Abstract
The micellization of mixed binary surfactant systems of sodium dodecylsulfate (SDS) and sodium dodecylbenzenesulfonate (SDBS) has been studied by conductometry, tensiometry, fluorimetry, and microcalorimetry at different mole fractional compositions. The counter-ion binding of micelles, micellar aggregation number, thermodynamics of micellization, interaction of components in the mixed micelles, and their compositions therein and amphiphile packing in micelles have been examined. The adsorption features of the surfactants at the air/solution interface have also been estimated. Correlation of the results and explanations of the findings have been presented. The difference in the head groups of SDS and SDBS has manifested interesting solution and interfacial behaviors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solution properties of mixed surfactant systems are more interesting than pure surfactants, both from physicochemical and application points of view. By virtue of differences in the tail and head groups of the surfactants, mixed surfactants may show composition-dependent micellization, mutual interaction, counter-ion binding, solvation, micellar shape, etc. These manifestations are also influenced by the type of solvent, temperature, and pressure. Because of the availability of different kinds of surfactants and varied properties of micelles, there remains ample scope for investigations in this field, which is supported by literature reports [1–10].
The bulk studies done on mixed surfactant systems have been mostly on ionic–nonionic and anionic–cationic mixtures in which binary mixtures have been investigated in plenty, and ternary mixtures have been examined only in limited cases [11–16]. The dependence of thermodynamics of the micellization process on system composition and type, and the synergistic or antagonistic interaction among the components in the light of different theories have been assessed quantitatively [17–20]. The mutual interactions among the surfactants in solution and in micelles therein essentially arise from the difference in type and length in the amphiphile chains and the electrostatic and steric interactions among their head groups. The issue is complex, and experimental results on various mixed combinations are wanted for verification of theories and assessment of the nature of interaction that might prevail.
Literature search has revealed that physicochemical investigations on anionic–anionic [20–22] binary surfactant mixtures are indeed much less compared to other kinds of combinations. This aspect of surface chemical studies involving different types of surfactant systems, therefore, remains fairly open for exploration. In recent studies, we have explored such processes using cationic surfactants of similar head groups but variable chain lengths viz. alkyltriphenylphosphonium bromides [2, 4], and alkyltrimethylammonium bromides [23]. Such studies with variable head groups and identical chain lengths are under way [15]. Herein, we have considered two representative anionic surfactants, sodium dodecylsulfate (SDS) and sodium dodecylbenzenesulfonate (SDBS) and their different molar combinations for the study. The type of their nonpolar tails and their head group regions are different to lend fair possibility of mutual interactions both at the air/solution interface and in the bulk. The study of their mixed systems comprised (1) mixed micelle formation, (2) counter-ion binding of micelles, (3) state of micellar aggregation, (4) mutual interaction between the components in the micelles, (5) amphiphile packing in the micelles, (6) thermodynamics of micellization, and (7) adsorption at the air/solution interface. The methodologies adopted were tensiometry, conductometry, microcalorimetry, and fluorimetry. The results have been analyzed in the light of physicochemical concepts and proposed theories. These are described and discussed in what follows.
Experimental section
Materials
The sodium dodecylsulfate and sodium dodecylbenzenesulfonate were both AR grade products from Merck (USA) and Fluka (Switzerland), respectively. Both of them produced no minima in the surface tension vs concentration plots indicating good purity of the compounds. Pyrene (Aldrich), the fluorescence probe, was a gift sample from the Polymer Science Laboratory, IACS, Kolkata (India), and the fluorescence quencher cetylpyridinium chloride (CPC) was a 99% pure product obtained from Sigma (USA). Pyrene was purified by vacuum sublimation followed by recrystallization from ethanol and then solubilized in 25% (by volume) ethanol–water mixture for use. All solutions were prepared and diluted by doubly distilled conductivity water (specific conductance 2–4 μS cm−1). All measurements were taken in a temperature-controlled thermostated water bath at 303 ± 0.1 K, if not stated otherwise.
Methods
Tensiometry
A calibrated Krüss (Germany) tensiometer was used to measure the surface tension (γ) at the air/solution interface of the surfactant solutions by the du Noüy ring detachment method. The concentration of surfactant solution was increased by progressively adding a concentrated solution of it into water with a Hamilton microsyringe. The surface tensions were measured allowing ∼20 min time for equilibration after each addition. The experiments were duplicated to check their reproducibility. The mean values of the measurements were considered for the data analysis.
Conductometry
A Jenway conductance bridge (UK) combined with a cell of unit cell constant was used to measure specific conductance of surfactant solutions. The concentration of the surfactant solution was increased progressively following the same protocol as in tensiometry. Measurements were taken after thorough mixing and allowing time for temperature equilibration.
