Abstract
The cationic photo-responsive polymeric micelles (mPEG-b-p(DEMA-co-MMA-CL)) with light-switchable cross-linker were designed and fabricated by atom transfer radical polymerization and quaternization. These micelles were well dispersed spherical with positive charge. The efficient release of encapsulated contents can be triggered by light irradiation. No significant toxicity was observed of copolymer and its photolysis residues at concentrations up to 250 μg/mL. The antitumor activity of camptothecin (CPT) in micelles demonstrated that light triggered the release of CPT, which dramatically improves the cytotoxicity after UV irradiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymeric micelles have been proved to be an effective strategy for the delivery of anticancer drugs due to their safety, biocompatibility and structural modification [1,2,3,4,5,6]. In past decades, in order to further improve the controlled release performance of the encapsulated material, micelles capable of responding to stimuli, such as pH [7, 8], redox [9, 10], temperature [11, 12], light [13] and enzymes [14], have been developed by incorporating functional components. Among these stimuli, light, which can control the action time, position and dose precisely by adjusting the wavelength and intensity, can control the drug release process more effectively [15, 16]. Therefore, lots of polymeric micelles self-assembled by photosensitive amphiphilic polymer, based on light-responsive functional groups such as spiropyrane [17, 18], azobenzene [19], nitrobenzyl [13, 20], pyrene [21, 22] and cinnamic acid groups [23], have been extensively investigated. As far as we know, most of the light-responsive drug carriers reported focus attention on a more novel structure, but seldom concern the intensity of ultraviolet light which directly affects the biological safety of vectors. In our earlier work, we have ever prepared nitrobenzyl-functionalized photo-responsive drug carriers and found that this type of micelles was sensitive to low-intensity UV illumination (365 nm, 5000 μW/cm2). [24].
Typically, the surface charge of numerous micelles is negatively charged [25, 26]. Although the drugs release efficiency of photosensitive micelles can be controlled by light, the therapeutic efficacy is always hampered by the poor cellular internalization because of the charge repulsion of micelles from negatively charged cell membranes [15, 26]. Therefore, lots of positively charged drug carriers are designed and widely used to enhance the cellular uptake [25, 27].
Here, we introduce a simple method to prepare cross-linked amphiphilic polymer for fabricating photo-responsive positively charged micelles by atom transfer radical polymerization (ATRP) and subsequent quaternization utilizing the nitrobenzyl derivative 1-(2-nitrophenyl) ethane-1, 2-diyl bis(4-chlorobutanoate) (CL) as a cross-linked agent. The cross-linked network was formed by quaternization proceeded between chlorine groups of CL and the tertiary amino groups of linear amphiphilic polymer. Nitrobenzyl groups in the micelles could be quickly cleaved under low-intensity UV illumination (365 nm, 5000 μW/cm2), and the drug loaded in micelles could be released quickly. The micelles formed by cross-linked amphiphilic polymer would be relatively steady in blood, phagocytized by cells, and then release the cargos in cells (Scheme 1). The structure and morphology of the polymer and micelles were characterized by 1HNMR, Fourier transform infrared spectroscopy (FT-IR), Dynamic light scattering (DLS), Scanning electron microscopy (SEM). Moreover, photosensitive cargo release behavior of the micelles was studied using nile red (NR) and camptothecin (CPT) as model drug.
Experimental
Materials
2-Nitroacetophenone was purchased from Guangdong Wengjiang Chemical Reagent Co., Ltd. Nile red (NR), 2-diethylaminoethyl methacrylate (DEMA), N,N,Nʹ,Nʺ,Nʺ-pentamethyldiethy-lenetriamine (PMDETA), sodium iodide (NaI), cuprous bromide (CuBr), pyrene (Py), aluminum oxide (Al2O3), methyl methacrylate (MMA) and 4-chlorobutyryl chloride were provided by Shanghai Aladdin Chemistry Co., Ltd. Camptothecin (CPT) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) was purchased from Energy Chemical. 1640 medium was purchased from Thermo Fisher Scientific. Tetrahydrofuran (THF), triethylamine (TEA), dichloromethane (DCM) and acetonitrile (AN) were distilled over CaH2.
