Abstract
In aqueous solution at 40 °C, the micellization behavior of the binary mixtures constituted by an amphoteric sodium n-dodecyl diamine sulfonate and a cationic octadecyltrimethyl ammonium bromide has been investigated by the tensiometric technology. The values of critical micelle concentration (cmc) were determined by surface tension measurement of aqueous solution. The experimental data were analyzed according to various mixing thermodynamic models within the framework of the pseudophase separation model. The values of mixed cmc have been predicted successfully by both the Rubingh’s and Rodenas’s treatments. Various parameters like the mixed cmc, interaction parameters between surfactants (β 12), the compositions and activity coefficients in mixed micelles as well as thermodynamic properties have been determined using Lang, Rubingh, Rodenas, and Rosen approach. Over the entire composition range investigated, the mixed micelle formation, non-ideal or ideal mixing, and synergistic effect or antagonism are explained by the electrostatic interaction between ionic headgroups of surfactants and the steric effect of surfactant. Thermodynamic parameters favor the process of micellization which is found to be entropy-driven.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The properties of a pure surfactant are substantially determined by its chemical structure and its geometrical arrangement within a micelle. In the most practical occasion, surfactant mixtures, rather than a pure surfactant, are used, and their solution properties are interesting from both physicochemical and application points of view. Surfactant mixtures can show some different behaviors in comparison to their components. The interaction between surfactants can lead to synergism or to antagonism, mainly depending on the kinds of surfactants [1–3]. The synergistic property is exhibited due to the attractive interaction between surfactants and the result of their non-ideal mixing [1–3]. The mixtures of unlike surfactants show more non-ideal behavior and are of both theoretical interest and practical importance [2–4]. The non-ideal mixing between surfactants may be favorable to form a effective mixed micelle in aqueous solution, consequently resulting in some unique properties, for example, a lower critical micelle concentration (cmc) than their components in mixed surfactant system, a reduction of both the cost and environmental impact, and so forth.
The non-ideal behavior of surfactant mixtures has been described reasonably by some models, e.g., Rubingh model [5], Rosen model [4], and so on. On the basis of the regular solution theory (RST), the Rubingh model [5] was applicable to focus on the interaction between any binary mixtures of surfactants. An interaction parameter (β 12), which takes account of the deviation from ideality, was introduced into the Rubingh model and helps us to predict the interaction or repulsion between surfactants. The negative value of β 12 means the synergistic effect and the non-ideal mixing. β 12 near to zero reveals little interaction between surfactants and the ideal mixing, whereas the positive value denotes the antagonism between surfactants.
Regarding the practical importance, the synergistic properties or non-ideal mixing between surfactants are expected to occur. Some researches for surfactant mixtures have indicated the favorable mixing properties or non-ideal mixing. Among a great number of researches, it is a overwhelming interest to focus on binary surfactant mixed systems including the mixtures of the surfactant types, anionic/cationic [3, 6], nonionic/anionic [7–9], cationic/cationic [10, 11], nonionic/cationic [12–14], and nonionic/nonionic [15]. In addition, some researchers have also paid some attentions to the effect of temperature, solvents, or some additives on the interaction behavior of the mixed surfactant systems [16, 17]. Except these, the binary surfactant systems of zwitterionic/anionic [2, 18–20] and zwitterionic/nonionic [21] have been reported to lesser extent, while the investigation related to the zwitterionic/cationic mixed system is rather rare. Hence, it is necessary to investigate the interaction between zwitterionic and cationic surfactants and their properties in aqueous solution.