Microcalorimetry
An Omega ITC microcalorimeter of Microcal, Northampton, MA (USA) was used for thermometric measurements. Concentrated degassed surfactant solution (∼20 times their critical micellar concentration, CMC) was taken in the microsyringe and added in steps, injecting 5 μl at each step after equal time intervals (210 s) to 1.325 ml degassed water taken in the calorimeter cell under constant stirring (300 rpm) condition. The calorimeter was set to the study temperature of 303 K, which fluctuated within the limit of ±0.01 K. The heat released at each step of dilution of the surfactant solution was recorded against 1.65 ml water taken in the reference cell, and the enthalpy per mole of injectant was calculated using the isothermal titration calorimetry (ITC) software. Each run was duplicated to check reproducibility. Further details of the experimental protocol can be found in our earlier reports [24, 25].
Fluorimetry
Fluorescence measurements using pyrene as the fluorescence probe were taken in a FluoroMax-3, JOBIN YVON, Horiba (Japan), using a 10-mm path length quartz cuvette. The samples were excited at 331.5 nm and emission was recorded in the 340 to 450 nm range. The slit widths for both excitation and emission were fixed at 0.4 nm. Surfactant solutions were taken ten times their CMCs, and pyrene concentration in solution was kept at 2 μM (below its solubility limit of 3 μM). At this concentration, dimerization of the probe molecules were absent and their distribution in the medium was considered to be of Poisson type as per the experimental conditions suggested by Turro [26] and others [27, 28]. No other components in the system other than pyrene absorbed at the wavelength of excitation so that the inner filter effect was absent. To determine the aggregation number, the quencher (CPC) was added progressively with a Hamilton microsyringe (concentration varied between 0.01 and 0.3 mM) into the surfactant solution of constant concentration containing pyrene, and the fluorescence spectra were recorded. The scan time was fixed at 0.8 s for scanning each wavelength.
Results and discussion
Critical micellar concentration
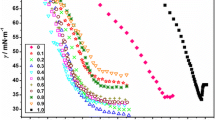
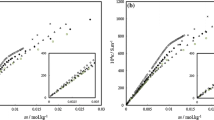
The ways of determining the CMCs of pure SDS, SDBS, and their mixtures by different methods (tensiometry, conductometry, and microcalorimetry) are graphically presented in Figs. 1, 2 and 3, and the results are shown in Table 1. In the conductometric procedure, the method of triple differentiation of the specific conductance with respect to concentration (\( {\operatorname{d} ^{3} k} \mathord{\left/ {\vphantom {{\operatorname{d} ^{3} k} {\operatorname{d} c^{3} = 0}}} \right. \kern-\nulldelimiterspace} {\operatorname{d} c^{3} = 0} \) at CMC) as proposed by Phillips [29] was used. Each method has provided unambiguous CMC values. Clear breaks (CMC points) were observed in tensiometric plots; ups and downs with crossing through zero (i.e., the CMC point) were observed in the third derivative plots for conductance. The calorimetric illustrations (enthalpograms) were sigmoidal in nature. The CMC was determined from the derivative plots [25]. In Fig. 3a (representing equimolar mixture of SDS and SDBS), the enthalpogram and its first derivative plot are exemplified, wherein the perpendicular (PP/) dropped on the [surfactant] axis passes through the CMC point. The data in Table 1 evidence higher values of CMC by calorimetry and conductometry; the tensiometric results were lower. The CMC is a method-dependent quantity and so are the other micellar parameters. The conductometry-, tensiometry-, and calorimetry-derived values have been considered for presentation since the methods are well-accepted, and besides CMC, conductometry can provide information on counter-ion binding, tensiometry on interfacial properties, and calorimetry on energetics of the micellization process. It may be mentioned that the Wilhelmy method produces CMC values lower than other methods. This has been recently observed and discussed by us [30] with reference to the surfactant MEGA 10 (decanoylmethylglucamine) by comparing the results by light scattering and calorimetry with tensiometry. Higher CMC values by calorimetry were also obtained for alkyltrimethylammonium bromides [23] and alkyltriphenylphosphonium bromides [2].
Differential enthalpy of dilution vs [surfactant] profiles at 303 K. a Curve 1, enthalpogram for X SDS = 0.5; curve 2, derivative plot of curve 1 to get CMC from the minimum (see text). b Curve I, enthalpogram for pure SDS. AB and CD denote the pre- and post-micellar slopes, respectively. Inset shows curves II and III. Enthalpograms for X SDS = 0 and 0.5. EF and QR denote pre-micellar slopes; GH and ST denote post-micellar slopes, respectively
The conductometric results were processed to determine the extents of counter-ion binding of the micelles (pure and mixed). The minimum head group areas of SDS, SDBS, and their combinations at CMC were obtained from tensiometry, and the thermodynamics of the micellization process was understood from calorimetry. The results will be discussed in subsequent sections.