Synthesis of 1-(2-nitrophenyl) ethane-1, 2-diyl bis(4-chlorobutanoate) (CL)
The synthetic route 1-(2-nitrophenyl)-1,2- ethanediol is shown in Scheme 2, which is synthesized according to published procedures [24].
CL was synthesized as follows (Scheme 3): Briefly, 1-(2-nitrophenyl)-1, 2-ethanediol (0.05 g, 0.27 mmol) was dried through azeotropic distillation with dry THF thrice. Subsequently, freshly distilled DCM (10 mL) and TEA (150 μL, 1.08 mmol) were added under N2 at 0 °C. Then, a solution of 4-chlorobutyryl chloride (80 µL, 0.715 mmol) in DCM (1 mL) was added dropwise to the reaction mixture. The reaction mixture was stirred for approximately 3 h at 30 °C, after which the reaction was quenched by the addition of an aqueous solution of NaHCO3 (5%, 3 × 30 mL) and extracted with CH2Cl2 (3 × 30 mL). The organic layer was dried over Na2SO4 for 24 h and concentrated. The crude product was purified by silica column chromatography to yield CL (0.073 g, 68.50%) as a yellow oil. 1HNMR δ (CDCl3) (Fig. S1) δ 8.00 (dd, J = 8.2, 1.3 Hz, 1H), 7.89 (dd, J = 7.9, 1.4 Hz, 1H), 7.72–7.68 (m, 1H), 7.50 (ddd, J = 8.6, 7.4, 1.5 Hz, 1H), 5.61–5.58 (q, 1H, CH), 4.41–4.36 (m, 2H, –CH2), 3.64–3.58(m, 4H, Cl–CH2), 2.59–2.55 (m, 4H, CH2), 2.13–2.08 (m, 4H, O=C–CH2), 13C-NMR (100 MHz, CDCl3) (Fig. S1) δ 177.89, 172.98, 147.85, 135.41, 133.69, 128.95, 128.77, 124.65, 68.38, 68.04, 43.97, 43.88, 31.00, 30.83, 27.47, 27.34.
Synthesis of polyethylene glycol-block-poly(2-diethylaminoethyl methacrylate-co-methyl methacrylate) (mPEG-b-p(DEMA-co-MMA))
mPEG-Br were synthesized according to published procedures [9].
The ATRP process of DEMA and MMA was carried out using mPEG-Br as the initiator, CuBr and PMDETA as the catalysts at 90 °C under N2. Briefly, mPEG-Br (200 mg, 0.1 mmol), CuBr (29 mg, 0.2 mmol) and PMDETA (42 µL, 0.2 mmol) were dissolved and dispersed in 2 mL 1, 4-dioxane, and then the mixture was charged in a Schlenk tube. The mixture was degassed by four freeze–pump–thaw cycles. After dissolution, the mixture was heated to 90 °C for 4 h with a magnetic stirrer. The reaction was quenched by exposing the mixture to air. Then, the mixture was passed through a neutral Al2O3 column with DCM as eluent to remove copper catalysts. The copolymer was precipitated into an excess of petroleum ether. The dissolution–precipitation cycle was repeated three times. The final product was dried in vacuum at 25 °C to give 200 mg yellow viscous polymer.
Synthesis of photosensitive cross-linked copolymer mPEG-b-p(DEMA- co-MMA-CL)
The photosensitive cross-linked copolymer mPEG-b-p(DEMA-co-MMA-CL) was synthesized via quaternization, as shown in Scheme 4. Typically, 100 mg mPEG-b-p(DEMA-co-MMA), 40 mg CL and 4 mg NaI were dispersed in 25 mL acetone with a magnetic stirrer. Then, the reaction mixture was refluxed at 60 °C for 16 h. Acetone was removed under vacuum, and the raw product was dissolved in DCM. The copolymer was purified by precipitated into petroleum ether. The dissolution–precipitation cycle was repeated three times. Thin-layer chromatography was used to confirm that the uncross-linked cross-linker had been completely removed. The final product was dried in vacuum at 25 °C to give 90 mg yellow powder.