Sodium n-dodecyl diamine sulfonate (C12AS) developed by our group represents amino acid-type amphoteric surfactants [2, 22–25]. Recently, the micellization behavior of mixing C12AS with nonionic or anionic surfactants has been reported by our group [2, 22–24], and the surfactant formula systems containing C12AS have been developed successfully and used as oil-displacing agent to enhance oil recovery [25]. In tertiary oil recovery, a cationic surfactant may be used sometimes as a sacrificial agent to modify the surface of rock in the reservoir before injecting the solutions containing surfactants as oil-displacing agent. The cationic surfactant added previously but not exhausted is very possible to mix with the surfactants injected later, which should be urgent to worry about some harmful case, such as, the occurrence of precipitate as mixing cationic with anionic or other surfactants, the reduction of surface/interfacial activity, and so forth. Therefore, on the basis of the above considerations, the micellization behavior of mixing of C12AS with cationic octadecyltrimethyl ammonium bromide (OTAB) was investigated in this paper, and a major aim focuses on the interaction behavior between C12AS and OTAB and on whether synergistic properties of the mixed surfactant solution and non-ideal mixing between two surfactants appear or not. Regarding this, the cmc values of the binary mixtures were obtained by the tensiometric method, some micellization parameters were calculated by using different mixing thermodynamic models, including RST, Rubingh model, and so on, and then the interaction between two surfactants was theoretically analyzed.
Experimental
Materials
The raw product of C12AS developed by our group [2] was washed and recrystallized by absolute ethanol and acetone for more than five times, and then dried to the constant weight in vacuum oven at 40 °C and under the pressure of 0.01 MPa, and consequently the purity of the treated product was over 96 % (the rest being less than 4 % water) [2], measured with Vario EL III Automatic Elementary Analyser made by Germany Elementar Co. OTAB is an analytical reagent (a purity of> 99 %) from Sinopharm Chemical Reagent Co., Ltd. The chemical structure formulas of C12AS and OTAB are depicted in Fig. 1. All these materials were used as supplied. Deionized triple distilled water was used to confect the solution of surfactant in this investigation.
Methods
The surface tension of surfactant solution was measured with a JK99C automatic surface tensiometer (Shanghai Zhongchen Digital Technic Apparatus Co., Ltd) using a Wilhelmy platinum plate [26]. Before measuring surface tension, all surfactant solutions prepared previously in the conical flasks sealed with rubber plug were allowed to stand in water bath of 40 ± 0.2 °C over 50 min, and so that all measurements could be carried out at near 40 °C. The pH values of all solutions were found to be close to 6.5 within the range of isoelectric point for C12AS. In order to check the compatibility of C12AS with OTAB, the turbidities of solution before and after standing in water bath were measured with a TN100 digital turbidity meter made by Thermo Fisher Scientific Inc. Over the entire surfactant concentrations range investigated, all solutions containing different amounts of C12AS and OTAB at 40 °C were visible to be transparent, and the turbidity change of solutions was not more than 2 %, indicating the well blend between two surfactants [26]. The value of surface tension listed in this paper is an average value from the twice experimental values. The measurement error of surface tension is within ±1 %. The cmc value of surfactant was determined from the inflection point in the curve of the surface tension versus the logarithm of the surfactant concentration in aqueous solution.
Results and discussion
Formation of mixed micelle and its critical concentration
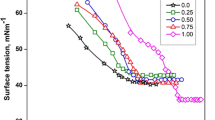
The surface tension data measured in aqueous solutions containing pure OTAB and different amounts of C12AS and OTAB at 40 °C are presented in Fig. 2. The surface tensions of single surfactant OTAB and the mixed C12AS/OTAB systems show some remarkable decreases with surfactant concentrations without any minimum close to the cmc or the mixed cmc, C M, indicating their higher purity. The value of C M at different mole fractions of C12AS in bulk solution (x 1) and the cmc value of OTAB were obtained as the break point of both straight lines of Fig. 2 and are listed in Table 1. The experimental cmc value for OTAB is in good agreement with the reported value [4]. In Table 1, the experimental cmc value for C12AS is obtained from our previous experimental result [2]. The mixed cmc values (C M) for all the mixed systems are lower than the cmc values of pure surfactants as well as their corresponding ideal cmc values (C Mideal ) obtained from the Lange equation [27]:
where C 1 and C 2 are the cmc values of the first (C12AS) and the second component (OTAB) in the mixed system, respectively. The Lange equation is based on the pseudophase thermodynamic formulation and predicts the nature of deviation from the ideal behavior. For all the binary mixed systems of C12AS/OTAB, the values of C M (in Table 1 or Fig. 4) indicate negative deviation from the ideal case, favoring the mixing of two unlike surfactants on the mixed state or their non-ideal mixing. The negative deviation may be attributed to the enhancement in the hydrophobicity of the mixed system from two aspects: (1) intercalation of amphoteric C12AS molecule into the micelles of cationic OTAB shields the repulsive interaction between ionic headgroups and improves the electrostatic stabilization of the mixed micelle; (2) attractive interaction between headgroups of cationic and amphoteric components may benefit the formation of mixed micelles, as shown in Fig. 3.