Micellar aggregation number (n)
The monomer aggregation extent in a micelle is an important parameter for the amphiphile self-aggregation process. But unambiguous results are seldom obtained using different methods and concepts. In most of the past works, static light scattering (SLS) method was used for determining n [31–36]. The estimated micellar molar mass was divided by the monomer molar mass to get n. Because of counter-ion binding of micelles and micellar solvation, the n values found from SLS in principle should be more than the actual one. The incongruence of results reported in the literature on n of a surfactant can be cited taking the most well-studied representative, SDS, discussed in details by Shanks et al. [37] In a recent publication [30], considerable overestimation of n of MEGA 10 by the SLS method due to hydration was shown, comparing the n values obtained from simulation of thermometric results. Other than SLS, the fluorescence quenching method has been fairly used to get n of micelles wherein counter-ion association and solvation hardly affect the emission characteristics of the probe and, hence, the magnitude of n. Both dynamic and static quenching methods are used, of which the former gives more accurate results than the latter although for the sake of simplicity and easier equipment availability, the latter method is most frequently used. But as per reports, the results obtained from fluorimetry also show variances. In this study, we have used static quenching method using pyrene as the probe and cetylpyridinium chloride (CPC) as the quencher. The equation proposed [27, 28] on the basis of Poisson statistics of distribution of the probe and quencher molecules among micelles in the tertiary system given below was considered.
where I 0 and I are the fluorescence intensities without and with quencher, respectively; [Q] and [S] are the concentrations of quencher and surfactant, respectively; n and CMC are already defined.
At a constant [S] (ten times CMC) and temperature (T), [Q] was varied and n was obtained from the slope of the least squares plot of \( \ln {\left( {{I_{0} } \mathord{\left/ {\vphantom {{I_{0} } I}} \right. \kern-\nulldelimiterspace} I} \right)} \)against [Q] (Fig. 4), an illustration of fluorescence spectra is shown in the inset. The results are presented in Table 2. It may be mentioned that the interaction between the cationic quencher and the anionic components in the micelle was considered not to affect the quenching efficacy of CPC. Available reports on n of SDS fall in the range of 40–100, and the value was found to depend on the concentration of the surfactant used in the experiment. This has been discussed in details by Shanks [37]. In a recent report [38], the n value of 51 has been obtained for SDBS micelle by the method of fluorimetry. A simulation procedure [39, 40] using the ITC-determined enthalpy values has been tried to estimate the n for the pure and mixed micelles of SDS and SDBS. Like our earlier attempts [23, 24] much reduced n values were obtained. For lack of a proper explanation of such findings, we refrain from discussing the results. Some recent literature reports of n for both SDS and SDBS micelles are also recorded in Table 2. The observed n values of SDBS micelles obtained by Das Burman et al. [38] by the steady-state fluorescence quenching method and those values that we have obtained were smaller than that of SDS micelles. Apart from the chain length difference, the head group types of the two amphiphiles were also different. The n values were affected by these two factors. The nonionic surfactant Triton X 100 [p-tert-butyl phenoxypolyoxyethylene(9.5)ether] with lower chain length and head group size than Tween 20 [polyoxyethylene (20) sorbitan monolaurate] produces micelles with higher n (130) [41] than Tween 20 (n = 80) [41]. The polarity index (I 1/I 3) values given in column 3 in Table 2 indicate fair nonpolarity of the studied micelles. The interior of SDS micelle is a bit less nonpolar than the interior of SDBS micelles. The mixed micelles produced composition-dependent intermediate nonpolarities. The highly nonpolar pyrene molecule tends to reside in the interior of micelles. The measured nonpolarity depends on its distance from the head group region. Thus, for a small micelle, the nonpolarity index is expected to be higher than a large micelle. Such was the experimental observation. For identical head groups, the nonpolarity indexed by pyrene in alkyltrimethylammonium bromide micelles decreased with decreasing chain length [42]. SDBS being larger than SDS, the I 1/I 3 of its micelle was thus lower than the micelle of SDS.
Counter-ion binding of micelles
By virtue of appreciable surface charge density, ionic micelles bind a fair fraction of their counter-ions. The SDS, SDBS and SDS-SDBS combinations, therefore, bind Na+ ions on their micelles, which can be estimated from the ratio of the post- and the pre-CMC slopes of specific conductance vs concentration plots using the relation,
where β is the fraction of counter ions bound to a micelle, and S 1 and S 2 are the pre- and post-CMC slopes in the specific conductance vs concentration plot, respectively. For m number of counter-ions bound to n number of monomers in a micelle, β = m / n.
Equation 2 is frequently used [43–47] for its simplicity and good evaluation potential. The results fairly agree with that by the EMF method [48–51]. The conductance increases with increasing concentration of a surfactant and undergoes a change (decrease) in course at CMC because of binding of a part of the free counter-ions at the micelle/solution interface. The bound ions become incapable of acting as charge carriers. The percent of counter-ions lost is considered equivalent to the percent of ions bound to the micelles. The phenomenon is manifested as a decrease in the slope of the post-CMC line to the same extent as that of the percent ions lost. This is a simple method and is considered to help evaluation of fairly accurate β. Of course, the other electrochemical method, using ion-selective membrane electrodes to monitor the activity of the counter-ions is a quantitative method of determining β [48–51]. We have herein considered the slope ratio method for the data analysis. A comparative study of this method with the ionometric method is worthwhile to assess its merits and demerits. In a recent study, Moriyama et al. [52] have measured electrical conductivity and counter-ion activity of ionic surfactants, dihydroperflouroalkyltrimethylammonium chlorides to estimate the aggregation numbers n and the counter-ion binding per micelle m in terms of theoretical analysis. Along with the activity of Cl−, equations for the pre- and post-CMC slopes of the conductance vs concentration plots were used in the evaluation procedure of β or m/n. We have used the pre- and post-micellar slopes of Moriyama et al. [52] to get m/n by the slope ratio method and have found them to fairly agree with the author’s calculation. This has strengthened the use of the slope ratio method for the evaluation of β.
