Abstract
Homeostasis is maintained within organisms through the physiological recycling process of autophagy, a catabolic process that is intricately involved in the mobilization of nutrients during starvation, recycling of cellular cargo, as well as initiation of cellular death pathways. Specific to the cardiovascular system, autophagy responds to both chemical (e.g. free radicals) and mechanical stressors (e.g. shear stress). It is imperative to note that autophagy is not a static process, and measurement of autophagic flux provides a more comprehensive investigation into the role of autophagy. The overarching themes emerging from decades of autophagy research are that basal levels of autophagic flux are critical, physiological stressors may increase or decrease autophagic flux, and more importantly, aberrant deviations from basal autophagy may elicit detrimental effects. Autophagy has predominantly been examined within cardiac or vascular smooth muscle tissue within the context of disease development and progression. Autophagic flux within the endothelium holds an important role in maintaining vascular function, demonstrated by the necessary role for intact autophagic flux for shear-induced release of nitric oxide however the underlying mechanisms have yet to be elucidated. Within this review, we theorize that autophagy itself does not solely control vascular homeostasis, rather, it works in concert with mitochondria, telomerase, and lipids to maintain physiological function. The primary emphasis of this review is on the role of autophagy within the human vasculature, and the integrative effects with physiological processes and diseases as they relate to the vascular structure and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
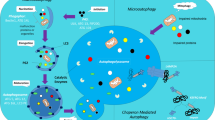
Autophagy is a cellular recycling process in response to various stressors (e.g. oxidative stress, hypoxia, and starvation) by which cells attempt to maintain homeostasis by providing recycled metabolic substrates, particularly during times of nutrient shortage/starvation. Functionally, autophagy facilitates switches in cellular phenotypes, such as the transition of smooth muscle cells from contractile to proliferative phenotypes [127], and the conversion of circulating monocytes to macrophages [166]. The process and signaling cascade of autophagy has been well described across many cell types in various species ranging from yeast to mammals, is relatively well-conserved evolutionarily, and importantly, disruption or excessive autophagy underlies the pathology of numerous chronic diseases (for reviews, see: [27, 40, 49, 103, 123]). Broadly, damaged or superfluous intracellular components are encapsulated within double-membraned autophagosomes, which fuse with lysosomes, and are subsequently degraded by acidic hydrolases into cellular metabolites that are then recycled for use in other physiological processes. Within these constraints and throughout this review, the term autophagy refers to macro-autophagy, being more prevalent than other forms of autophagy- micro-autophagy and chaperone-mediated autophagy. Micro-autophagy directly envelops vesicles via invaginations within the membrane, while chaperone-mediated autophagy occurs in mammalian cells and involves direct targeting and transportation of organelles that express a specific molecular target [5, 142]. The focus of this review is on macro-autophagy and cardiovascular disease with special emphasis on the role of autophagy in vascular health and function.
Autophagy is a key regulator of cardiovascular homeostasis, responding to physiological and pathophysiological stimuli (Fig. 1). Autophagy has been studied within cardiac tissue (e.g. cardiomyocytes), vascular smooth muscle (VSM), and cultured endothelial cells [162]. While recycling of cellular organelles is generally viewed as a beneficial process, insufficient and excessive levels of autophagy can lead to premature cell death (apoptosis). VSM and endothelial cells are plastic tissues responding to environmental factors that elicit changes in phenotypes [108, 137] therefore autophagy is critical in the maintenance of cellular homeostasis. Surprisingly, far less is known about how autophagy influences vascular function, specifically the microvasculature, or how it contributes to vascular pathologies. The primary focus of this review is to provide evidence on novel roles for autophagy within the vasculature from a functional perspective, and relevance to other physiological processes.
Conceptual overview of shear-induced signaling pathways to elicit vasodilation in health. Laminar shear stress confers adaptive autophagy within the endothelium and vascular smooth muscle by enhancing production of NO from eNOS, minimizing mitochondria-derived ROS, ultimately eliciting NO-mediated vasodilation. NO nitric oxide, ROS reactive oxygen species, PI3K Phosphoinositide 3-kinase, eNOS endothelial nitric oxide synthase, L-Arg l-arginine, sGC soluble guanylate cyclase, GTP guanosine triphosphate, cGMP cyclic guanosine monophosphate, PKG protein kinase g
Mechanisms of autophagy
Autophagy is highly sensitive to nutrient conditions and ATP levels [30]. Under conditions where nutrients are depleted or ATP diminished, autophagy is activated to conserve and regenerate metabolic substrates, thereby sustaining homeostasis. The most widely recognized regulators of autophagy are the mechanistic target of rapamycin complex 1 (mTORC1), which acts as a negative regulator of autophagy, sensing amino acid levels and inhibiting autophagy when levels are high, and 5′ adenosine monophosphate-activated protein kinase (AMPK), an energy sensing pathway that regulates autophagy by detecting the intra-cellular ratio of adenosine triphosphate (ATP) to adenosine monophosphate (AMP). Protein kinase B (AKT) regulates mTOR via class 1 phosphoinositide 3-kinase (PI3K). An integral part of the autophagy signaling cascade is the interaction between various autophagy related proteins to elongate the double-membrane autophagosome that encapsulates damaged or superfluous cells/organelles. It should be stressed that autophagy is not a static process, but rather dynamic, responding to various physiological stressors. Many of the methods to investigate autophagy are predicated on measuring snapshots of this dynamic process such as genetic markers or protein quantification in response to pharmacological/physiological interventions. With developing imaging technology, autophagic flux has become more easily measured and is a more robust indicator of the autophagy process. Readers are referred to recent reviews on measuring autophagic flux in various tissues [43, 72].
Autophagy and cardiovascular health and disease
Autophagy holds an essential role within development and progression of cardiovascular disease [12, 38, 39, 78, 122]. Excessive or insufficient levels of autophagic flux contribute to cardiovascular disease pathologies. Data from experimental animal models utilizing genetic deletions of various autophagy-related genes demonstrate structural and functional changes within the developing cardiovascular system, including defective development of valves and chambers of the heart, as well as development of atherosclerotic plaques within coronary arteries [81]. Various cardiovascular-related stressors such as aging, ischemia–reperfusion injury [93], biological and lifestyle factors (such as genetics, smoking, hypertension, and low physical activity) impact autophagy related genes, and proteins (e.g. LC3B, Atg12, Atg3), ultimately contributing to cardiovascular disease development and progression. Collectively, these factors are associated with an increase in reactive oxygen species (ROS), which is holds an important role in cardiovascular function in health and disease.
Free radical species, reactive oxygen species and regulation of autophagy
Under healthy basal conditions, ROS and reactive nitrogen species (RNS) are maintained at physiological levels by anti-oxidants, and by superoxide dismutases (SOD’s) [153]. NO quenches superoxide in an almost diffusion-limited manner, and much faster than spontaneous or enzymatically facilitated conversion to hydrogen peroxide (H2O2) by SOD [55]. Elevations in NADPH oxidase expression also generate elevated levels of ROS [33]. Overproduction of, prolonged exposure to ROS, or insufficient production of anti-oxidants ultimately results in oxidative stress, altering mitochondrial structure (e.g. membrane potential) and function (e.g. respiration, fission/fusion) resulting in protein modifications/aggregation and ultimately cell death [67, 141]. Free radical species are well known to modify mitochondrial proteins and function creating a precipitous cycle wherein cytosolic or mitochondria-derived ROS generate further ROS release from the mitochondria [172].
Under pathological conditions where cellular organelles are damaged by free radical species, autophagy/mitophagy is activated to degrade and recycle damaged organelles. If the damaged organelle is efficiently degraded and recycled, this results in cellular survival and maintenance of homeostasis. If the organelle is only partially, or incompletely degraded, this can result in further oxidative stress, accelerated by ROS-induced ROS-release cycle [99, 145, 162]. Autophagy is also activated by exposure to endothelial shear stress, enhancing phosphorylation of endothelial nitric oxide synthase (eNOS) and production of NO, but also generating ROS [9]. Thus free radicals and autophagy influence the vasculature resulting in adaptive and maladaptive outcomes, highlighting the role of free radicals as critical regulators of autophagy specific to the vasculature.
Role of autophagy and vascular function with health and disease
Autophagy within vascular smooth muscle
The vascular media is a plastic tissue as vascular smooth muscle cells (VSMC) may exhibit multiple phenotypes in response to environmental factors contributing to the development and progression of atherosclerotic plaque [6]. Readers are referred to a recent review by Salabei and Hill [127] for a comprehensive molecular overview of the role of autophagy in VSM.
Autophagy and atherosclerosis
Development of atherosclerosis is associated with VSM phenotype switch, smooth muscle cell death, plaque instability of arterial wall lesions, and importantly, vascular calcification [45]. Activation of autophagy in VSM is generally adaptive promoting VSMC survival, plaque stabilization [84] and reducing vascular calcification [23, 89]. Osonoi, et. al [110] demonstrated that within murine cultured smooth muscle cells, genetic deletion of Atg7 (involved autophagosome formation) increases atherosclerotic burden and results in maladaptive arterial remodeling with descending aortic ruptures being the most common cause of death [110]. Macrophage autophagy plays a protective role within the early phase of atherosclerosis as genetic deletion of Atg5 is demonstrated to accelerate atherosclerosis progression within murine models [87, 117]. Inefficient autophagy as a result of Atg5 deletion may result in further foam cell development and exaggerated inflammatory markers. Conversely, enhancement of autophagy within VSM and macrophages via trehalose or overexpression of transcription factor EB (TFEB) exerts athero-protective effects, reducing plaque burden and reductions in inflammatory markers [32, 149].