Variation of surface tension with the logarithm of concentration (logc) for pure OTAB and the binary mixtures of C12AS and OTAB in aqueous solution at 40 °C:  purple solid line with filled circle relates to the data of pure OTAB; other lines relate to the binary mixtures with the molar fraction of C12AS in aqueous solution (x
1): 0.10(
purple solid line with filled circle relates to the data of pure OTAB; other lines relate to the binary mixtures with the molar fraction of C12AS in aqueous solution (x
1): 0.10(  ), 0.20(
), 0.20(  ), 0.40(
), 0.40(  ), 0.60(
), 0.60(  ), 0.80(
), 0.80(  ), and 0.90(
), and 0.90(  ), respectively
), respectively
Interaction of surfactants and their components in mixed micelles
The formation of mixed micelles may be represented by the following relation [5]
where f 1 and f 2 are the activity coefficients of the first (C12AS) and the second component (OTAB) in the mixed system, respectively. f 1 and f 2 represent the degree of participation of surfactant in mixed micelles. On the basis of the RST, the values of f 1 and f 2 can be calculated by the Rubingh’s treatment [5] as follows:
where X 1 and X 2 denote the molar fractions of the first and the second component in the mixed micelle, respectively. Combining Eq. (3) with Eq. (2), it easily deduces the following equation
According to Eq. (4), X 1 can be obtained by an iterative calculation. Then, β 12 can be evaluated from the equation
Without considering the activity coefficients in the Rubingh’s treatment, Rodenas et al. [23, 28] have given other treatment as the following equation
This Eq. (6) can calculate X 1 from dlnC M/dx 1, and then calculate the values of β 12 and f 1 (or f 2) from Eqs. (5) and (3), respectively. The Rodenas’s treatment is based on Lange’s model [25] and considers the Gibbs–Duhem equation, and then it is not subjected to the restrictions of the regular theory of real solution. According to the Rubingh’s treatment and the Rodenas’s treatment, some parameters (e.g., X 1, β 12, f 1, and f 2) have been obtained and listed in Table 1 (wherein X ideal1 was obtained by the Lange’s model [27]).
A negative value of β 12 accounts for synergism arising from attractive interactions between unlike surfactants in mixed micelle formation, while a positive value is indicative of antagonism [2, 10, 22–24, 28, 29]. According to the Rubingh’s treatment, it is found from the data in Table 1 that the value of β 12 is negative, indicating some synergistic effects between C12AS and OTAB. However, the value of β 12 is dependent on the compositions in aqueous solution (x 1), due to the unlike surfactants in the mixed systems. Similar results have been obtained with other surfactant mixtures [2, 10, 22–24, 28, 29]. In solutions enriched with C12AS or OTAB (such as x 1 = 0.10 or x 1 = 0.90), it should be noted that β 12 calculated by the Rubingh’s treatment near to zero and the criterion of synergism according to Rosen [4] determined by the condition of |β 12| > |ln(C 1/C 2)|(= 0.194) are not fulfilled, indicating little interaction between two surfactants and ideal mixing. According to the Rubingh’s treatment, the composition in real mixed micelles (X 1) at x 1 = 0.10 or x 1 = 0.90 singularly approaches to that on ideal state, confirming their ideal mixing. However, according to the Rodenas’s treatment, a larger negative value of β 12 at x 1 = 0.10 and the fulfillment in the condition of |β 12| > |ln(C 1/C 2)| mean synergistic effect, while a larger positive value of β 12 at x 1 = 0.90 indicates antagonism between two surfactants. Obviously, there are some deviations of β 12 calculated by the Rodenas’s treatment from the Rubingh’s treatment, which may be resulted mainly from the difference in their theoretical background. Nevertheless, in solutions at x 1 = 0.20, 0.40, 0.60, and 0.80, the values of β 12 obtained from both the Rubingh’s and Rodenas’s treatment are negative and fulfill the criterion of synergism, suggesting the consistency and validity of two treatments within some given conditions.