The β values obtained for the SDS and SDBS systems by the use of Eq. 2 are shown in Table 1, wherein results on the parent components obtained from other sources are also presented. The experimental β values from conductance measurements obtained in this study on SDBS were low (only 21% counter-ions were bound to the micelles) as against 51% in the case of SDS (∼50–80% counter ion binding can be found in literature [37, 53–55]). In agreement with our observation, Das Burman et al. [38] have also reported ∼22% counter-ion binding for SDBS micelles by the slope ratio method. Low values of β for ionic surfactants are not commonly found. The micelles of AOT (sodium bis-2-ethylhexylsulfosuccinate) have been reported to bind ∼10% Na+ ions [56]. The primary micelles of decyltriphenylphosphonium bromide and its 1:1 mixtures with dodecyl-, tetradecyl- and hexadecyl- analogues have also evidenced fairly low (∼25%) counter-ion binding.
In a recent study, Dai and Tam [57] have reported a procedure for calculating β from the ITC enthalpograms in aqueous medium. Like conductometry, the post- and pre-micellar slopes of the enthalpograms were used to calculate β of SDS at different temperatures and that of dodecyltrimethylammonium bromide, which agreed with literature reports. In Fig. 3b, curves I, II, and III (curves II and III in inset) denote the ITC enthalpograms for SDS, SDS–SDBS (1:1 mole ratio), and SDBS, respectively. In them, the lines corresponding to the pre- and post-micellar slopes are indicated by AB, CD; EF, GH; and QR, ST respectively. The computed β values by this method are shown in Table 1. They are low but to a certain extent higher than the β obtained by conductometry. A supportive evidence in favor of low β values for pure and mixed micelles of SDBS with SDS was obtained. In Fig. 5, the dependence of β (by both the methods) on stoichiometric X SDS is illustrated. The points fitted to two-degree polynomial equations; the correlation for β cal was better than that for β cond. The mutual correspondence between β cal and β cond, presented in the inset of Fig. 5, has evidenced a good correlative fitting to a two-degree polynomial equation,
Plot of β vs X SDS for the micelles of SDS, SDBS, and their mixtures at 303 K. Open square calorimetry, full circle conductometry. Inset shows the correlation between β cond and β cal for the systems depicted in the main illustration. The fitted polynomial equation: \( \beta _{{{\text{cond}}}} = {\left( {0.379 \pm 0.066} \right)} - {\left( {1.500 \pm 0.396} \right)}\beta _{{{\text{cal}}}} + {\left( {3.458 \pm 0.549} \right)}\beta ^{2}_{{{\text{cal}}}} \) (correlation coefficient: 0.9950)
Interfacial behavior of SDS and SDBS in pure and mixed states
From tensiometric measurements, the Gibbs surface excess at the CMC point (Γ max) was estimated from the relation,
where γ is the surface tension, C is the surfactant concentration in the solution, i is the number of species formed in the solution by way of dissociation of a surfactant molecule, T is the absolute temperature and R is the gas constant. In the studied mixed SDS–SDBS system (each of uni-univalent type electrolyte with common counter-ion), the value of i was 2 for the pure systems and 3 for the binary mixtures.
From the slopes of the linear plots between γ and \( \log C \), the surface excess values were calculated at the CMC points and are presented in Table 3. The minimum area per head group (A min) of the surfactants at CMC at the interface was obtained from the relation,
The standard Gibbs energy of adsorption \( {\left( {\Delta G^{0}_{{{\text{ad}}}} } \right)} \) at the interface was obtained from the relation,
where \( \Delta G^{0}_{{\text{m}}} \) is the standard Gibbs energy change for the micellization process (to be discussed later), and π CMC is the surface pressure (surface tension of water minus surface tension of solution) at the CMC point. The standard state was considered as the hypothetical state of ideal solution of unit mole fraction.