An important factor predisposing to cardiovascular events is the propensity for an atherosclerotic plaque to rupture vs. remain stable [4]. Basal autophagy has been demonstrated to be important in maintaining the integrity of the fibrous cap and restricting lipid accumulation [45, 44, 132, 144, 146]. Insufficient or inhibition of autophagy accelerates plaque burden or renders it unstable, while activation of autophagy generally maintains stability [32, 44, 65, 87, 132, 147, 159]. In this context, arteries continuously exposed to laminar or high shear stress are protected against plaque formation [65, 147], and intact autophagy (that is, not insufficient nor hyperactive) is required for limiting plaque formation within these areas, attenuating cell death and release of inflammatory markers [157]. Blood flow within an artery may modulate plaque stability through autophagy via endothelial cell to VSM transmission [65, 147]. For example, shear-induced secretion of platelet derived growth factor (PDGF) isoforms by the endothelium act on PDGF receptors in VSM to determine proliferative/migratory activity and VSM phenotype in an autophagy dependent manner [48, 113].
Autophagy is a potent target for the treatment of atherosclerosis, as well as for limiting the detrimental effects of plaque rupture and release of plaque debris and thrombotic factors. This concept is demonstrated by the anti-atherosclerotic beneficial effects (e.g. reduced VSM proliferation) of drug-eluding stents containing first and second generation mTOR inhibitors such as sirolimus and everolimus [76, 95]. Some data suggests that mTOR inhibitor-coated stents result in favorable clinical outcomes (reduced neointimal hyperplasia or repeat revascularization) when compared to bare metal stents chronically [11, 42, 102, 138]. In the short term, however, drug-eluting stents may promote thrombosis at the level of stent placement, necessitating dual anti-platelet therapy. This is due to delayed endothelialization which is associated with an increase in platelet aggregation [35, 41, 58]. Stent placement within blocked arteries is designed to limit ischemia of downstream tissue, however deployment of the stent may induce localized endothelial injury from balloon inflation resulting in plaque rupture and release of particles eliciting downstream coronary microvascular dysfunction [52, 70, 71]. Thus, there appears to be both athero-protective and detrimental effects of autophagy stimulation. The specific role of autophagy in mediating these events is unclear, and represents a future area of investigation.
Disease severity may play a role in the effectiveness of autophagy in drug-eluting stents, however direct evidence for this is lacking. For example, Zhao, et al. [168]. utilizing peripheral blood monocytes demonstrated that acute myocardial infarction decreased beclin-1 and LC3II levels relative to control patients, or stable angina pectoris. In this study, patients with unstable angina pectoris also demonstrate reductions in beclin-1 and LC3II relative to stable angina and controls, but greater than those with acute myocardial infarction. It should be noted that this study did not investigate autophagic flux per se and requires further investigation. Given this evidence it appears that acute cardiovascular events and heightened disease severity result in reductions in autophagy. On the other hand, it is well established that autophagy is beneficial for survival in response to ischemia, while reperfusion stimulates excess autophagy resulting in more cell death. Overall, the effects of autophagy appear to be context dependent [160].
It should be noted that while current generation drug eluting stents containing mTOR inhibitors confer some beneficial effect through autophagy, systemic administration of mTOR inhibitors result in side-effects such as dyslipidemia, hyperglycemia, hypertension and immuno-suppressive effects [77, 95, 132]. Targeting macrophage autophagy for treatment of atherosclerosis has emerged as a promising approach. Activation of autophagy via TFEB in macrophages reverses plaque-induced reductions in autophagy and protects against plaque development within animal models [32, 135]. Other cardiovascular drugs such as statins and calcium channel blockers may induce autophagy. For a review on current cardiovascular drugs and their potential use in autophagy, readers are directed to a review by Salabei and Conklin [124].
Autophagy and hypertension
Hypertension shares an etiological relationship with cardiovascular disease. Evidence links autophagy to the development and progression of systemic arterial hypertension independent of effects on cardiac tissue [24, 109, 131, 133, 170, 171]. Specific to the VSM, increases in autophagy have been demonstrated to promote the switch in VSM phenotype from contractile to macrophage-like and synthetic/proliferative. At the same time, hypertrophy, proliferation and calcification VSM increase contractile tone, subsequently increasing peripheral resistance and systemic blood pressure [127, 126]. In obese, hypertensive Zucker diabetic fatty rats, excessive autophagy is associated with hypertension and endothelial dysfunction, while administration of a resveratrol analogue ameliorated these detrimental effects, which was blocked by co-administration of rapamycin [29]. Induction of hypertension with angiotensin II activates mTORC1 leading to hypertrophic responses in vascular smooth muscle [47, 140], while, inhibition of mTORC1 in rats fed a high-salt diet ameliorates salt-induced hypertension [75]. More recently, McCarthy, et al. [98]. demonstrated that autophagic activity is reduced in spontaneously hypertensive relative to normotensive Wistar rats which was associated with endothelial dysfunction. Interestingly, restoration of autophagy with trehalose improved endothelial function in SHR independent of improvements in blood pressure. Most data examining autophagy in hypertension has been collected in large vessels, however as blood pressure and organ perfusion are regulated by small resistance vessels. As such, the role of autophagy in arterial hypertension within the vasculature remains on ongoing area of study.
Pulmonary hypertension is characterized by hyperproliferation of VSM within pulmonary arteries (resistance arteries) leading to reductions in lumen diameter, dramatic increases in pulmonary artery pressure, eventually resulting in right heart failure. These VSM and microcirculatory changes are a result of an over-activation of autophagy. Indeed, markers of autophagy are elevated in the lungs of patients with pulmonary hypertension, as well as within various animal models of pulmonary hypertension [82]. Inhibition of autophagy by blocking lysosomal degradation within these various models impedes the development and progression of pulmonary hypertension [91]. Additionally, pulmonary hypertension results in mitochondrial fragmentation, inducing a hyperproliferative state within VSM [121]. Thus autophagy contributes to both systemic and pulmonary hypertension through modulation of VSM phenotypes within both large and smaller arteries, although divergent effects further highlight the complex, and dichotomous nature of autophagy.
Influence of autophagy on endothelial function with health and disease
Endothelial function is a critical barometer of cardiovascular health influenced by numerous factors including age, oxidative stress, genetics, and lifestyle factors. “Conduit artery” (i.e. macrovasculature) refers to the large elastic capacitance arteries that conduct blood under relatively high pressure to the distal vessels, and include the aorta, and primary arterial branches to visceral organs and somatic tissues. More distal to the heart, the composition of arteries changes from elastic to more muscular in content, allowing for greater vasomotion and regulation of perfusion and blood pressure. As arteries branch, both the number and summed cross-sectional area increases, peaking with capillaries characterized by low flow, low pressure, but high volume of distribution (e.g. microvasculature) [21]. Both the macro- and microvasculature exhibit vasodilation to various pharmacological stimuli, as well as shear stress (flow-mediated dilation, FMD) with the primary endothelium-dependent mechanism of dilation under physiological conditions being NO in health (Fig. 1) [57]. Extensive data from our lab indicates that development of coronary artery disease [88, 116], physical (increased intraluminal pressure) [7, 31] or chemical stressors (e.g. increase in ceramide, or lysophosphatidic acid) [14, 36] switches the primary mechanism of microvascular dilation from NO to the H2O2 largely due to an increase in mitochondria-derived ROS (Fig. 2) [88, 116]. The specific role of autophagy on microvascular function in health and disease is an emerging area of research.
Disturbed shear stress, decreases in TERT, as well as elevations in LPA and ceramide confer maladaptive autophagy. Both excessive and insufficient, minimizes NO formation from eNOS, preferentially producing H2O2 and ultimately driving the primary mechanism of vasodilation to H2O2 in response to shear stress. Maladaptive autophagy may not sufficiently degrade the cellular cargo, ultimately eliciting further elevations in ROS. LPA lysophosphatidic acid, TERT telomerase reverse transcriptase, NO nitric oxide, ROS reactive oxygen species, PI3K Phosphoinositide 3-kinase, eNOS endothelial nitric oxide synthase, O2− superoxide, H2O2 hydrogen peroxide, BKCa large conductance calcium activated potassium channel, VSM vascular smooth muscle
Autophagy and endothelial-(dys)function
The endothelium is a critical regulator of vascular health and function and is constantly exposed to varying levels of shear stress via blood flow which in turn releases various vasoactive compounds to regulate vascular tone and vascular cell phenotype. The autophagy signaling cascade has typically been studied within cultured endothelial cells. Cultured endothelial cells are proliferative, and have divergent responses to physical and chemical stressors when compared to quiescent cells within the vasculature, and thus may exhibit differential autophagy responses as well. Nevertheless, systemically circulating compounds/chemicals, as well as processes within endothelial cells themselves regulate autophagy and promote endothelial dysfunction. As discussed previously, atherosclerosis is a hallmark of vascular aging, with endothelial dysfunction as a precursor within the development of atherosclerosis. Indeed, oxidized low-density lipoprotein [165], advanced glycation end-products [158], and various lipid compounds may promote autophagosome formation within endothelial cells which are mediated in part by ROS [56].