Figure 4 shows the dependence of the mixed cmc (C M) on the composition of C12AS in solutions. It can be observed from Fig. 4 that there is no difference between the values of C M calculated by both the Rubingh’s and Rodenas’s treatment, and the calculated values of C M are close to the experimental values, implying the validity of two treatments for the binary mixed systems of C12AS/OTAB investigated in this paper. Figure 5 depicts the variation of micellar composition of C12AS (X 1) with its content in solution. The composition of C12AS in mixed micelles on ideal state (X ideal1 ) is more than its contents in bulk solution over the range of composition investigated, resulting from the stronger micellization of C12AS. The sign of the deviation of X 1 in real micelles from X ideal1 in ideal micelles is reversed. In solution enriched with cationic OTAB, the values of X 1 in real micelles turn out to be larger than calculated in the case of ideal mixing. And while in solutions enriched with C12AS, the content of cationic OTAB in real micelles even exceeds its content in ideal micelles. It is also found easily from Fig. 5 that at x 1 < 0.67, the values of X 1 in real micelles are more than its content in bulk solutions, while at x 1 > 0.67, there is a reverse trend. These cases may be explained by the electrostatic effect of surfactant headgroups and their steric effect. In the first case, in solutions enriched with OTAB, an increase of the molar fraction of C12AS means that C12AS becomes more available for the intercalation of C12AS in the mixed micelles, largely shielding the electrostatic repulsion between ionic headgroup of surfactant, thus promoting micellization, as shown in Fig. 3. In the formation of the mixed micelles, other cause may result partly from a feeble electrostatic attraction between a cationic headgroup of OTAB and an oppositely charged headgroup of C12AS with a charge density of q 2 = 0.21 [2]. And among the first case, as the content of C12AS in bulk solution is too small (x 1 ≤ 0.10), the shield effect does not effectively make contribution on the formation of mixed micelles, resulting in little interaction between two surfactants and even ideal mixing, such as β 12 near to zero in Table 1. In the second case, in solutions enriched with C12AS (especially x 1 > 0.67), the steric effect of C12AS headgroup [2, 22–24] may play a vital role in the micellization, disfavoring the formation of mixed micelles and consequently resulting in a lower value of X 1 than x 1. And especially at x 1 ≥ 0.90, the steric effect for both C12AS headgroup and OTAB headgroup may overwhelmingly control the formation of mixed micelles, then resulting in the ideal mixing and even antagonism between two surfactants.