The results for A min and \( \Delta G^{0}_{{ad}} \) are also presented in Table 3. The experimentally determined A min values obtained from tensiometry were higher for the mixtures. The higher values of A min than their individual A min values were due to antagonistic behavior between DS− and DBS− anions at the interface. This effect might also be prevalent in the mixed micelles to be discussed in a subsequent section. Γ max (SDS) was found to be greater than Γ max (SDBS); greater mutual repulsion between the ions of the second kind than the first was envisaged at the interface. The combinations have produced Γ max values lower than Γ max (SDBS) with a decreasing trend up to X SDS = 0.5 after it increased. The A min values were complementary to Γ max. The \( \Delta G^{0}_{{{\text{ad}}}} \) (SDBS) was more spontaneous than \( \Delta G^{0}_{{{\text{ad}}}} \) (SDS); the former has shown preference for the interface than the latter. With increasing X SDS, the free energy of adsorption became continuously less spontaneous up to X SDS = 0.5, and then the spontaneity increased. For the binary combinations compared to \( \Delta G^{0}_{{\text{m}}} \), the magnitudes of \( \Delta G^{0}_{{{\text{ad}}}} \) were greater; the transfer of surfactant monomers at the interface was thermodynamically more favorable than their transfer from the bulk into the micelles. On the average, \( {\Delta G^{0}_{{{\text{ad}}}} } \mathord{\left/ {\vphantom {{\Delta G^{0}_{{{\text{ad}}}} } {\Delta G^{0}_{{\text{m}}} \approx 2.5}}} \right. \kern-\nulldelimiterspace} {\Delta G^{0}_{{\text{m}}} \approx 2.5} \), which was indicative of the difference in the degree of spontaneity between the two processes.
Thermodynamics of mixed micelle formation
The thermodynamic parameters for the micellization of SDS, SDBS, and their binary combinations were estimated using both mass action and pseudo phase models [55]. The following relations (Eqs. 10 and 14) were used. Thus,
where \( \Delta G^{0}_{{\text{m}}} {\left( {{\text{MM}}} \right)} \) is the standard Gibbs energy change of micellization process by the mass action model; the other terms are already defined.
where \( \Delta G^{0}_{{\text{m}}} {\left( {{\text{PM}}} \right)} \) is the standard Gibbs energy change of micellization process by the pseudo phase model.
The calorimetrically determined enthalpy change [ΔH m (CAL)] was considered as the standard enthalpy change of micellization [24, 25]. The procedure for the evaluation of enthalpy is presented with reference to Fig 3b, curve I. The straight line drawn through the transition region in the thermogram met the pre- and post-CMC dilution enthalpy courses at points p and q, respectively. The vertical distance between p and r (as shown in the diagram) was considered as the enthalpy for the micellization process. As per the previous statement,
The following equations were used to evaluate the entropy change of the process:
where \( \Delta S^{0}_{{\text{m}}} {\left( {{\text{PM}}} \right)} \) is the mass action model-based standard entropy change for micellization. Again,
where \( \Delta S^{0}_{m} {\left( {{\text{PM}}} \right)} \) represents the pseudo phase model-based standard entropy change for micellization.
The thermodynamic parameters are shown in Table 4. To be consistent with the calculations, calorimetric CMC and β cal were used in the equations. Both MM and PM models were used in the evaluation. In the MM, n values obtained from fluorimetry were used. The MM results were a little less spontaneous than PM. Similar was our earlier observations on SDBS [24] and alkyltrimethylammonium bromides and their binary mixtures [23]. The maximum difference between \( \Delta G^{0}_{{\text{m}}} \) estimated with β cal and that with β cond was within ±5% in both MM and PM models. The enthalpy values were small and negative, and the entropy values were all fairly positive. The variable enthalpy for the mixed systems with constant entropy suggested unaffected structural organization of the overall system during the process of micellization. The antagonistic interactions between the two surfactants made the micellization process less spontaneous and less exothermic.
The interdependence of \( \Delta G^{0}_{{\text{m}}} \) and \( \Delta H^{0}_{{\text{m}}} \) on X SDS and that of \( \Delta H^{0}_{{\text{m}}} \) on \( \Delta G^{0}_{{\text{m}}} \) has been presented in Fig. 6. The dependence of \( \Delta G^{0}_{{\text{m}}} \) was dome-shaped and broad with a maxima at X SDS = 0.5. The \( \Delta H^{0}_{{\text{m}}} \) was also broadened, roughly maximized at the same mole fraction. In thermodynamic sense, the micellization process was least spontaneous and least exothermic at equimolar proportion of the components. The \( \Delta H^{0}_{{\text{m}}} - \Delta G^{0}_{{\text{m}}} \) correlation (inset) was narrow and maximized at X SDS = 0.5. It revealed the composition-dependent enthalpy-free energy compensation for the SDS–SDBS binary system under the condition of constant entropy, a rarely observed process.
Mutual interaction of SDS and SDBS in mixed micelles
The ideal behavior of mixed surfactants producing mixed micelles can be rationalized by the equation of Clint [58]. Thus,
where X i and CMC i are the mole fraction of the ith species and its CMC, respectively, and CMCmix is the CMC in the mixed state.
For the studied two-component system, SDS and SDBS, the Clint equation takes the form,
A nonideal mixture (components interacting) would show deviation from the equation, and CMCmix would be either lower or higher than Clint’s prediction for synergistic or antagonistic interactions. In Fig. 7, the correlation of the CMCs with Clint equation is illustrated. In the plot, the CMC values by calorimetry, tensiometry, and conductometry are presented in curves I, II, and III, respectively. It is seen that while calorimetry and conductometry have diagonized antagonism, tensiometry has evidenced closeness with Clint’s formalism with minor deviations. None of the calorimetric CMCs fitted to the ideal course, non-ideality ran throughout. The A min values have evidenced antagonism between DS− and DBS− at the interface. We have processed the calorimetric results for quantification of the antagonism effect.