Mechanical forces are an important modulator of endothelial autophagy [68]. It is important to note that autophagy within endothelial cells serves as a renewal function, protecting against endothelial cell injury, and plays an integral role in mediating the progression of vascular diseases. Shear stress generated by the frictional forces (blood flow) along the endothelium induces autophagy in a reversible manner via mechano-transduction, and is critical for endothelial cell alignment [85, 147] NO production, and excessive ROS mitigation (Figs. 1, 2) [15, 54]. Shear stress along endothelial cells may be laminar (smooth flow) or turbulent/oscillatory, and is usually pulsatile in larger vessels that dampens with transmission to the microvasculature. High levels of laminar shear stress (> 15 dynes/cm2) promote autophagy within endothelial cells, and up-regulate expression of endothelial NO synthase (eNOS) while inhibiting expression of the potent vasoconstrictor endothelin-1 (ET-1) [46]. This physiological response to shear is sustained suggesting a chronic beneficial effect on the vasculature [90]. Pre-treatment with rapamycin, an mTOR-dependent autophagy activator, further enhances eNOS expression and suppresses ET-1 expression, while pre-treatment with an inhibitor of autophagy (3-MA), enhances ET-1 expression. In contrast, low levels of shear stress (e.g. regions of artery curvature, arterial segments distal to carotid artery ligation) reduce autophagy and promote pro-atherogenic responses [147, 167]. Disturbed/turbulent flow increases autophagosome formation but impair p62-mediated clearance of autophagosomes and promotes endothelial dysfunction [85, 147]. Bharath et al. demonstrated that within bovine aortic endothelial cells exposed to physiological levels of shear stress, autophagy markers increased due to an increased phosphorylation of AMPK [9]. These elevations in autophagy indicators and phosphorylated AMPK regulate mTOR signaling and thereby autophagy [9, 163]. Guo et al. [46]. demonstrated that physiological levels of laminar shear stress increase markers of autophagy which were associated with up-regulation of eNOS expression, and parallel down-regulation of the vasoconstrictor ET-1. Importantly, autophagy activation (rapamycin) enhanced eNOS expression and reduced ET-1 expression in response to shear, while inhibition of autophagy with 3-MA exerted the opposite effect. Inhibition of mTOR via rapamycin treatment in transgenic mice (designed to express fluorescent labeled human eNOS isoforms), increased eNOS expression in regions of the carotid artery exposed to low shear stress, while reducing maximal eNOS expression at regions of high shear stress [19]. The authors attributed the paradoxical reduction in maximal eNOS expression in areas of high shear stress to rapamycin having a shear-stress dependent impact on protein synthesis, however this was not tested directly [19].
These findings in cells and animals have been recently translated into humans utilizing novel approaches. Park et al. [114] demonstrated that the hyperemic response to dynamic handgrip exercise elevated primary endothelial cell markers of autophagy in parallel with NO production within the radial artery. In subjects with diabetes, Fetterman et al. [34] reported reductions in autophagy along with endothelial dysfunction, and impaired eNOS activation) at the level of autophagosome-lysosome fusion. The same investigators demonstrated within cultured endothelial cells that exposure to high glucose concentrations impaired autophagy similar to that observed in vivo, and inhibiting autophagy with bafilomycin (inhibits lysosome acidification) abrogated insulin-mediated eNOS activation, but did not change basal eNOS phosphorylation. Activation of autophagy with spermidine improved insulin-mediated eNOS phosphorylation in both primary and cultured endothelial cells, but had no effect in cells exposed to high glucose [34]. These results demonstrate a prominent role for autophagy in vascular function both in health and disease, and highlight the role for mechanical (shear) and chemical (high glucose) stress in contributing to function of the endothelium at least in part through autophagy.
While the endothelium releases NO in response to shear stress to elicit vasodilation, ROS are also released, particularly from within diseased endothelium. H2O2, an endothelial derived hyperpolarizing factor, is preferentially formed and released from the endothelium of arterioles from subjects with CAD, or from non-CAD arterioles subjected to telomerase reverse transcriptase inhibition (TERT), ceramide, LPA, as well as acute pressure stress in response to flow [7, 8, 13, 14, 31, 36, 88, 116]. Shear-induced H2O2 can damage mitochondrial DNA and enhance superoxide generation which sequesters NO by forming peroxynitrite a potent inhibitor of endothelial NOS and prostacyclin synthase [112]. Deletion/knockdown of Atg3 in endothelial cells reduces NO release and exaggerates ROS production [9]. Liu et al. [90] demonstrated using gain/loss of function experiments that shear-induced autophagy markers are sensitive to redox status. The authors further demonstrated that under flow conditions, endothelial cells were more resistant to oxidant-induced injury, but this protective effect was abolished with inhibition of autophagy [90]. Similarly, Bharath et al. demonstrated that shear-induced autophagy within endothelial cells maintains NO generation via glycolytic dependent purinergic signaling [10].
Data from pre-hypertensive spontaneously hypertensive rats and obese Zucker diabetic fatty rats suggest that excessive autophagy may contribute to microvascular endothelial dysfunction (e.g. reduced endothelium- dependent dilation to acetylcholine) demonstrated by reduced phosphorylation of mTOR concomitant with increased ratio of LC3 II/I and p62 within the microvasculature (3rd order mesenteric arterioles). Increases in LC3 II/I and elevated p62 indicate a high rate of formation of autophagosomes, but impaired fusion of the autophagosome with the lysosome, impeding the clearance of damaged cargo. These effects were ameliorated via activation of mTOR, subsequently suppressing autophagy, suggesting at least pre-hypertension is associated with impaired autophagic flux [28, 29]. These data highlight and provide proof of concept concerning the critical role of autophagy within endothelial cells in response to shear stress as well as the dual nature of autophagy within the development of cardiovascular disease. Future investigations should examine the role of autophagic flux in determining the primary mechanism of microvascular FMD within health and disease, as well as the role of autophagic flux in development of vascular disease pathologies.
Sirtuins (SIRT) have a broad range of physiological effects ranging from cell survival to mediation of signaling pathways, with several SIRT proteins identified in humans [66]. SIRT1 exerts a variety of cellular effects such as regulation of cell cycle, and longevity effects as a positive regulator of autophagy [69]. SIRT1 may directly deacetylate specific autophagy related proteins critical for the autophagy signaling cascade, directly activating the AMPK-mTOR-autophagy axis, as well as deacetylate eNOS, enhancing NO production and promoting NO-mediated endothelium dependent vasodilation [96]. Laminar shear stress enhances SIRT1-induced eNOS deacetylation; however, it appears that a basal level of phosphorylated eNOS via AMPK is necessary for this interaction [17]. Within the context of vascular regulation by autophagy, Liu et al. demonstrated that physiological levels of flow on cultured endothelial cells promotes autophagy via SIRT1 activation in a redox sensitive manner [90], while administration of the exogenous activator of autophagy, spermidine, exerts its protective effects on the vasculature independent of SIRT1 activity [105]. Loss or impairment of another NAD-dependent deacetylase sirtuin, SIRT3, results in cardiac hypertrophy, accelerated development of angiotensin II induced hypertension, along with elevated mitochondrial superoxide production concomitant with reduced eNOS production of NO [26]. SIRT3 deficiency or deletion does not impact native endothelial function, but does exacerbate angiotensin II induced endothelial and cardiac dysfunction [26, 151]. Interestingly, loss of SIRT3 impairs autophagic flux as demonstrated by reduced autophagosome formation and reduction in autolysosome degradation [86]. Conversely, overexpression of SIRT3 enhances autophagy, specifically, mitophagy and results in reduced mitochondrial ROS, and decreased fibrosis [151]. Collectively, these findings further highlight the role of autophagy as a critical regulator of vascular function, and highlight the role for upstream modulators of autophagy on vascular function. The interplay and role for SIRT and autophagy on cardiovascular function remains an ongoing area of investigation.
Aging, endothelial dysfunction and autophagy
Advancing age is associated with exaggerated ROS concomitant with reductions in NO bioavailability, directly impacting the health and function of the vasculature [134]. Vascular aging is characterized by stiffening of conduit arteries, endothelial dysfunction, atherosclerosis, and increases in cardiovascular disease risk [100]. Several lines of evidence demonstrate that autophagy mediates some of these age-associated maladaptive responses. Within primary endothelial cells from older adults, autophagy markers are reduced relative to young adults [79]. Furthermore, atherosclerotic plaques are found in regions of low shear stress, or within the curvature of arteries, and enhancing autophagy, or increasing shear stress in these areas is generally beneficial [10, 9, 19, 46, 65, 114, 147]. Enhancing autophagy within the vasculature is an attractive target for treatment of cardiovascular disease. LaRocca et al. [80] demonstrated that four weeks of supplementation with the autophagy inducer spermidine reduced large artery arterial stiffness (assessed by carotid-femoral pulse-wave velocity), improved endothelial function (enhanced dilation to intra-arterial acetylcholine), and reduced markers of oxidative stress in aged rats. These improvements in older rats paralleled elevations in proteins associated with markers of autophagy suggesting that enhancing autophagy in a model of cardiovascular aging improves conduit vascular responses, effectively recapitulating a younger cardiovascular phenotype. This same group demonstrated that primary endothelial cells harvested from the brachial artery of older adults exhibit reductions in autophagy markers that are associated with markedly reduced endothelial function [79]. In a parallel mouse model, trehalose, a non-reducing natural disaccharide that induces autophagy through nuclear translocation of TFEB and inhibition of cellular glucose transport [25], restored endothelium-dependent dilation and reduced markers of oxidative stress [79]. Kaplon et al. [63] translated these findings into humans, demonstrating that trehalose supplementation improved both endothelium-dependent and independent dilation within the microvasculature (reactive hyperemia), but not within conduit arteries. Although these studies demonstrate improvement in vascular health through enhancement of autophagy, they should be interpreted with caution as concentrations of trehalose used exceeded 100 mmol/L, while animal data utilizing i.p. administration demonstrates that as little as 1 mM exerts beneficial effects [135]. Additionally, in the study by Kaplon et al. vascular improvements were restricted to subjects who gained less than 5 lb [63]. In contrast to these studies, Headland et al [51]. examined endothelial function in response to short-term energy restriction (a potent inducer of autophagy), demonstrating that brachial artery FMD exhibited no changes in response to consecutive days of calorie restriction. It should be emphasized that involvement of autophagy within this experimental design is only implied, and examination of conduit artery function requires further investigation. Collectively, trehalose and caloric restriction represent potential avenues for improvement of vascular function, however this area requires further investigation.