Variation of the mixed cmc (C
M) with the molar fraction of C12AS in aqueous solution (x
1) at 40 °C:  red dashed line with open circles according to the ideal behavior;
red dashed line with open circles according to the ideal behavior;  black solid line with filled circles according to the experimental data;
black solid line with filled circles according to the experimental data;  green solid line with open triangles according to the Rubingh’s treatment;
green solid line with open triangles according to the Rubingh’s treatment;  blue solid line with open stars according to the Rodenas’s treatment
blue solid line with open stars according to the Rodenas’s treatment
Variation of the compositions in mixed micelles (X
1) with the molar fraction of C12AS in aqueous solution (x
1) at 40 °C:  black dashed line with open circles according to the ideal behavior (Lange treatment);
black dashed line with open circles according to the ideal behavior (Lange treatment);  red solid line with open triangles according to the Rubingh’s treatment;
red solid line with open triangles according to the Rubingh’s treatment;  blue solid line with open stars according to the Rodenas’s treatment;
blue solid line with open stars according to the Rodenas’s treatment;  green solid line is a diagonal
green solid line is a diagonal
The data in Table 1 show that activity coefficients of C12AS in mixed micelles (f 1) increase continuously with the content of C12AS in bulk solution, and while there is an opposite tendency for OTAB, which implies a negative deviation from ideality as well as their participation in mixed micelles. There is not obviously a deviation in the values of f 1 or f 2 obtained from both the Rubingh’s and Rodenas’s treatment, indicating a consistency for two treatments. The values of f 2 at x 1 = 0.10 or f 1 at x 1 = 0.90 near to 1 may partly confirm the ideal mixing between two surfactants.
Thermodynamics
Thermodynamic parameters can be used to be characteristic of the process of micellization and a deviation of non-ideal mixing from ideal mixing. On the basis of RST, thermodynamic parameters of micellization can be calculated from the following equations [2, 22–24]
where \( \Delta G_{\text{ideal}}^{{^{\text{M}} }} \), ΔG M, ΔH M, and ΔS M denote Gibbs free energy change on ideal state, free energy change, enthalpy change, and entropy change in the process of micellization, respectively. Thermodynamic parameters of micellization obtained by both the Rubingh’s and Rodenas’s treatments are listed in Table 2. It is found from the data in Table 2 that though a small deviation between the values obtained by two treatments is found, it should not influence the description of phenomena investigated in this paper. According to thermodynamics, the values of ΔH M and ΔG M (or \( \Delta G_{\text{ideal}}^{{^{\text{M}} }} \)) are negative, but ΔS M is positive, implying the spontaneous process of micellization [2, 3, 7, 22–24]. For all the mixed systems, the absolute values of TΔS M are obviously larger than the absolute values of ΔH M, and the values of entropy are always positive, suggesting entropically a favorable process in the mixed micelles formation.
Figure 6 shows the variation of Gibbs free energy change with the mole fraction of C12AS in bulk solution (x 1). It can be observed from Fig. 6 that in solutions in the range of x 1 from 0.20 to 0.80, the Gibbs free energy change of micellization (ΔG M) displays a larger negative deviation from the ideal case, revealing non-ideal mixing and the stability of mixed micelle. While in solutions lacked with C12AS or OTAB (at x 1 < 0.10 or x 1 > 0.90), the values of ΔG M are abnormally close to the ideal values, implying the ideal mixing. This can be explained by the electrostatic effect of surfactant headgroups and the steric effect described as previously. However, in comparison to the micelles of individual components (such as OTAB or C12AS), the mixed micelles may be more stable, mainly due to the occurrence of the electrostatic attraction between the headgroups of unlike surfactant ions as shown in Fig. 3, consequently promoting the formation of compact mixed micelles. And while as shown in the I or V stage in Fig. 3, the electrostatic repulsion between headgroups of same surfactant ions may result in the unconsolidated structure for the micelle of individual component, disfavoring the formation of stable micelles. It is also found that the Gibbs free energy change in the process of micellization is an asymmetrical function of the components in aqueous solution (in Fig. 6), due to the unlike structures of two surfactants and the difference in their abilities of micellization. Some similar investigations have been reported by Ren et al. [2, 22–24] and Hines et al. [18].