Preparation of NR/CPT-loaded mPEG-b-p(DEMA-co-MMA-CL) micelles
NR was used as fluorescent probes to assess the photo-controlled release behaviors of micelles. Typically, 600 µL mPEG-b-p(DEMA-co-MMA-CL) solution in THF was mixed with 100 µL NR solution in THF (0.4 mg/mL) and another 300 µL THF, after which 234 µL water was added dropwise to form the micelles. After 30 min stirring, 4 mL water was added to precipitate the unloaded NR. The precipitated NR and copolymer were removed by filtration through 0.45 μm membrane. THF and most of the water were volatilized at 40 °C until the volume reaches 2 mL.
CPT was used as a model drug to assess the photo-controlled release behaviors of micelles. Typically, 2 mg CPT and 8 mg mPEG-b-p(DEMA-co-MMA) or mPEG-b-p(DEMA-co-MMA-CL) were dissolved in 600 µL DMSO with 30 min stirring, and then 10 mL distilled water was added and stirred for 10 min. Then, the aqueous solution was dialyzed against water for 12 h. The medium was exchanged every 1 h to remove DMSO and unloaded CPT. Yellow powder (CPT-loaded micelles) was obtained after freeze-dried 24 h.
The drug-loading contents (DLC) and entrapment efficiency (EE) were analyzed by dissolving the CPT-loaded micelles in methanol and measured by fluorescence spectrophotometer (PerkinElmer, LS55) at an excitation wavelength of 364 nm. DLC and EE were calculated according to the following formula:
Photo-triggered NR/CPT release study
To evaluate the photo-triggered release behavior of the encapsulated liposoluble molecules, the fluorescence spectra of NR-loaded micelles were monitored upon irradiation (365 nm, 5000 μW/cm2) with pH value (pH 7.4) at an excitation wavelength of 557 nm using a fluorescence photometer (PerkinElmer, LS55).
CPT release from cross-linked micelles was carried out before and after 15 min UV irradiation. Typically, 0.9 mg CPT-loaded mPEG-b-p(DEMA-co-MMA-CL) micelles were dispersed in 2 mL PBS buffer (pH 7.4) and the solution was divided into two equal aliquots marked as A sample and B sample. After 15 min UV Illumination for A sample, the two samples were all transferred into a dialysis bag that was dialyzed against 50 mL PBS buffer (pH 7.4) at 37 °C. At regular time intervals, 1 mL of the solution was exchanged with fresh PBS buffer. The concentration of CPT in the media was determined by fluorescence photometer at an excitation wavelength of 364 nm.
Measurement
1HNMR spectra (Bruker AVANCEIII, 400 MHz) of CL, mPEG-b-p(DEMA-co-MMA) and mPEG-b-p(DEMA-co-MMA-CL) were recorded using CDCl3 as the solvent. FT-IR spectra of the mPEG-b-p(DEMA-co-MMA) and mPEG-b-p(DEMA-co-MMA-CL) were characterized using a FT-IR spectrometer (Bruker TENSOR 27). The morphology of the micelles was characterized by scanning electron microscopy (SEM, FEI, Quanta-450-FEG). The dynamic size and zeta potential of micelles in PBS buffer were measured using dynamic laser light scattering (DLS; Malvern, ZEN3600). UV light (UVP, B-100SP, 365 nm, 5000 μW/cm2) was used in the study of photosensitivity. Gel permeation chromatography (GPC, Waters, 1515) measurements were carried out to determine the average molecular weight (Mw) and polydispersity index (PDI) with THF as eluent.
In vitro cell assays
SGC7901 cells were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 mg/mL streptomycin and 100 U/mL penicillin) at 37 °C with 5% CO2.
The in vitro cytotoxicity of particles against SGC7901 cells was evaluated using the MTT assay. First, SGC7901 cells were seeded in 96-well plates at a density of 1 × 105 cells/mL and incubated for 24 h. The cells were then treated with various amounts of copolymer, CPT and CPT-loaded micelles. After incubation for a certain length of time, MTT solution (0.5 mg/mL) was added to the wells, and the cells were incubated for 4 h at 37 °C. Next, 200 μL DMSO was added to dissolve the formazan crystals. The absorbance of each well at 490 nm was measured using a microplate reader (Thermo, Multiskan GO). The cell viability was determined by comparing the absorbance of particle-incubated cells to that of control cells.