Caution is warranted when interpreting the influence of shear stress on autophagy in cultured endothelial cells as this experimental design does not truly mimic the arterial system, which is comprised of both straight segments (exposed to laminar flow) as well as bifurcations and bulges (e.g. carotid sinus). Pestana et al. [115] demonstrated that inhibition of starvation-induced autophagy via chloroquine promoted NO-mediated vasodilation in a dose–response manner, and protected against superoxide generation in HUVEC’s and rat aortic rings. These results are in direct contrast to previous studies discussed above showing that enhancing autophagy elicits NO-mediated vasodilation. Reconciliation of these divergent results may depend on how autophagy is activated/inhibited. In this regard, proximal inhibition of autophagy with the class III PI3K inhibitor 3 methyladenine (3-MA) (inhibition of elongation of phagophore) may paradoxically induce autophagy under nutrient rich conditions, while suppressing autophagy under starvation conditions [62, 154]. Conversely, bafilomycin A and chloroquine act more distal within the autophagy signaling cascade to inhibit fusion of the autophagosome with the lysosome, neutralizing the acidic hydrolases within the lysosome, respectively [107]. Collectively, these divergent findings of pharmacological modulation of autophagy highlight the dichotomous nature of autophagy and the necessity to interpret these results within the appropriate experimental design. Recently, more specific sensors/probes have been developed to more accurately reflect autophagic flux [43, 62, 72].
Integration of autophagy, signaling pathways, and future directions as they relate to vascular function
Autophagy, mitophagy and the mitochondria
Mitochondrial biogenesis is regulated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), while recycling is mediated by mitochondrial specific autophagy (mitophagy), which has a distinct signaling cascade [12, 150]. Mitochondrial function is a key regulator of cardiovascular health, as mitochondrial disturbances contribute to development and progression of disease. As mitochondria are the main source of cellular ATP, optimal function is of key importance to maintaining homeostasis, particularly in response to physiological stressors. Upon exposure to shear stress, cultured endothelial cell mitochondria release ATP and enhance intracellular calcium signaling [161], ultimately eliciting vasodilation. Mitochondria continuously undergo fluctuations in structure and function. Mitochondrial fission induced by activation of cellular stress pathways and cytosolic proteins permeabilize the mitochondrial membrane, changing the morphological structure to a more spherical shape and fragmented organization, forming a small bud that is then tagged for encapsulation by an autophagosome. Mitochondrial specific proteins mark damaged mitochondria for encapsulation and degradation by mitophagy. PTEN-induced kinase 1 (PINK1) and Parkin are integral synergistic regulators of mitophagy. Depolarized mitochondria causes PINK1 to accumulate on the outer membrane, recruiting Parkin, which marks damaged mitochondria for mitochondrial specific autophagy [53, 106]. Conversely, mitochondrial fusion activated by various GTPases prevents autophagic degradation of mitochondria and are associated with repair of damaged mitochondria, however the exact mechanism for this is unclear.
Damage to mitochondria, whether structural or functional, alters mitochondrial respiration in favor of production of ROS such as superoxide, H2O2, as well as reactive aldehydes such as 4-hydroxy-2-nonenal through lipid peroxidation [59]. Mitochondrial damage starts a vicious cycle of ROS-induced ROS-release [172]. Aging itself is associated with elevated basal levels of ROS [59], as well as alterations to mitochondria structure and function [143]. Mitophagy selectively degrades damaged mitochondria, thus limiting ROS production and accumulation in response to pathophysiological stressors. Attenuated or impaired formation of new mitochondria, and accumulation of damaged mitochondria are a cornerstone in the development of chronic diseases associated with aging, such as heart failure [64, 73].
Vascular effects of mitophagy
Mitochondria are critical in the development and function of the cardiovascular system, providing ATP for maintenance of vascular homeostasis. PGC1α is expressed in endothelial cells and helps protect against oxidative stress, limiting inflammation and maintaining/improving NO bioavailability [22]. Given that mitochondrial dysfunction is associated with vascular dysfunction and atherosclerosis [12, 83, 88, 152] autophagy/mitophagy generally has a protective role in response to stress in VSM and the endothelium (Tables 1, 2). Mitochondria-derived ROS promote damage and degradation of mitochondrial DNA, contributing to inflammation and progression of atherosclerosis. For example, within VSM, mitochondrial fission has been demonstrated to play a critical role in limiting intimal hyperplasia weakening mitochondrial membrane integrity and enhancing mitochondria-derived ROS formation [148]. In further support of this, expression of the key regulator of mitochondrial fission, dynamin-related protein 1 (DRP-1) is upregulated within vascular calcification formations, while inhibition of DRP-1 in VSMC limited the phenotype transition [118]. Mitochondrial fusion as a result of phosphorylation of the outer mitochondrial membrane protein mitofusion-2 suppresses VSMC proliferation and phenotype switching, ultimately mitigating ROS-induced damage [169].
Elevations in mitochondria-derived ROS have been demonstrated to contribute to endothelium-dependent vasodilation [16, 88, 152]. Mitochondria-derived H2O2 is the primary mechanism for FMD in patients with CAD, and loss of PGC1α is implicated in contributing to CAD pathology. Data from our laboratory demonstrate that upregulating PGC1α in isolated arterioles from patients with CAD creates a unique FMD phenotype mediated by both NO and H2O2, protects against acute exposure to increased intraluminal pressure and is important for exercise-induced protection against vascular stress. Conversely, in non-CAD tissue inhibition of PGC1α switches the primary mechanism of FMD to H2O2 [61, 60]. Inhibition of mitochondrial fission within rat aorta and mesenteric arteries, attenuates the vasoconstrictor effects of ET-1 [18], while exposure to a high glucose induces mitochondria fission and fragmentation. These increases in fission were associated with enhanced mitochondria-derived ROS concomitant to reductions in NO signaling [136]. Ultimately, mitochondrial dynamics regulate mitochondrial turnover that is intimately involved in not only autophagic/mitophagy flux, but also strongly influence vascular function.
Autophagy and telomerase
While the vasculature is typically non-replicative, mechanical (e.g. oscillatory/turbulent shear stress) and chemical (e.g. oxLDL) injury to the vascular wall elicits replication and proliferation that precede atherosclerosis. Continued replication or division of cells is mediated by specialized structures called telomeres, located on the end of chromosomes that act to maintain stability and functionality while undergoing replication or division [111]. Subsequent release of ROS due to telomere dysfunction (shortening/uncapping) necessitates that autophagy responds to damaged cellular organelles. Telomerase is comprised of an RNA component, TERC and a catalytic component, telomerase reverse transcriptase (TERT), that work in concert to counteract telomere shortening and maintaining telomere length. Telomerase and its subunits have been described as nuclear-specific, however more recent evidence has demonstrated non-canonical roles for TERT within the mitochondria [128, 129]. TERT may be reversibly shuttled from the nucleus and translocated to the mitochondria where it exerts a protective effect in response to oxidative stress [1, 139]. Administration of anti-oxidants and statins prevent this shuttle [1]. This movement of TERT from the nucleus to mitochondria appears to be dependent upon mTOR signaling as dietary restriction and administration of rapamycin (activate autophagy via mTOR suppression) results in mitochondrial localization of TERT and decreased ROS within mouse brain homogenates and fibroblasts [101]. Taken together, TERT and autophagy hold integral supportive roles within intracellular signaling and cell survival in response to physiological stressors and there appears to be significant intersections between them (Tables 3, 4).
Telomerase and autophagy in the vasculature
In the vasculature, telomere shortening/uncapping induces mitochondria DNA damage, ROS production and is inversely related to forearm endothelium-dependent dilation [104, 120], inflammation, and reductions in NO [130]. Beyer et al. [8] examined whether telomerase activity influences flow-mediated dilation within the microvasculature in subjects with and without coronary artery disease (CAD). Inhibition of telomerase activity with BIBR 1532 in non-CAD vessels had no effect on the magnitude of FMD, but switched the mechanism of dilation from NO to H2O2, similar to that seen with CAD. Conversely, in CAD vessels treated with an activator of telomerase, the primary mechanism of dilation switched from H2O2 to NO identifying a critical role for telomerase in flow-mediated dilation phenotype (Fig. 2) [8].
Mechanistic evidence for the cross-talk between autophagy and telomerase stems from convergence of signaling pathways regarding metabolism. Data from animal models of telomerase deficiency indicate telomere shortening impairs glucose tolerance and insulin secretion [74]. Overexpression of TERT induces autophagy via inhibition of mTOR complex 1 kinase activity under basal and nutrient deprivation conditions, while TERT deficiency impairs autophagic flux [3]. Miwa et al. [101] demonstrated that within the brain of aged mice, TERT protein expression is reduced concomitant with excessive mitochondria-derived ROS, while administration of rapamycin, or dietary restriction ameliorated these effects. These autophagic effects elicited mitochondrial localization of TERT to reduce mitochondria-derived ROS, and more importantly had beneficial effects on memory and learning [101]. Caloric restriction increases both telomerase activity and autophagy resulting in improvements in cardiac function within the heart of diabetic rats [94]. Taken together, autophagy and extra-nuclear telomerase activity appear to share common metabolic pathways with hexokinase 2, the rate limiting catalyst for glucose metabolism, demonstrated to be the molecular transducer connecting these two processes [119]. Future investigations examining the cross-talk between telomerase and autophagy within the context of vascular function would reveal insight into whether these two pathways run in parallel (e.g. both effect vascular function) or whether telomerase acts upstream of autophagy to exert its vascular effects.
