YJ, Wang Y, Ji J (2014) Zwitterionic drug nanocarriers: a biomimetic strategy for drug delivery. Colloids Surf B: Biointerfaces 124:80–86
Ishihara K, Ueda T, Nakabayashi N (1990) Preparation of phospholipid polylners and their properties as polymer hydrogel membranes. Polym J 22:355–360
Iwasaki Y, Ishihara K (2005) Phosphorylcholine-containing polymers for biomedical applications. Anal Bioanal Chem 381:534–546
Wang HB, Wang Y, Chen YJ, Jin Q, Ji J (2014) A biomimic pH-sensitive polymeric prodrug based on polycarbonate for intracellular drug delivery. Polym Chem 5:854–861
Wang HB, Xu FM, Wang Y, Liu XS, Jin Q, Ji J (2013) pH-responsive and biodegradable polymeric micelles based on poly(β-amino ester)-graft-phosphorylcholine for doxorubicin delivery. Polym Chem 4:3012–3019
Raymo FM, Giordani S (2001) Signal processing at the molecular level. J Am Chem Soc 123:4651–4652
Bonné TB, Lüdtke K, Jordan R, Štěpánek P, Papadakis CM (2004) Aggregation behavior of amphiphilic poly(2-alkyl-2-oxazoline) diblock copolymers in aqueous solution studied by fluorescence correlation spectroscopy. Colloid Polym Sci 282:833–843
Garcia A, Marquez M, Cai T, Rosario R, Hu Z, Gust D, Hayes M, Vail SA, Park CD (2007) Photo-, thermally, and pH-responsive microgels. Langmuir 23:224–229
Cheng R, Wang X, Chen W, Meng F, Deng C, Liu H, Zhong Z (2012) Biodegradable poly(ε-caprolactone)-g-poly(2-hydroxyethyl methacrylate) graft copolymer micelles as superior nano-carriers for “smart” doxorubicin release. J Mater Chem 22:11730–11738
Zhao J, Chen C, Li D, Liu X, Wang H, Jin Q, Ji J (2014) Biocompatible and biodegradable supramolecular assemblies formed with cucurbit[8]uril as a smart platform for reduction-triggered release of doxorubicin. Polym Chem 5:1843–1847
Bansal A, Zhang Y (2014) Photocontrolled nanoparticle delivery systems for biomedical applications. Acc Chem Res 47:3052–3060
Acknowledgments
Financial support from the Special Social Commonweal Research Programs of the Scientific Institution of Ministry of Science and Technology (No.GY2012G-3) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, H., Zhou, M., Zhang, Q. et al. Zwitterionic light-responsive polymeric micelles for controlled drug delivery. Colloid Polym Sci 293, 1685–1694 (2015). https://doi.org/10.1007/s00396-015-3550-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3550-7