Results and discussion
Synthesis and characterization of copolymers
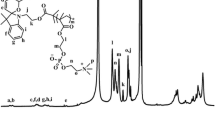
The photo-responsive cationic block copolymer mPEG-b-p(DEMA-co-MMA-CL) was synthesized by ATRP and subsequent quaternization utilizing the nitrobenzyl derivative 1-(2-nitrophenyl) ethane-1,2-diyl bis(4-chlorobutanoate) (CL) as a cross-linked agent, as depicted in Scheme 1. The successful synthesis of mPEG-b-p(DEMA-co-MMA-CL) was verified by 1HNMR and FT-IR. The typical 1HNMR spectra of each step in the synthesis are shown in Fig. 1. By comparing the well-defined peak integrals of DEMA (δ 2.60) and MMA (δ 3.60) for mPEG-b-p(DEMA-co-MMA) (Fig. 1b) with those of the ATRP initiator fragment (δ 3.39) (Fig. 1a), the polymerization degree of MMA block and DEMA block was 41 and 50, calculated from Fig. 1. The synthesis of mPEG-b-p(DEMA-co-MMA-CL) was also verified by 1HNMR. The CL characteristic signals were found at δ 8.0-7.5, δ 5.75-5.5 and δ 4.65 (Fig. 1c) which showed sectional quaternization of DEMA.
The FT-IR spectra of mPEG-OH, mPEG-Br, copolymer mPEG-b-p(DEMA-co-MMA), mPEG-b-p(DEMA-co-MMA-CL) and CL were detected and are presented in Fig. 2. In Fig. 2A, comparing with mPEG-OH, a new peak at 1732 cm−1 in mPEG-Br attributed to ester carbonyl suggested that bromine groups are introduced via ester bonds. The strong peak at 2890 cm−1 was attributed to the C-H stretching vibration of alkanes in the PEG structure. The strong peak at 1151–1160 cm−1 was the C–O–C stretching vibration of saturated aliphatic ethers in the mPEG structure. For mPEG-b-p(DEMA-co-MMA) and mPEG-b-p(DEMA-co- MMA-CL) (Fig. 2A), the peaks at 2970 and 2923 cm−1 corresponded to the carbon skeleton. The peak at 1732 cm−1 belongs to ester C=O, and the peaks at 1452 and 1384 were attributed to CH3 and CH2. The FT-IR spectra of CL were detected and are shown in Fig. 2B. The characteristic absorption peaks at 1534 cm−1 and 1350 cm−1 attributed to aromatic –NO2 antisymmetrical stretching vibration and symmetrical stretching vibration were obviously visible. In addition, the peaks at 1534 cm−1 and 1350 attributed to the –NO2 of CL appear in mPEG-b-p(DEMA-co-MMA-CL), which is completely unobservable in mPEG-b-p(DEMA-co-MMA), suggesting that CL was cross-linked in copolymer.
The copolymers were characterized by GPC to determine the molecular weight. As shown in the chromatogram (Fig. 2C), the retention time of mPEG-b-p(DEMA-co-MMA-CL) is lower than that of mPEG-b-p(DEMA-co-MMA), which indicates an increase in the molecular weight for cross-linked sample. The Mw value for mPEG-b-p(DEMA-co-MMA) and mPEG-b-p(DEMA-co-MMA) obtained via calibration is 8539 g/mol and 14702 g/mol, respectively. The increase in molecular weight is due to the cross-linker (CL) linking mPEG-b-p(DEMA-co-MMA), and there are about 1.72 linear molecules in a PEG-b-p(DEMA-co-MMA-CL) structure. In addition, the cross-linking effect of the structure is also reflected in the polydispersity (PDI) of polymer. The PDI value increased from 1.19 (PEG-b-p (DEMA-co-MMA)) to 1.42 (PEG-b-p (DEMA-co-MMA-CL)).