Questions, perspectives and future directions
Unanswered questions remain regarding autophagy and vascular function. These include defining the mechanisms by which physiological stressors and pharmacological agents positively and negatively influence vascular autophagic flux, as modulation of autophagic flux in vascular diseases (macro and microvascular disease) may ameliorate disease pathology [37, 107]. As previously highlighted, one challenge in targeting autophagy is its dichotomous nature, whereby the goal is to target maladaptive autophagy while maintaining basal cell survival levels to optimize the beneficial effect. For example, trehalose, a natural disaccharide, is implicated in reducing age and disease-associated endothelial dysfunction and atherosclerotic plaque burden in human and animal models, possibly through nuclear translocation of TFEB [32, 63, 98]. How trehalose/TFEB exert vascular protective effects remains to be investigated. In addition, whether pharmacologically activating autophagy exerts an additive effect in conjunction with a physiological stressor that induces autophagy remains to be interrogated. Activation of autophagy via trehalose ameliorates age-induced endothelial dysfunction. Whether exercise-induced autophagy influences vascular structure and function is a future area of exploration, with one group providing compelling evidence that handgrip-induced increases in shear rate activates endothelial cell autophagy, resulting in increases in NO as well as ROS in young adult males [114]. How this is altered with aging, disease, or exercising training or whether further activation of autophagy enhances the vaso-protective effects of exercise remains to be investigated. Given the evidence that a basal level of autophagy is critical to maintain homeostasis, it is unclear whether activation of autophagy in healthy individuals would impart maladaptive effects. Interrogation of these questions would shed light onto the adaptive vs. maladaptive role of autophagy within the vasculature.
Within skeletal muscle, autophagy holds a critical role in adaptions to exercise. Given that the beneficial effects of exercise and physical activity on the vasculature are mediated by shear stress, it would be interesting to examine whether vascular autophagy (endothelial or VSM) is involved in the structural and functional adaptations to physical activity and exercise training. Cardiac-specific exercise induced protection was recently reviewed elsewhere, concluding that exercise training would enhance basal autophagy and preventing cardiovascular disease in otherwise healthy populations, while exercise training in diseased populations may mitigate excessive autophagy, thereby decreasing the severity of disease [156]. Future directions within the field of autophagy in the vasculature should focus on the functional role of autophagy, specifically delineating the role of autophagic flux in vascular health and function in response to physiological stressors.
Conclusions
Autophagy is an evolutionarily conserved recycling process critical in the maintenance of homeostasis across organ systems. The autophagy signaling cascade is complex, and historically, autophagy has been examined with a focus on cardiomyocytes. Novel translational evidence is emerging on the role of autophagy and the functional ramifications specific to the vasculature system. While autophagy is generally viewed as beneficial, excessive as well as insufficient levels of autophagy may exert detrimental effects within the cardiovascular system. Specific to the vasculature, autophagy may be the crux in determining vascular health and function due to its numerous interactions with other physiological pathways in determining cell fate and survival.
Abbreviations
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- CAD:
-
Coronary artery disease
- eNOS:
-
Endothelial nitric oxide synthase
- FMD:
-
Flow-mediated dilation
- SIRT:
-
Sirtuins
- H2O2 :
-
Hydrogen peroxide
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- mTOR:
-
Mammalian target of rapamycin
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TERT:
-
Telomerase reverse transcriptase
- TFEB:
-
Transcription factor EB
- VSM:
-
Vascular smooth muscle
References
Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G (2008) Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121:1046–1053. https://doi.org/10.1242/jcs.019372
Ait-Aissa K, Heisner JS, Norwood Toro LE, Bruemmer D, Doyon G, Harmann L, Geurts A, Camara AKS, Beyer AM (2019) Telomerase deficiency predisposes to heart failure and ischemia-reperfusion injury. Front Cardiovasc Med 6:31. https://doi.org/10.3389/fcvm.2019.00031
Ali M, Devkota S, Roh JI, Lee J, Lee HW (2016) Telomerase reverse transcriptase induces basal and amino acid starvation-induced autophagy through mTORC1. Biochem Biophys Res Commun 478:1198–1204. https://doi.org/10.1016/j.bbrc.2016.08.094
Arroyo LH, Lee RT (1999) Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res 41:369–375. https://doi.org/10.1016/s0008-6363(98)00308-3
Bejarano E, Cuervo AM (2010) Chaperone-mediated autophagy. Proceedings of the American Thoracic Society 7:29–39. https://doi.org/10.1513/pats.200909-102JS
Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118:692–702. https://doi.org/10.1161/circresaha.115.306361
Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD (2014) An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. American journal of physiology. Heart Circ Physiol 307:H1587–1593. https://doi.org/10.1152/ajpheart.00557.2014
Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD (2016) Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 118:856–866. https://doi.org/10.1161/circresaha.115.307918
Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD (2014) Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol 92:605–612. https://doi.org/10.1139/cjpp-2014-0017
Bharath LP, Cho JM, Park SK, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD (2017) Endothelial cell autophagy maintains shear stress-induced nitric oxide generation via glycolysis-dependent purinergic signaling to endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 37:1646–1656. https://doi.org/10.1161/atvbaha.117.309510
Bonaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygard O, Nilsen DW, Klow NE, Uchto M, Trovik T, Bendz B, Stavnes S, Bjornerheim R, Larsen AI, Slette M, Steigen T, Jakobsen OJ, Bleie O, Fossum E, Hanssen TA, Dahl-Eriksen O, Njolstad I, Rasmussen K, Wilsgaard T, Nordrehaug JE (2016) Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med 375:1242–1252. https://doi.org/10.1056/NEJMoa1607991
Bravo-San Pedro JM, Kroemer G, Galluzzi L (2017) Autophagy and mitophagy in cardiovascular disease. Circ Res 120:1812–1824. https://doi.org/10.1161/circresaha.117.311082
Buchanan CE, Kadlec AO, Hoch AZ, Gutterman DD, Durand MJ (2017) Hypertension during weight lifting reduces flow-mediated dilation in nonathletes. Med Sci Sports Exerc 49:669–675. https://doi.org/10.1249/mss.0000000000001150
Chabowski DS, Kadlec AO, Ait-Aissa K, Hockenberry JC, Pearson PJ, Beyer AM, Gutterman DD (2018) Lysophosphatidic acid acts on LPA1 receptor to increase H2 O2 during flow-induced dilation in human adipose arterioles. Br J Pharmacol 175:4266–4280. https://doi.org/10.1111/bph.14492
Chatterjee S, Fisher AB (2014) Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal 20:899–913. https://doi.org/10.1089/ars.2013.5624
Chen Y, Azad MB, Gibson SB (2009) Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16:1040–1052. https://doi.org/10.1038/cdd.2009.49
Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY (2010) Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 107:10268–10273. https://doi.org/10.1073/pnas.1003833107
Chen C, Gao JL, Liu MY, Li SL, Xuan XC, Zhang XZ, Zhang XY, Wei YY, Zhen CL, Jin J, Shen X, Dong DL (2017) Mitochondrial fission inhibitors suppress endothelin-1-induced artery constriction. Cel Physiol Biochem Int J Exp Cel Physiol Biochem Pharmacol 42:1802–1811. https://doi.org/10.1159/000479536
Cheng C, Tempel D, Oostlander A, Helderman F, Gijsen F, Wentzel J, van Haperen R, Haitsma DB, Serruys PW, van der Steen AF, de Crom R, Krams R (2008) Rapamycin modulates the eNOS vs. shear stress relationship. Cardiovasc Res 78:123–129. https://doi.org/10.1093/cvr/cvm103
Cheng H, Fan X, Lawson WE, Paueksakon P, Harris RC (2015) Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int 88:85–94. https://doi.org/10.1038/ki.2015.69
Chien S (2007) Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. American journal of physiology. Heart Circ Physiol 292:H1209–1224. https://doi.org/10.1152/ajpheart.01047.2006
Craige SM, Kroller-Schon S, Li C, Kant S, Cai S, Chen K, Contractor MM, Pei Y, Schulz E, Keaney JF Jr (2016) PGC-1alpha dictates endothelial function through regulation of eNOS expression. Sci Rep 6:38210. https://doi.org/10.1038/srep38210
Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, Kong W, Zhu WG, Xu MJ, Wang X (2013) Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int 83:1042–1051. https://doi.org/10.1038/ki.2012.482
De Meyer GR, De Keulenaer GW, Martinet W (2010) Role of autophagy in heart failure associated with aging. Heart Fail Rev 15:423–430. https://doi.org/10.1007/s10741-010-9166-6
DeBosch BJ, Heitmeier MR, Mayer AL, Higgins CB, Crowley JR, Kraft TE, Chi M, Newberry EP, Chen Z, Finck BN, Davidson NO, Yarasheski KE, Hruz PW, Moley KH (2016) Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci Signaling 9:ra21. https://doi.org/10.1126/scisignal.aac5472
Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, Fessel JP, Gamboa JL, Harrison DG, Dikalov SI (2017) Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ Res 121:564–574. https://doi.org/10.1161/circresaha.117.310933
Dodson M, Darley-Usmar V, Zhang J (2013) Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radical Biol Med 63:207–221. https://doi.org/10.1016/j.freeradbiomed.2013.05.014
Dong Q, Xing W, Fu F, Liu Z, Wang J, Liang X, Zhou X, Yang Q, Zhang W, Gao F, Wang S, Zhang H (2016) Tetrahydroxystilbene glucoside inhibits excessive autophagy and improves microvascular endothelial dysfunction in prehypertensive spontaneously hypertensive rats. Am J Chin Med 44:1393–1412. https://doi.org/10.1142/s0192415x16500786