In this direction, different theories have been proposed [15–17] of which Rubingh’s approach [15] is well-recognized and used for its relatively simple premise. We have analyzed our results in the light of Rubingh’s equation. Thus,
The equation was iteratively solved for \( X^{{\text{R}}}_{{{\text{SDS}}}} \), which was used in the following relation to get the interaction parameter g.
The calculated g and \( X^{{\text{R}}}_{{{\text{SDS}}}} \) values are given in Table 5. Positive g values indicated antagonistic interaction. The activity coefficients of both SDS and SDBS ions in the mixed micelles were obtained from the following equations,
The positive g values showed an increasing trend with X SDS in the mixture. The mole fractions of SDS in the mixed micelles \( {\left( {X^{{\text{R}}}_{{{\text{SDS}}}} } \right)} \) were much lower than the stoichiometric mole fraction (X SDS). Thus, SDBS constituted the major portion of the mixed micellar species. The \( f^{{\text{R}}}_{{{\text{SDS}}}} \) and \( f^{{\text{R}}}_{{{\text{SDBS}}}} \) values obtained at different X SDS were fairly different; the former was ∼2.3–2.8 times greater than the latter except at X SDS = 0.9. The sparsely populated component (SDS) was thermodynamically more active in the mixed micelle. Such higher component activity was observed in the past by our group and others [8, 20, 57, 68].
Like Rubingh, the Lange–Shinoda formalism [59] also evidenced non-ideality in the mixed surfactant system comprising SDS and SDBS. Similar was the case with C12PyBr–C12QBr analyzed by Maeda [60]. On the basis of Gibbs–Duhem relation and the proposal of Hall with consideration of counter-ion binding, Maeda has advanced a general thermodynamic analysis of charged binary mixed micelles of nonionic–ionic and like-charged ionic–ionic types. We have tested our results on SDS–SDBS mixed system also in the light of Maeda’s analysis. The micellar mole fraction of the higher CMC-yielding component SDS \( {\left( {X^{{\text{M}}}_{{{\text{SDS}}}} } \right)} \) in relation to CMCmix, the Corrin–Harkins [61] constant K (which is β in the present notation), and the stoichiometric mole fraction of SDS (X SDS) can be expressed as:
The activity coefficients of the components (\( f^{{\text{M}}}_{{{\text{SDS}}}} \) and \( f^{{\text{M}}}_{{{\text{SDBS}}}} \)) and the resultant excess free energy (G ex) can be calculated from the following relations:
The nonelectric activity coefficients and the excess free energy can be obtained from the relations shown below:
In the above equations, f terms are activity coefficients, and the G ex terms are excess free energies. They were computed for all the compositions of the binary mixtures of SDS and SDBS.
The β SDS (0.505) and β SDBS (0.210) are widely different. In such a case for mixed systems, the use of average value of β is recommended [59]. Since the individual β values for the binary combinations are available to us, we have used them in the calculation. The results are given in Table 6. Up to X SDS = 0.5, the \( X^{{\text{M}}}_{{{\text{SDS}}}} \), \( f^{{\text{M}}}_{{{\text{SDS}}}} {\left( {f^{{{\text{M, $ \times $ non}}}}_{{{\text{SDS}}}} } \right)} \) were all negative, and G ex and (G ex,non) values were all very small, but positive (except that at X SDS = 0.5). With greater proportion of SDS in the mixture than 0.5, all the parameters were positive and of appreciable magnitude. Repulsive or antagonistic interaction between the components was envisaged in the micelles as also found from Rubingh’s treatment (Table 5). Chemical structural difference between the head groups of SDS and SDBS or the mismatch might have caused mutual antagonism. Nonideal behavior throughout the whole range of compositions has been reported by Maeda for the binary C12PyBr–C12QBr system. There, the excess free energies were positive. The Rubingh’s formalism has been found to be applicable for the whole composition range of the mixed SDS and SDBS, whereas Maeda’s approach was clearly valid only for X SDS > 0.5. This might have arisen because of the observed CMCSDS / CMCSDBS ratio = 4.6, which was greater than 4.0, the maximum CMC ratio of the mixed components required for the Regular Solution Theory to hold [17]. More experiments on different systems and data analysis are required to establish the generality of the Maeda’s proposition.
Amphiphile packing in micelle
The packing of SDS and SDBS in pure and mixed micelles was examined in the light of Israelachvili’s model [62], in which a packing parameter (P) has been defined by the relation,
where A is the head-group cross-sectional area of the surfactant monomer, l is the critical length per surfactant in the micelle (given in Eq. 9), and v is the volume of the hydrocarbon chain of the monomer. The l and v of the hydrophobic chain of a surfactant monomer were calculated using Tanford’s expression [63],
In case of mixed micelles, v was calculated by the relation,
In Eq. 27, the subscript i refers to the ith species. The \( X^{{\text{R}}}_{i} \) values obtained from Rubingh’s equation (Eq. 19 and given in Table 5) were used in the calculation. In the case of a mixture, l was considered to be that of the component with higher tail length (SDBS in the present case) since l SDBS > l SDS. In the calculation of l SDBS, C n was taken to be 15.5 considering the contributions of three and a half carbon atoms for the benzene ring [64].