Characterization of micelles
The critical micelle concentration (CMC) of the copolymer mPEG-b-p(DEMA-co-MMA-CL) was measured by fluorescent method using pyrene (Py) as a fluorescence probe. The emission intensity ratio (I391/372) of Py versus logc (c is the concentration of copolymer in aqueous) is shown in Fig. 3. The CMC value of mPEG-b-p(DEMA-co-MMA-CL) in aqueous solution was determined to be about 0.04 mg/mL, as shown in Fig. 3.
The morphology of the mPEG-b-p(DEMA-co-MMA-CL) micelles was investigated by SEM. As shown in Fig. 4a–c, the SEM image with different magnifications indicated that the micelles were spherical, and there was no adhesion between micelles. The mean diameter of about 1 μm was similar to the hydrodynamic diameter (Dh) measured by DLS (1000 nm). The surface charges of micelles were investigated by zeta potential. The zeta potential for mPEG-b-p(DEMA-co-MMA) and mPEG-b-p(DEMA-co- MMA-CL) micelles was 29.2 mV and 37.9 mV, respectively. An increase of the zeta potential of cross-linked micelles was observed, which might be ascribed to the partial quaternary ammonium groups. This characteristic may promote the cellular uptake of micelles, because the cell membrane is usually negatively charged.
Photo-triggered degradation of copolymer
Figure 5a shows the light-dependent FT-IR spectra changes for mPEG-b-p(DEMA-co-MMA-CL) micelles. Upon UV irradiation, the typical peaks at 1534 cm−1 attributed to aromatic –NO2 antisymmetrical stretching vibration disappeared, and the peak at 1350 cm−1 attributed to –NO2 symmetrical stretching vibration reduced significantly, suggesting that –NO2 groups were vanished by the breakage of the ester group (Scheme 1). The absorption spectrum variations of CL in acetonitrile were detected in detail. The structure of CL is light dependent. As shown in Fig. 5b, the peak at 258 nm belonged to the chromophore nitrobenzene weakened upon illumination, which indicated that the structure of nitrobenzene has changed during the illumination process. As shown in Fig. 5c, o-nitrobenzyl ester for CL will undergo photocleavage to form 2-(2-nitrosophenyl)-2-oxoethyl 4-chlorobutanoate. The formation of an isosbestic points at 287 and 313 nm indicates the transition of CL from the ester to the ketone carbonyl [24, 28]. Besides, the blank micelles in aqueous are colorless, and the color changed into light yellow after 15 min UV irradiation (Fig. S2).
In vitro controlled release of NR and CPT from micelles under stimulation
The self-assembled polymeric micelles were designed to be a hydrophobic molecule carrier which could control the release of the cargos under the stimulation of relatively weak UV light. Here, NR was used as a model molecule. The fluorescence spectra of NR in mPEG-b-p(DEMA-co-MMA-CL) micelles were investigated under UV light irradiation in PBS (pH 7.4), and the release efficiency of NR from micelles was calculated from the fluorescence decrease divided by the primary fluorescence intensity. As shown in Fig. 6, a dramatic decrease for fluorescence intensity can be observed for mPEG-b-p(DEMA-co-MMA-CL) micelles. Upon UV irradiation, about 80% of the encapsulated NR could be released from micelles after 20 min UV light.
The cumulative release percentages of CPT released from the micelles were further investigated. As illustrated in Fig. 7, CPT was released from mPEG-b-p(DEMA-co-MMA-CL) micelles rapidly, in which 98% CPT was released in 1320 min. Conversely, low drug release efficiency (35%) for mPEG-b-p(DEMA-co-MMA) micelles was observed in 1320 min. These results indicated that mPEG-b-p(DEMA-co-MMA-CL) micelles were unstable upon low-intensive UV irradiation.
In vitro cell assays
The cell viability upon low-intensive UV irradiation against SGC7901 gastric carcinoma cell lines was evaluated using MTT assays. As we expected, the cell viability was not declined both for 24 h incubation and 48 h incubation after 15 min UV irradiation as shown in Fig. 8a, which suggests that the low-intensive UV irradiation (5000 μW/cm2) is safe for cells.