Dong Q, Xing W, Su F, Liang X, Tian F, Gao F, Wang S, Zhang H (2017) Tetrahydroxystilbene glycoside improves microvascular endothelial dysfunction and ameliorates obesity-associated hypertension in obese ZDF rats via inhibition of endothelial autophagy. Cel Physiol Biochem 43:293–307. https://doi.org/10.1159/000480410
Dunlop EA, Tee AR (2014) mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol 36:121–129. https://doi.org/10.1016/j.semcdb.2014.08.006
Durand MJ, Dharmashankar K, Bian JT, Das E, Vidovich M, Gutterman DD, Phillips SA (2015) Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension 65:140–145. https://doi.org/10.1161/hypertensionaha.114.04540
Evans TD, Jeong SJ, Zhang X, Sergin I, Razani B (2018) TFEB and trehalose drive the macrophage autophagy-lysosome system to protect against atherosclerosis. Autophagy 14:724–726. https://doi.org/10.1080/15548627.2018.1434373
Fernandez-Marcos PJ, Nobrega-Pereira S (2016) NADPH: new oxygen for the ROS theory of aging. Oncotarget 7:50814–50815. https://doi.org/10.18632/oncotarget.10744
Fetterman JL, Holbrook M, Flint N, Feng B, Breton-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, Vita JA (2016) Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 247:207–217. https://doi.org/10.1016/j.atherosclerosis.2016.01.043
Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R (2005) Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 112:270–278. https://doi.org/10.1161/circulationaha.104.508937
Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD (2014) Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115:525–532. https://doi.org/10.1161/circresaha.115.303881
Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G (2017) Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discovery 16:487–511. https://doi.org/10.1038/nrd.2017.22
Gatica D, Chiong M, Lavandero S, Klionsky DJ (2015) Molecular mechanisms of autophagy in the cardiovascular system. Circ Res 116:456–467. https://doi.org/10.1161/circresaha.114.303788
Giampieri F, Afrin S, Forbes-Hernandez TY, Gasparrini M, Cianciosi D, Reboredo-Rodriguez P, Varela-Lopez A, Quiles JL, Battino M (2018) Autophagy in human health and disease: novel therapeutic opportunities. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7234
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Gori T (2018) Endothelial function: a short guide for the interventional cardiologist. Int J Mol Sci. https://doi.org/10.3390/ijms19123838
Goryo Y, Kume T, Ueda T, Watanabe M, Yamada R, Neishi Y, Saito Y, Uemura S (2018) Vascular healing response after everolimus-eluting stent implantation in acute coronary syndrome culprit lesions: comparison with implantation in stable angina pectoris. Acta Cardiol Sin 34:124–129. https://doi.org/10.6515/acs.201803_34(2).20171115a
Gottlieb RA, Andres AM, Sin J, Taylor DP (2015) Untangling autophagy measurements: all fluxed up. Circ Res 116:504–514. https://doi.org/10.1161/circresaha.116.303787
Grootaert MOJ, Roth L, Schrijvers DM, De Meyer GRY, Martinet W (2018a) Defective autophagy in atherosclerosis: to die or to senesce? Oxidative Med Cel longevity 2018:7687083. https://doi.org/10.1155/2018/7687083
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY (2018b) Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 114:622–634. https://doi.org/10.1093/cvr/cvy007
Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX (2014) Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng 42:1978–1988. https://doi.org/10.1007/s10439-014-1033-5
Hafizi S, Wang X, Chester AH, Yacoub MH, Proud CG (2004) ANG II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. American journal of physiology. Heart Circ Physiol 287:H1232–1238. https://doi.org/10.1152/ajpheart.00040.2004
Haga M, Yamashita A, Paszkowiak J, Sumpio BE, Dardik A (2003) Oscillatory shear stress increases smooth muscle cell proliferation and Akt phosphorylation. J Vasc Surg 37:1277–1284. https://doi.org/10.1016/s0741-5214(03)00329-x
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. https://doi.org/10.1146/annurev-genet-102808-114910
He L, Zhou Q, Huang Z, Xu J, Zhou H, Lv D, Lu L, Huang S, Tang M, Zhong J, Chen JX, Luo X, Li L, Chen L (2019) PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKalpha and exacerbates atherosclerotic lesions. J Cell Physiol 234:8668–8682. https://doi.org/10.1002/jcp.27527
Headland ML, Clifton PM, Keogh JB (2018) Effect of intermittent energy restriction on flow mediated dilatation, a measure of endothelial function: a short report. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15061166
Horn P, Baars T, Kahlert P, Heiss C, Westenfeld R, Kelm M, Erbel R, Heusch G, Kleinbongard P (2015) Release of intracoronary microparticles during stent implantation into stable atherosclerotic lesions under protection with an aspiration device. PLoS ONE 10:e0124904. https://doi.org/10.1371/journal.pone.0124904
Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T, Matoba S (2013) Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun 4:2308. https://doi.org/10.1038/ncomms3308
Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL (2014) Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci 21:3. https://doi.org/10.1186/1423-0127-21-3
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. https://doi.org/10.1146/annurev.biochem.77.061606.161055
Jiang F (2016) Autophagy in vascular endothelial cells. Clin Exp Pharmacol Physiol 43:1021–1028. https://doi.org/10.1111/1440-1681.12649
Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF (1995) Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91:1314–1319
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48:193–202. https://doi.org/10.1016/j.jacc.2006.03.042
Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C (2005) Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19:419–421. https://doi.org/10.1096/fj.04-2622fje
Kadlec AO, Chabowski DS, Ait-Aissa K, Hockenberry JC, Otterson MF, Durand MJ, Freed JK, Beyer AM, Gutterman DD (2017) PGC-1alpha (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) overexpression in coronary artery disease recruits NO and hydrogen peroxide during flow-mediated dilation and protects against increased intraluminal pressure. Hypertension 70:166–173. https://doi.org/10.1161/hypertensionaha.117.09289
Kadlec AO, Barnes C, Durand MJ, Gutterman DD (2018) Microvascular adaptations to exercise: protective effect of PGC-1 alpha. Am J Hypertens 31:240–246. https://doi.org/10.1093/ajh/hpx162
Kaizuka T, Morishita H, Hama Y, Tsukamoto S, Matsui T, Toyota Y, Kodama A, Ishihara T, Mizushima T, Mizushima N (2016) An autophagic flux probe that releases an internal control. Mol Cell 64:835–849. https://doi.org/10.1016/j.molcel.2016.09.037
Kaplon RE, Hill SD, Bispham NZ, Santos-Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB, Seals DR (2016) Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging 8:1167–1183. https://doi.org/10.18632/aging.100962
Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, Tian R (2011) Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail 4:707–713. https://doi.org/10.1161/circheartfailure.111.961474
Kheloufi M, Vion AC, Hammoutene A, Poisson J, Lasselin J, Devue C, Pic I, Dupont N, Busse J, Stark K, Lafaurie-Janvore J, Barakat AI, Loyer X, Souyri M, Viollet B, Julia P, Tedgui A, Codogno P, Boulanger CM, Rautou PE (2018) Endothelial autophagic flux hampers atherosclerotic lesion development. Autophagy 14:173–175. https://doi.org/10.1080/15548627.2017.1395114
Kida Y, Goligorsky MS (2016) Sirtuins, cell senescence, and vascular aging. Can J Cardiol 32:634–641. https://doi.org/10.1016/j.cjca.2015.11.022
Kiffin R, Bandyopadhyay U, Cuervo AM (2006) Oxidative stress and autophagy. Antioxid Redox Signal 8:152–162. https://doi.org/10.1089/ars.2006.8.152
King JS, Veltman DM, Insall RH (2011) The induction of autophagy by mechanical stress. Autophagy 7:1490–1499
Kitada M, Ogura Y, Koya D (2016) The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging 8:2290–2307. https://doi.org/10.18632/aging.101068
Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, Elter-Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G (2011) Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res 108:344–352. https://doi.org/10.1161/circresaha.110.235713
Kleinbongard P, Konorza T, Bose D, Baars T, Haude M, Erbel R, Heusch G (2012) Lessons from human coronary aspirate. J Mol Cell Cardiol 52:890–896. https://doi.org/10.1016/j.yjmcc.2011.06.022
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algul H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso C, Altan-Bonnet N, Altieri DC, Alvarez S, Alvarez-Erviti L, Alves S, Amadoro G, Amano A, Amantini C, Ambrosio S, Amelio I, Amer AO, Amessou M, Amon A, An Z, Anania FA, Andersen SU, Andley UP, Andreadi CK, Andrieu-Abadie N, Anel A, Ann DK, Anoopkumar-Dukie S, Antonioli M, Aoki H, Apostolova N, Aquila S, Aquilano K, Araki K, Arama E, Aranda A, Araya J, Arcaro A, Arias E, Arimoto H, Ariosa AR, Armstrong JL, Arnould T, Arsov I, Asanuma K, Askanas V, Asselin E, Atarashi R, Atherton SS, Atkin JD, Attardi LD, Auberger P, Auburger G, Aurelian L, Autelli R, Avagliano L, Avantaggiati ML, Avrahami L, Awale S, Azad N, Bachetti T, Backer JM, Bae DH, Bae JS, Bae ON, Bae SH, Baehrecke EH, Baek SH, Baghdiguian S, Bagniewska-Zadworna A et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222. https://doi.org/10.1080/15548627.2015.1100356
Kubli DA, Quinsay MN, Gustafsson AB (2013) Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commu Integr Biol 6:e24511. https://doi.org/10.4161/cib.24511
Kuhlow D, Florian S, von Figura G, Weimer S, Schulz N, Petzke KJ, Zarse K, Pfeiffer AF, Rudolph KL, Ristow M (2010) Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging 2:650–658. https://doi.org/10.18632/aging.100200
Kumar V, Wollner C, Kurth T, Bukowy JD, Cowley AW Jr (2017) Inhibition of mammalian target of rapamycin complex 1 attenuates salt-induced hypertension and kidney injury in dahl salt-sensitive rats. Hypertension 70:813–821. https://doi.org/10.1161/hypertensionaha.117.09456