Variation of the Gibbs free energy change of micellization with the molar fraction of C12AS in aqueous solution (x
1) at 40 °C:  and
and  Gibbs free energy change on ideal state and in real mixed micelles according to the Rubingh’s treatment, respectively;
Gibbs free energy change on ideal state and in real mixed micelles according to the Rubingh’s treatment, respectively;  and
and  Gibbs free energy change on ideal state and in real mixed micelles according to the Rodenas’s treatment, respectively
Gibbs free energy change on ideal state and in real mixed micelles according to the Rodenas’s treatment, respectively
Conclusions
The experimental cmc values (C M) for the binary mixtures of C12AS and OTAB in aqueous solutions at 40 °C were measured by the tensiometric technology. Mixed micelle formation for the C12AS/OTAB mixed system was observed. The results showed that the C M of mixed systems were found to be dependent on the molar fraction of C12AS in the bulk, with the values of C M always inferior to the ideal values obtained by the Lange equation over the entire composition range investigated, partly indicating their non-ideal mixing. The predicted values of C M calculated from both the Rubingh’s and Rodenas’s treatment can be in good agreement with the experimental values, suggesting the validity of both treatments. In solutions with nearby equal-proportional mixing of C12AS and OTAB (x 1 from 0.20 to 0.80), the interaction parameters between two surfactants (β 12) always show larger negative values and are fulfilled to the criterion of synergism suggested by Rosen, implying synergistic effect of interaction between two surfactants. And, while in solution lacked with C12AS or OTAB, the values of β 12 are near to zero or even positive, indicating the little interaction between surfactants or even antagonism. These cases can be explained rationally by the electrostatic repulsion between ionic headgroups of OTAB and the steric effect of C12AS headgroups. Thermodynamic data show that the process of micellization is spontaneous once the cmc has been reached for the C12AS/OTAB mixed system. For all the mixed solutions, the mixed micelle formation may be an entropy-driven process. These favorable micellization behaviors between C12AS and OTAB may play a vital role on some practical occasions, such as tertiary oil recovery.
References
Chakraborty T, Ghosh S, Moulik SP (2005) Micellization and related behavior of binary and ternary surfactant mixtures in aqueous medium: cetyl pyridinium chloride (CPC), cetyl trimethyl ammonium bromide(CTAB), and polyoxyethylene (10) cetyl ether (Brij-56) derived system. J Phys Chem B 109:14813–14823
Ren ZH, Luo Y, Shi DP (2013) Mechanism on the interaction between amimo sulfonate amphoteric surfactant and sodium dodecyl benzene sulfonate in aqueous solution. Colloids Surf A 428:18–24
Parekh P, Varade D, Parikh J, Bahadur P (2011) Anionic-cationic mixed surfactant systems: Micellar interaction of sodium dodecyl trioxyethylene sulfate with cationic gemini surfactants. Colloids Surf A 385:111–120
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, Hoboken
Holland PM, Rubingh DN (1983) Nonideal multicomponent mixed micelle model. J Phys Chem 87:1984–1990
Bhat M, Gaikar VG (1999) Characterization of interaction between butyl benzene sulfonates and cetyl trimethylammonium bromide in mixed aggregate systems. Langmuir 15:4740–4751
Danov KD, Kralchevsky PA, Ananthapadmanabhan KP (2014) Micelle–monomer equilibria in solutions of ionic surfactants and in ionic–nonionic mixtures A generalized phase separation model. Adv Colloid Interface Sci 206:17–45
Lu SH, Wu J, Somaundaran P (2012) Micellar evolution in mixed nonionic-anionic surfactant systems. J Colloid Interface Sci 367:272–279
Das C, Chakraborty T, Ghosh S, Das B (2008) Mixed micellization of anionic–nonionic surfactants in aqueous media: a physicochemical study with theoretical consideration. Colloid Polym Sci 286:1143–1155