In the calculation of P, choice of A is important. In earlier work, [15] the head group cross-sectional area at CMC, i.e., A min was used, which refers to a property of the surfactant monolayer at the air/water interface. The actual value of A at the micellar surface is expected to be different. Here, in the calculation of P, we have used both A min and A from the geometrical relation (A = 4πl 2/n). In the calculation of A (values in parenthesis in column 3, Table 3), n determined by fluorimetry was used. The values of v and P are reported in Table 7. The shape and type of amphiphile aggregates can be predicted [62] from the magnitude of P. For spherical bodies, P < 0.333; for nonspherical shape, 0.333 < P < 0.50; for vesicles and bilayers, 0.50 < P < 1, and for inverted structures, P > 1. The P values herein obtained by the use of A min and A, although differed in the values, were all less than 0.333 and suggested spherical micellar shape in all the cases. Since pure micelles of both SDS and SDBS appeared to be spherical, their mixtures also resulted in spherical species. In the light of packing parameter, nonspherical micelles of Brij-56 and spherical micelles of both CPC and CTAB were reported [15]. Their mixed compositions with fair shares of Brij-56 produced nonspherical assemblies whereas with lower fraction of Brij-56, the mixed micelles were almost spherical [14]. Such predictions of micellar shapes from the packing parameter (P) require experimental support by small-angle neutron scattering and/or dynamic light scattering measurements [65]. Such facilities are not presently available to us.
Conclusions
The following conclusions can be drawn from the results.
-
1.
The CMC of mixed SDS–SDBS obtained from conductometry and calorimetry fairly agreed with each other; the tensiometric values were lower.
-
2.
The aggregation number of SDBS was approximately 20% lower than that of SDS because of differences between the hydrophobic tails and the polar heads. Their mixtures produced intermediate values depending on micellar compositions.
-
3.
The counter-ion binding (β) for the SDBS micelle was much lower than that of SDS; the β for the mixed micelles were consequently low.
-
4.
The thermodynamic treatment has shown large and more or less constant entropy of micellization and variable free energy and enthalpy. An overall comparable environmental condition prevailed during the micellization of the mixed species in solution.
-
5.
SDS and SDBS were found to interact antagonistically in the mixed micelles and yielded \( f^{{\text{R}}}_{{{\text{SDS}}}} > 1 \) like other reported antagonistically interacted binary surfactant combinations.
-
6.
The packing parameter of the amphiphiles in the micelles (both pure and mixed) advocated spherical geometry of the assemblies.
References
Sulthana SB, Rao PVC, Bhat SGT, Nakano TY, Sugihara G, Rakshit AK (2000) Langmuir 16:980
Prasad M, Moulik SP, Palepu R (2005) J Colloid Interface Sci 284:658
Junquera E, Aicart E (2002) Langmuir 18:9250
Prasad M, Moulik SP, McDonald A, Palepu R (2004) J Phys Chem B 108:355
Treiner C, Makayssi A (1992) Langmuir 8:794
Kashimoto K, Matshubata H, Takahara H, Nakano T, Takine T, Aratono M (2004) Colloid Polym Sci 283:329
Aswal VK, Goel PS (1998) Physica B 245:73
Shivaji Sharma K, Patil SR, Rakshit AK, Glenn K, Doiron M, Palepu RM, Hassan PA (2004) J Phys Chem B 108:12804
Haque ME, Das AR, Moulik SP (1995) J Phys Chem 99:14032
Barry BW, Gray GMT (1975) J Colloid Interface Sci 52:327
Holland PM, Rubingh DN (1983) J Phys Chem 87:1984
Graciaa A, Ghoulam Mben, Marion G, Lachaise J (1989) J Phys Chem 93:4167
Ghosh S, Moulik SP (1998) J Colloid Interface Sci 208:357
Moulik SP, Ghosh S (1997) J Mol Liq 72:145
Chakraborty T, Ghosh S, Moulik SP (2005) J Phys Chem B 109:14813
Ghosh S (2001) J Colloid Interface Sci 244:128
Rosen MJ (1989) Surfactants and interfacial phenomenon, 2nd edn. Wiley, New York
Sarmoria C, Puvvada S, Blankschtein D (1992) Langmuir 8:2690