Kurdi A, De Meyer GR, Martinet W (2016) Potential therapeutic effects of mTOR inhibition in atherosclerosis. Br J Clin Pharmacol 82:1267–1279. https://doi.org/10.1111/bcp.12820
Kurdi A, Martinet W, De Meyer GRY (2018) mTOR inhibition and cardiovascular diseases: dyslipidemia and atherosclerosis. Transplantation 102:S44–s46. https://doi.org/10.1097/tp.0000000000001693
Lampert MA, Gustafsson AB (2018) Balancing autophagy for a healthy heart. Cur Opin Physiol 1:21–26. https://doi.org/10.1016/j.cophys.2017.11.001
LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR (2012) Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590:3305–3316. https://doi.org/10.1113/jphysiol.2012.229690
LaRocca TJ, Gioscia-Ryan RA, Hearon CM Jr, Seals DR (2013) The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134:314–320. https://doi.org/10.1016/j.mad.2013.04.004
Lavandero S, Chiong M, Rothermel BA, Hill JA (2015) Autophagy in cardiovascular biology. J Clin Investig 125:55–64. https://doi.org/10.1172/jci73943
Lee SJ, Smith A, Guo L, Alastalo TP, Li M, Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, Feghali-Bostwick C, Ryter SW, Kim HP, Rabinovitch M, Choi AM (2011) Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 183:649–658. https://doi.org/10.1164/rccm.201005-0746OC
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540. https://doi.org/10.1042/bj20111451
Li H, Li J, Li Y, Singh P, Cao L, Xu LJ, Li D, Wang Y, Xie Z, Gui Y, Zheng XL (2012) Sonic hedgehog promotes autophagy of vascular smooth muscle cells. American journal of physiology. Heart Circ Physiol 303:H1319–1331. https://doi.org/10.1152/ajpheart.00160.2012
Li R, Jen N, Wu L, Lee J, Fang K, Quigley K, Lee K, Wang S, Zhou B, Vergnes L, Chen YR, Li Z, Reue K, Ann DK, Hsiai TK (2015) Disturbed flow induces autophagy, but impairs autophagic flux to perturb mitochondrial homeostasis. Antioxid Redox Signal 23:1207–1219. https://doi.org/10.1089/ars.2014.5896
Li J, Chen T, Xiao M, Li N, Wang S, Su H, Guo X, Liu H, Yan F, Yang Y, Zhang Y, Bu P (2016) Mouse Sirt3 promotes autophagy in AngII-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget 7:86648–86659. https://doi.org/10.18632/oncotarget.13429
Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I (2012) Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15:545–553. https://doi.org/10.1016/j.cmet.2012.01.022
Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD (2003) Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93:573–580. https://doi.org/10.1161/01.Res.0000091261.19387.Ae
Liu D, Cui W, Liu B, Hu H, Liu J, Xie R, Yang X, Gu G, Zhang J, Zheng H (2014) Atorvastatin protects vascular smooth muscle cells from TGF-beta1-stimulated calcification by inducing autophagy via suppression of the beta-catenin pathway. Cel Physiol Biochem 33:129–141. https://doi.org/10.1159/000356656
Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ, Liu GS, Zhang Y, Bu P, Jiang F (2015) Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis 6:e1827. https://doi.org/10.1038/cddis.2015.193
Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW (2013) Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112:1159–1170. https://doi.org/10.1161/circresaha.111.300483
Lugus JJ, Ngoh GA, Bachschmid MM, Walsh K (2011) Mitofusins are required for angiogenic function and modulate different signaling pathways in cultured endothelial cells. J Mol Cell Cardiol 51:885–893. https://doi.org/10.1016/j.yjmcc.2011.07.023
Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochem Biophys Acta 1852:271–276. https://doi.org/10.1016/j.bbadis.2014.05.010
Makino N, Oyama J, Maeda T, Koyanagi M, Higuchi Y, Tsuchida K (2015) Calorie restriction increases telomerase activity, enhances autophagy, and improves diastolic dysfunction in diabetic rat hearts. Mol Cell Biochem 403:1–11. https://doi.org/10.1007/s11010-015-2327-0
Martinet W, De Loof H, De Meyer GR (2014) mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 233:601–607. https://doi.org/10.1016/j.atherosclerosis.2014.01.040
Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K (2007) SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104:14855–14860. https://doi.org/10.1073/pnas.0704329104
Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M (2006) Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99:156–164. https://doi.org/10.1161/01.RES.0000233315.38086.bc
McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B, Webb RC (2019) Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats. American journal of physiology. Heart Circ Physiol 317:H1013–h1027. https://doi.org/10.1152/ajpheart.00227.2019
Mei Y, Thompson MD, Cohen RA, Tong X (2015) Autophagy and oxidative stress in cardiovascular diseases. Biochem Biophys Acta 1852:243–251. https://doi.org/10.1016/j.bbadis.2014.05.005
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121:505–511. https://doi.org/10.1161/circulationaha.109.886655
Miwa S, Czapiewski R, Wan T, Bell A, Hill KN, von Zglinicki T, Saretzki G (2016) Decreased mTOR signalling reduces mitochondrial ROS in brain via accumulation of the telomerase protein TERT within mitochondria. Aging 8:2551–2567. https://doi.org/10.18632/aging.101089
Mizoguchi T, Sawada T, Shinke T, Yamada S, Okamoto H, Kim SS, Takarada A, Yasaka Y (2014) Detailed comparison of intra-stent conditions 12 months after implantation of everolimus-eluting stents in patients with ST-segment elevation myocardial infarction or stable angina pectoris. Int J Cardiol 171:224–230. https://doi.org/10.1016/j.ijcard.2013.12.021
Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132. https://doi.org/10.1146/annurev-cellbio-092910-154005
Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ (2013) Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. American journal of physiology. Heart Circ Physiol 305:H251–258. https://doi.org/10.1152/ajpheart.00197.2013
Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, Lopez-Otin C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G (2011) Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 192:615–629. https://doi.org/10.1083/jcb.201008167
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. https://doi.org/10.1083/jcb.200809125
Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA (2011) Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol 51:584–593. https://doi.org/10.1016/j.yjmcc.2011.06.010
Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, Giachelli CM, Parmacek MS, Raines EW, Rusch NJ, Speer MY, Sturek M, Thyberg J, Towler DA, Weiser-Evans MC, Yan C, Miano JM, Owens GK (2013) Smooth muscle cell plasticity: fact or fiction? Circ Res 112:17–22. https://doi.org/10.1161/circresaha.112.281048
Nussenzweig SC, Verma S, Finkel T (2015) The role of autophagy in vascular biology. Circ Res 116:480–488. https://doi.org/10.1161/circresaha.116.303805
Osonoi Y, Mita T, Azuma K, Nakajima K, Masuyama A, Goto H, Nishida Y, Miyatsuka T, Fujitani Y, Koike M, Mitsumata M, Watada H (2018) Defective autophagy in vascular smooth muscle cells enhances cell death and atherosclerosis. Autophagy 14:1991–2006. https://doi.org/10.1080/15548627.2018.1501132
O'Sullivan RJ, Karlseder J (2010) Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11:171–181. https://doi.org/10.1038/nrm2848
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424. https://doi.org/10.1152/physrev.00029.2006
Palumbo R, Gaetano C, Antonini A, Pompilio G, Bracco E, Ronnstrand L, Heldin CH, Capogrossi MC (2002) Different effects of high and low shear stress on platelet-derived growth factor isoform release by endothelial cells: consequences for smooth muscle cell migration. Arterioscler Thromb Vasc Biol 22:405–411. https://doi.org/10.1161/hq0302.104528
Park SK, La Salle DT, Cerbie J, Cho JM, Bledsoe AD, Nelson AD, Morgan DE, Richardson RS, Shiu YT, Boudina S, Trinity JD, Symons JD (2018) Elevated arterial shear rate increases indices of endothelial cell autophagy and nitric oxide synthase activation in humans Heart and circulatory physiology. Am J Physiol. https://doi.org/10.1152/ajpheart.00561.2018
Pestana CR, Oishi JC, Salistre-Araujo HS, Rodrigues GJ (2015) Inhibition of autophagy by chloroquine stimulates nitric oxide production and protects endothelial function during serum deprivation. Cel Physiol Biochem 37:1168–1177. https://doi.org/10.1159/000430240
Phillips SA, Hatoum OA, Gutterman DD (2007) The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. American journal of physiology. Heart Circ Physiol 292:H93–100. https://doi.org/10.1152/ajpheart.00819.2006
Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF (2012) Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 15:534–544. https://doi.org/10.1016/j.cmet.2012.02.011
Rogers MA, Maldonado N, Hutcheson JD, Goettsch C, Goto S, Yamada I, Faits T, Sesaki H, Aikawa M, Aikawa E (2017) Dynamin-related protein 1 inhibition attenuates cardiovascular calcification in the presence of oxidative stress. Circ Res 121:220–233. https://doi.org/10.1161/circresaha.116.310293
Roh JI, Kim Y, Oh J, Kim Y, Lee J, Lee J, Chun KH, Lee HW (2018) Hexokinase 2 is a molecular bridge linking telomerase and autophagy. PLoS ONE 13:e0193182. https://doi.org/10.1371/journal.pone.0193182
Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ (2017) Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. American journal of physiology. Heart Circ Physiol 313:H890–h895. https://doi.org/10.1152/ajpheart.00416.2017
Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL (2015) Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med (Berlin, Germany) 93:229–242. https://doi.org/10.1007/s00109-015-1263-5
Ryter SW, Lee SJ, Smith A, Choi AM (2010) Autophagy in vascular disease. Proc Am Thorac Soc 7:40–47. https://doi.org/10.1513/pats.200909-100JS
Saha S, Panigrahi DP, Patil S, Bhutia SK (2018) Autophagy in health and disease: a comprehensive review. Biomed Pharmacother Biomed Pharmacother 104:485–495. https://doi.org/10.1016/j.biopha.2018.05.007
Salabei JK, Conklin DJ (2013) Cardiovascular autophagy: crossroads of pathology, pharmacology and toxicology. Cardiovasc Toxicol 13:220–229. https://doi.org/10.1007/s12012-013-9200-8