Moore SE, Mohareb M, Moore SA, Palepu RM (2006) Conductometric and fluorometric investigations on the mixed micellar systems of cationic surfactants in aqueous media. J Colloid Interface Sci 304:491–496
Rub MA, Kumar D, Azum N, Khan F, Asiri AM (2014) Study of the interaction between promazine hydrochloride and surfactant (conventional/gemini) mixtures at different temperatures. J Solut Chem 43:930–949
Nandni D, Mahajan RK (2013) Micellar and interfacial behavior of cationic benzalkonium chloride and nonionic polyoxyethylene alkyl ether based mixed surfactant systems. J Surfactants Deterg 16:587–599
Ruiz CC, Aguiar J (2003) Mixed micellization of octaoxyethylene monododecyl ether and n-alkyltrimethylammonium bromides. Colloids Surf A 224:221–230
Matsubara H, Muroi S, Kameda M, Ikeda N, Ohta A, Aratono M (2001) Interaction between ionic and nonionic surfactants in the adsorbed film and micelle. 3. sodium dodecyl sulfate and tetraethylene glycol monooctyl ether. Langmuir 17:7752–7757
Haque ME, Das AR, Rakshit AK, Moulik SP (1996) Properties of mixed micelles of binary surfactant combinations. Langmuir 12:4084–4089
Chauhan S, Sharma K (2014) Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution: a conductometric study. J Chem Thermodyn 71:205–211
Khimani M, Vora S (2011) Effect of inorganic additives on a conventional anionic–nonionic mixed surfactants system in aqueous solution. J Surfactants Deterg 14:545–554
Hines JD, Thomas RK, Garrett PR, Rennie GK, Penfold J (1998) Investigation of mixing in binary surfactant solutions by surface tension and neutron reflection: strongly interacting anionic/zwitterionic mixtures. J Phys Chem B 102:8834–8846
Abdel-Rahem R (2009) Synergism in mixed anionic–amphoteric surfactant solutions: influence of anionic surfactant chain length. Tenside Surfactants Deterg 46:298–305
Safonova EA, Alexeeva MV, Smirnova NA (2009) The effect of acidity on micellization in dodecyldimethylamine oxide–sodium dodecyl sulfate aqueous mixtures. Colloid J 71:717–724
Goloub TP, Pugh RJ, Zhmud BV (2000) Micellar interactions in nonionic ionic mixed surfactant systems. J Colloid Interface Sci 229:72–81
Ren ZH (2014) Interacting behavior between amino sulfonate amphoteric surfactant and octylphenol polyoxyethylene ether (7) in aqueous solution and pH effect. J Ind Eng Chem 20:3649–3657
Ren ZH, Luo Y, Zheng YC, Shi DP, Mei P, Li FS (2014) Interacting behavior between amino sulfonate surfactant and octylphenol polyoxyethylene ether (10) in aqueous solution. J Solut Chem 43:853–869
Ren ZH (2014) Interacting behavior between amino sulfonate surfactant and octylphenol polyoxyethylene ether in aqueous solution and effect of hydrophilicity. Ind Eng Chem Res 53:10035–10040
Ren ZH, Luo Y (2013) Dynamic interfacial tension behavior of alkyl amino sulfonate in crude oil-brine system. Tenside Surfactants Deterg 50:369–375
Ren ZH, Chen DJ, Luo Y, Huang J (2010) Investigation of influence of inorganic salt on the critical micelle concentration of sodium octylphenol polyoxyethylenated ethylsulfonate. Acta Chim Sinica 68:1771–1775
Lange H, Beck KH (1973) Zur mizellbildung in mischlösungen homologer und nichthomologer tenside. Kolloid Z Z Polym 251:424–431
Rodenas E, Valiente M, Villafruela MS (1999) Different theoretical approaches for the study of the mixed tetraethylene glycol mono-n-dodecyl etherhexadecyltrimethylammonium bromide micelles. J Phys Chem 87:4549–4554
Sehgal P, Kosaka O, Doe H, Otzen DE (2009) Interaction and stability of mixed micelle and monolayer of nonionic and cationic surfactant mixtures. J Dispers Sci Technol 30:1050–1058
Acknowledgements
Funding for this work was provided by the National Science Foundation of China (51304029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, Z.H., Luo, Y., Zheng, Y.C. et al. Micellization behavior of the mixtures of amino sulfonate amphoteric surfactant and octadecyltrimethyl ammonium bromide in aqueous solution at 40 °C: a tensiometric study. J Mater Sci 50, 1965–1972 (2015). https://doi.org/10.1007/s10853-014-8761-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8761-7