Motomura K, Aratono M, Ogino K, Abe M (1993) In: Ogino K, Abe M (eds) Mixed surfactant systems. Dekker, New York, p 99
Haque ME, Das AR, Rakshit AK, Moulik SP (1996) Langmuir 12:4084
Jana PK, Moulik SP (1991) J Phys Chem 95:9525
Kawamura H, Manabe M, Saio H, Takahashi H, Takunaga S (1989) Niihama kogyo koto senmon gakko kiyo rikogaku-hen 25:86
Basu Ray G, Chakraborty I, Ghosh S, Moulik SP, Palepu R (2005) Langmuir 21:10958
Hait SK, Majhi PR, Blume A, Moulik SP (2003) J Phys Chem B 107:3650
Majhi PR, Moulik SP (1998) Langmuir 14:3986
Turro NJ, Yekta A (1978) J Am Chem Soc 100:5952
Waka Y, Hamamoto K, Mataga N (1979) Chem Phys Lett 62:364
Waka Y, Hamamoto K, Mataga N (1978) Chem Phys Lett 53:242
Philips JN (1955) Trans Faraday Soc 51:561
Prasad M, Chakraborty I, Rakshit AK, Moulik SP (2006) J Phys Chem B 110:9815
Stigter D, Mysels KJ (1955) J Phys Chem 59:45
Tartar HV (1955) J Phys Chem 59:1195
Tartar HV (1959) J Colloid Sci 14:115
Kushner LM, Hubbard WD (1955) J Colloid Sci 10:428
Parfit GD, Wood JA (1969) Kolloid Z Z Polym 229:55
Huisman HF (1964) Proc K Ned Akad Wet B67:367, 388, 407
Shanks PC, Franses EI (1992) J Phys Chem 96:1794
Das Burman A, Dey T, Mukherjee B, Das AR (2000) Langmuir 16:10020
Garidel P, Hildebrand A, Neubert R, Blume A (2000) Langmuir 16:2567
Majhi PR, Blume A (2001) Langmuir 17:3844
Das HT, Bhattacharya SC, Moulik SP (1993) J Photochem Photobiol A Chem 71:257
Basu Ray G, Chakraborty I, Moulik SP (2006) J Colloid Interface Sci 294:248
Abu-Hamdiyyah M, Al-Mansour L (1979) J Phys Chem 83:2236
Hoffmann H, Platz G, Rehage H, Schott W, Ulbricht W Ber (1981) Bunsen Ges Phys Chem 85:255
Benrraou M, Bales BL, Zana R (2003) J Phys Chem B 107:13432
Ribiero ACF, Lobo VMM, Valente AJM, Azevedo EFG, Miguel MdaG, Burrows HD (2004) Colloid Polym Sci 283:277
Delisi R, Inglese A, Milioto S, Pellerito A (1997) Langmuir 13:192
Evans DF, Allen M, Ninham BW, Fonda A (1984) J Solution Chem 13:87
Kale KM, Cussler EL, Evans DF (1980) J Phys Chem 84:593
Kale KM, Cussler EL, Evans DF (1983) J Sol Chem 11:581
Bandyopadhyay A, Moulik SP (1988) Colloid Polym Sci 266:455
Moriyama E, Lee J, Moroi Y, Abe Y, Takahashi T (2005) Langmuir 21:13
Konop AJ, Colby RH (1999) Langmuir 15:58
Barchini R, Pottel R (1994) J Phys Chem 98:7899
Moroi Y (1992) Micelle: theoretical and applied aspects. Plenum, New York
Mukherjee K, Mukherjee DC, Moulik SP (1994) J Phys Chem 98:4713
Dai S, Tam KC (2004) Langmuir 20:2177
Clint JH (1975) J Chem Soc Faraday Trans 71:1327
Shinoda K (1954) J Phys Chem 58:541
Maeda H (2005) J Phys Chem 109(33):15933
Corrin ML, Harkins WD (1947) J Am Chem Soc 69:683
Israelachvili JN (1991) Intermolecular and surface forces, 2nd Edn. Academic, London
Tanford C (1980) The hydrophobic effect: formation of micelles and biological membranes. Wiley, New York
Myers D (1988) Surfactant science and technology. VCH, New York
Garg G, Hassan PA, Aswal VK, Kulshreshtha SK (2005) J Phys Chem B 109:1340
Griffiths PC, Hirst N, Paul A, King SM, Heenan RK, Farley R (2004) Langmuir 20(16):6904
Chatterjee A, Moulik SP, Sanyal SK, Mishra BK, Puri PM (2001) J Phys Chem B 105:12823
Moulik SP, Haque ME, Jana PK, Das AR (1996) J Phys Chem 100:701
Acknowledgements
GBR and IC acknowledge the Council of Scientific and Industrial Research (CSIR, Govt. of India) for the grants of a JRF and an SRF, respectively. SPM thanks the Indian National Science Academy (INSA) for a Senior Scientist position. The authors are thankful to Dr. S. Mukherjee and Ms. M. Mukhopadhyay of IACS, Kolkata, for providing fluorimetric measurement facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basu Ray, G., Chakraborty, I., Ghosh, S. et al. A critical and comprehensive assessment of interfacial and bulk properties of aqueous binary mixtures of anionic surfactants, sodium dodecylsulfate, and sodium dodecylbenzenesulfonate. Colloid Polym Sci 285, 457–469 (2007). https://doi.org/10.1007/s00396-006-1589-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-006-1589-1