Salabei JK, Hill BG (2013a) Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol 1:542–551. https://doi.org/10.1016/j.redox.2013.10.011
Salabei JK, Hill BG (2013b) Implications of autophagy for vascular smooth muscle cell function and plasticity. Free Radical Biol Med 65:693–703. https://doi.org/10.1016/j.freeradbiomed.2013.08.003
Salabei JK, Hill BG (2015) Autophagic regulation of smooth muscle cell biology. Redox Biol 4:97–103. https://doi.org/10.1016/j.redox.2014.12.007
Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B (2004) Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3:399–411. https://doi.org/10.1111/j.1474-9728.2004.00124.x
Santos JH, Meyer JN, Van Houten B (2006) Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Genet 15:1757–1768. https://doi.org/10.1093/hmg/ddl098
Sato I, Morita I, Kaji K, Ikeda M, Nagao M, Murota S (1993) Reduction of nitric oxide producing activity associated with in vitro aging in cultured human umbilical vein endothelial cell. Biochem Biophys Res Commun 195:1070–1076. https://doi.org/10.1006/bbrc.1993.2153
Schiattarella GG, Hill JA (2016) Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol 95:86–93. https://doi.org/10.1016/j.yjmcc.2015.11.019
Schrijvers DM, De Meyer GR, Martinet W (2011) Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 31:2787–2791. https://doi.org/10.1161/atvbaha.111.224899
Sciarretta S, Maejima Y, Zablocki D, Sadoshima J (2018) The role of autophagy in the heart. Annu Rev Physiol 80:1–26. https://doi.org/10.1146/annurev-physiol-021317-121427
Seals DR, Jablonski KL, Donato AJ (2011) Aging and vascular endothelial function in humans. Clin Sci (London, England: 1979) 120:357–375. https://doi.org/10.1042/cs20100476
Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, Javaheri A, Crowley JR, Ballabio A, Schilling JD, Epelman S, Weihl CC, Diwan A, Fan D, Zayed MA, Razani B (2017) Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat Commun 8:15750. https://doi.org/10.1038/ncomms15750
Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA (2011) Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124:444–453. https://doi.org/10.1161/circulationaha.110.014506
Shi N, Chen SY (2014) Mechanisms simultaneously regulate smooth muscle proliferation and differentiation. J Biomed Res 28:40–46. https://doi.org/10.7555/jbr.28.20130130
Simsek C, Magro M, Boersma E, Onuma Y, Nauta ST, Gaspersz MP, van der Giessen WJ, van Domburg RT, Serruys PW (2010) The unrestricted use of sirolimus- and paclitaxel-eluting stents results in better clinical outcomes during 6-year follow-up than bare-metal stents: an analysis of the RESEARCH (Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital) and T-SEARCH (Taxus-Stent Evaluated At Rotterdam Cardiology Hospital) registries. JACC Cardiovasc Interv 3:1051–1058. https://doi.org/10.1016/j.jcin.2010.08.003
Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC (2013) Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 8:e52989. https://doi.org/10.1371/journal.pone.0052989
Stuck BJ, Lenski M, Bohm M, Laufs U (2008) Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem 283:32562–32569. https://doi.org/10.1074/jbc.M801904200
Swiader A, Nahapetyan H, Faccini J, D'Angelo R, Mucher E, Elbaz M, Boya P, Vindis C (2016) Mitophagy acts as a safeguard mechanism against human vascular smooth muscle cell apoptosis induced by atherogenic lipids. Oncotarget 7:28821–28835. https://doi.org/10.18632/oncotarget.8936
Tekirdag K, Cuervo AM (2018) Chaperone-mediated autophagy and endosomal microautophagy: Joint by a chaperone. J Biol Chem 293:5414–5424. https://doi.org/10.1074/jbc.R117.818237
Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT (2010) Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal 12:503–535. https://doi.org/10.1089/ars.2009.2598
Torisu K, Singh KK, Torisu T, Lovren F, Liu J, Pan Y, Quan A, Ramadan A, Al-Omran M, Pankova N, Boyd SR, Verma S, Finkel T (2016) Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell 15:187–191. https://doi.org/10.1111/acel.12423
Vara D, Pula G (2014) Reactive oxygen species: physiological roles in the regulation of vascular cells. Curr Mol Med 14:1103–1125
Verheye S, Martinet W, Kockx MM, Knaapen MW, Salu K, Timmermans JP, Ellis JT, Kilpatrick DL, De Meyer GR (2007) Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol 49:706–715. https://doi.org/10.1016/j.jacc.2006.09.047
Vion AC, Kheloufi M, Hammoutene A, Poisson J, Lasselin J, Devue C, Pic I, Dupont N, Busse J, Stark K, Lafaurie-Janvore J, Barakat AI, Loyer X, Souyri M, Viollet B, Julia P, Tedgui A, Codogno P, Boulanger CM, Rautou PE (2017) Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc Natl Acad Sci USA 114:E8675–e8684. https://doi.org/10.1073/pnas.1702223114
Wang L, Yu T, Lee H, O'Brien DK, Sesaki H, Yoon Y (2015) Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovasc Res 106:272–283. https://doi.org/10.1093/cvr/cvv005
Wang YT, Li X, Chen J, McConnell BK, Chen L, Li PL, Chen Y, Zhang Y (2019) Activation of TFEB ameliorates dedifferentiation of arterial smooth muscle cells and neointima formation in mice with high-fat diet. Cell Death Dis 10:676. https://doi.org/10.1038/s41419-019-1931-4
Wei H, Liu L, Chen Q (2015) Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochem Biophys Acta 1853:2784–2790. https://doi.org/10.1016/j.bbamcr.2015.03.013
Wei T, Huang G, Gao J, Huang C, Sun M, Wu J, Bu J, Shen W (2017) Sirtuin 3 deficiency accelerates hypertensive cardiac remodeling by impairing angiogenesis. J Am Heart Assoc. https://doi.org/10.1161/jaha.117.006114
Widlansky ME, Gutterman DD (2011) Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15:1517–1530. https://doi.org/10.1089/ars.2010.3642
Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A (1998) Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem 273:28510–28515
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM (2010) Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285:10850–10861. https://doi.org/10.1074/jbc.M109.080796
Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, Cui Z, Cui T, Wang XL, Shen YH (2015) PINK1-Parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PLoS ONE 10:e0132499. https://doi.org/10.1371/journal.pone.0132499
Wu NN, Tian H, Chen P, Wang D, Ren J, Zhang Y (2019) Physical exercise and selective autophagy: benefit and risk on cardiovascular health. Cells. https://doi.org/10.3390/cells8111436
Xiao Q, Che X, Cai B, Tao Z, Zhang H, Shao Q, Pu J (2020) Macrophage autophagy regulates mitochondria-mediated apoptosis and inhibits necrotic core formation in vulnerable plaques. J Cell Mol Med 24:260–275. https://doi.org/10.1111/jcmm.14715
Xie Y, You SJ, Zhang YL, Han Q, Cao YJ, Xu XS, Yang YP, Li J, Liu CF (2011) Protective role of autophagy in AGE-induced early injury of human vascular endothelial cells. Mol Med Rep 4:459–464. https://doi.org/10.3892/mmr.2011.460
Xu K, Yang Y, Yan M, Zhan J, Fu X, Zheng X (2010) Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J Lipid Res 51:2581–2590. https://doi.org/10.1194/jlr.M005702
Xu Q, Li X, Lu Y, Shen L, Zhang J, Cao S, Huang X, Bin J, Liao Y (2015) Pharmacological modulation of autophagy to protect cardiomyocytes according to the time windows of ischaemia/reperfusion. Br J Pharmacol 172:3072–3085. https://doi.org/10.1111/bph.13111
Yamamoto K, Imamura H, Ando J (2018) Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca(2+) signaling in vascular endothelial cells heart and circulatory physiology. Am J Physiol. https://doi.org/10.1152/ajpheart.00204.2018
Yan Y, Finkel T (2017) Autophagy as a regulator of cardiovascular redox homeostasis. Free Radical Biol Med 109:108–113. https://doi.org/10.1016/j.freeradbiomed.2016.12.003
Young A, Wu W, Sun W, Benjamin Larman H, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G (2009) Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol 29:1902–1908. https://doi.org/10.1161/atvbaha.109.193540
Yu EPK, Reinhold J, Yu H, Starks L, Uryga AK, Foote K, Finigan A, Figg N, Pung YF, Logan A, Murphy MP, Bennett M (2017) Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol 37:2322–2332. https://doi.org/10.1161/atvbaha.117.310042
Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T, Xiao GD, Yang YP, Liu CF (2010) The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun 394:377–382. https://doi.org/10.1016/j.bbrc.2010.03.026
Zhang Y, Morgan MJ, Chen K, Choksi S, Liu ZG (2012) Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 119:2895–2905. https://doi.org/10.1182/blood-2011-08-372383
Zhang JX, Qu XL, Chu P, Xie DJ, Zhu LL, Chao YL, Li L, Zhang JJ, Chen SL (2018) Low shear stress induces vascular eNOS uncoupling via autophagy-mediated eNOS phosphorylation. Biochimica et biophysica acta. Mol Cell Res 1865:709–720. https://doi.org/10.1016/j.bbamcr.2018.02.005
Zhao K, Xu XS, Meng X, Li YL, Li JF, Chen WQ (2013) Autophagy of monocytes attenuates the vulnerability of coronary atherosclerotic plaques. Coron Artery Dis 24:651–656. https://doi.org/10.1097/mca.0000000000000035
Zhou W, Chen KH, Cao W, Zeng J, Liao H, Zhao L, Guo X (2010) Mutation of the protein kinase A phosphorylation site influences the anti-proliferative activity of mitofusin 2. Atherosclerosis 211:216–223. https://doi.org/10.1016/j.atherosclerosis.2010.02.012
Zhou L, Ma B, Han X (2016) The role of autophagy in angiotensin II-induced pathological cardiac hypertrophy. J Mol Endocrinol 57:R143–r152. https://doi.org/10.1530/jme-16-0086
Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA (2007) Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Investig 117:1782–1793. https://doi.org/10.1172/jci27523
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950. https://doi.org/10.1152/physrev.00026.2013
Funding
This work was supported by the National Institutes of Health Grants T32GM089586 and AHA 20POST35050017 (W.E.H); R01-HL-133029 (A.M. Beyer); R01-HL-135901-01 (D.D. Gutterman).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Hughes, W.E., Beyer, A.M. & Gutterman, D.D. Vascular autophagy in health and disease. Basic Res Cardiol 115, 41 (2020). https://doi.org/10.1007/s00395-020-0802-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-020-0802-6