In order to assess the toxicity of the mPEG-b-p(DEMA-co-MMA-CL) copolymer and its photolysis production, SGC7901 cells were incubated with varying concentrations (0–250 μg/mL) of mPEG-b-p(DEMA-co-MMA-CL) copolymer for 24 h. As seen in Fig. 8b, there was no remarkable cytotoxicity against SGC7901 cells over the range of concentrations up to 250 μg/mL. Another investigation was carried out using the same method as above, while a 15 min UV irradiation was proceeded after the copolymer was added in the cells. Upon irradiation, the cell viabilities did not change observably at the concentration as high as 250 μg/mL. Therefore, we confirm that the copolymer and its irradiated residues have low cytotoxicity and high cellular biocompatibility at the tested concentrations.
The efficiency of free CPT, CPT-loaded micelles and CPT-loaded micelles upon 15 min irradiation on the viability of SGC7901 cells was evaluated for 48 h. The concentrations range of CPT was adjusted from 0 to 20 μg/mL. As displayed in Fig. 9, the viabilities of SGC7901 cells in the 48 h treatment of free CPT decreased to 45.5 ± 2.56%, with a CPT dose of 20 μg/mL. The encapsulated CPT resulted in almost the same antitumor activity to SGC7901 cells. The viabilities of cells after 48 h of CPT-loaded mPEG-b-p(DEMA-co-MMA-CL) micelles co-incubation were 39.92 ± 3.65% with a CPT dose of 20 μg/mL. It is noteworthy that the cell viability for cell upon 15 min irradiation before co-incubation is significantly declined. The viabilities of cells were 19.15 ± 2.03% with a CPT dose of 20 μg/mL. The results demonstrated that a short and low-intensive UV irradiation could accelerate the destabilization of the mPEG-b-p(DEMA-co-MMA-CL) micelles and result in the rapid drug release.
Conclusion
In summary, we have synthesized a novel cross-linked photo-responsive copolymer (mPEG-b-p(DEMA-co-MMA-CL) by quaternization between the nitrosobenzyl derivative (CL) and DEMA units of mPEG-b-p(DEMA-co-MMA). The copolymer was characterized by 1HNMR and FT-IR, and the photolysis properties of copolymers and CL were studied by FT-IR and UV spectroscopy. A photo-responsive polymeric micelles can be assembled by mPEG-b-p(DEMA-co-MMA-CL) as hydrophobic drug carriers for efficient intracellular drug delivery. The obtained micelles are well dispersed spherical. Zeta potential of the mPEG-b-p(DEMA-co-MMA-CL) micelles was 37.9 mV, which was advantageous to cellular internalization. Furthermore, in vitro NR and CPT release showed that light irradiation caused the crash of drug release from micelles. NR release efficiency was approximately 80% after 20 min light stimulus based on fluorescence intensity of NR. Cell viability evaluation showed low toxicity for copolymer and its photolysis residues at concentrations up to 250 μg/mL. The antitumor activity of CPT in micelles demonstrated that light triggered the release of CPT, which dramatically improves the cytotoxicity. These results indicated that the novel (mPEG-b-p(DEMA-co-MMA-CL) micelles can be invoked as a promising strategy for photo-switchable intracellular drug delivery.
References
Simone EA, Dziubla TD, Muzykantov VR (2008) Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv 5:1283–1300
Gaspar R, Duncan R (2009) Polymeric carriers: preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv Drug Deliv Rev 61:1220–1231
Jiang G et al (2017) Reduction-sensitive N,Nʹ-dimethacryloylcystine nanohydrogel for triggered drug release. Mater Lett 189:122–125
Mochida Y, Cabral H, Kataoka K (2017) Polymeric micelles for targeted tumor therapy of platinum anticancer drugs. Expert Opin Drug Deliv 14:1–16
Zhang R et al (2017) Co-delivery of doxorubicin and AS1411 aptamer by poly(ethylene glycol)-poly(β-amino esters) polymeric micelles for targeted cancer therapy. J Nanopart Res 19:224
Cao Q et al (2017) Self-assembled nanostructures from amphiphilic globular protein–polymer hybrids. Polymer Bull 75:2627–2639
Chen J et al (2015) Polyion complex micelles with gradient pH-sensitivity for adjustable intracellular drug delivery. Polymer Chem 6:397–405
Huang F et al (2015) Micelles based on acid degradable poly(acetal urethane): preparation, pH-sensitivity, and triggered intracellular drug release. Biomacromol 16:2228–2236
Lili Y et al (2016) Intracellular doxorubicin delivery of a core cross-linked, redox-responsive polymeric micelles. Int J Pharm 498:195–204
Huo M et al (2016) Redox-sensitive micelles based on O, N-hydroxyethyl chitosan-octylamine conjugates for triggered intracellular delivery of paclitaxel. Mol Pharm 13:1750–1762
Li YY et al (2006) Thermosensitive Y-shaped micelles of poly(oleic acid-Y-N-isopropylacrylamide) for drug delivery. Small 2:917–923
Yang Z et al (2009) Temperature sensitivity and drug encapsulation of star-shaped amphiphilic block copolymer based on dendritic poly(ether-amide). J Biomed Mater Res A 89:988–1000
Yu L et al (2011) Photosensitive cross-linked block copolymers with controllable release. Photochem Photobiol 87:646–652
Harnoy AJ et al (2017) Modular synthetic approach for adjusting the disassembly rates of enzyme-responsive polymeric micelles. Biomacromol 18:1218–1228
Zhao Y et al (2009) Photo-cross-linkable polymer micelles in hydrogen-bonding-built layer-by-layer films. Langmuir 25:13151–13157
Wang Y et al (2007) Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with alpha-cyclodextrin. Angew Chem Int Ed Engl 46:2823–2826
Zhao Y, Tremblay L, Zhao Y (2010) Doubly photoresponsive and water-soluble block copolymers: synthesis and thermosensitivity. J Polym Sci Part A: Polym Chem 48:4055–4066
Lee HI et al (2007) Light-induced reversible formation of polymeric micelles. Angew Chem Int Ed Engl 46:2453–2457
Patra D et al (2013) Dual stimuli-responsive, rechargeable micropumps via “host-guest” interactions. ACS Nano 7:7674–7679
Han D, Tong X, Zhao Y (2011) Fast photodegradable block copolymer micelles for burst release. Macromolecules 44:437–439
Wang H, Zhang W, Gao C (2015) Shape transformation of light-responsive pyrene-containing micelles and their influence on cytoviability. Biomacromolecules 16:2276–2281
Hosseini AG, Bagheri M, Mohammad-Rezaei R (2016) Synthesis and fluorescence studies of dual-responsive nanoparticles based on amphiphilic azobenzene-contained poly (monomethyl itaconate). J Polym Res 23:161
Ding J, Liu G (1998) Polystyrene-b lock-poly (2-cinnamoylethyl methacrylate) nanospheres with cross-linked shells. Macromolecules 31:6554–6558
Lili Y et al (2016) Photo/pH dual-responsive biocompatible poly(methacrylic acid)-based particles for triggered drug delivery. J Appl Polym Sci 133:44003
Yan X et al (2017) Positively charged combinatory drug delivery systems against multi-drug-resistant breast cancer: beyond the drug combination. ACS Appl Mater Interfaces 9:6804–6815
Jin Q et al (2014) Light-responsive polyion complex micelles with switchable surface charge for efficient protein delivery. ACS Macro Lett 3:679–683
Chirio D et al (2014) Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment. Eur J Pharm Biopharm 88:746–758
Wang Z et al (2013) Photoresponsive cross-linked polymeric particles for phototriggered burst release. Photochem Photobiol 89:552–559
Acknowledgements
This work was financially supported by Natural Science Basic Research Project of Shaanxi Provincial Department of Science and Technology (2018JQ8041), the Association of Young Supporting Project of Shaanxi (20170410), the Provincial Key Discipline Construction Project of Pharmacy of Xi’an Medical University (2016YXXK03), the National Natural Science Foundation of China (81302706), Natural Science Basic Research Program of Shaanxi (2017JM8124) and the Shaanxi Provincial Education Department Project (17JK0663).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, L., Yao, L., Li, L. et al. Photo-responsive polymeric micelles bearing ammonium salts cross-linked for efficient drug delivery. Polym. Bull. 76, 2215–2231 (2019). https://doi.org/10.1007/s00289-018-2488-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2488-6