Abstract
Purpose
Genistein (GEN) has been reported to have diverse biological activities, including antioxidant, hypolipidemic, and antidiabetic effects. This study investigated whether the ameliorative effects of GEN on inflammation and insulin resistance were associated with the modulation of gut microbiota composition in type 2 diabetic (T2D) mice.
Methods
C57BL/6J mice were treated with a high-fat diet/streptozotocin to induce T2D and then gavaged with GEN (20 and 40 mg/kg) for 8 weeks. Then, oral glucose tolerance, fasting blood glucose, serum insulin, glucagon, lipid profiles, and pro-inflammatory factors were measured. After this, hepatic function and histopathological analysis and inflammation-related indices of the liver and colon were determined, along with short-chain fatty acid (SCFA) and gut microbiota composition.
Results
GEN treatment decreased hyperglycemia, hyperlipidemia, and serum pro-inflammatory factor levels and attenuated hepatic dysfunction, pathological changes, inflammation-related protein expression, and hepatocyte apoptosis. It also ameliorated colonic pathological changes, tight junction-associated protein expression, and pro-inflammatory factor increases. Furthermore, high-dose GEN treatment increased the concentrations of SCFAs and down-regulated the ratio of Firmicutes/Bacteroidetes and the abundance of Proteobacteria at the phylum level. However, GEN increased the abundances of Bacteroides and Prevotella and decreased the levels of Helicobacter and Ruminococcus at the genus level in T2D mice.
Conclusion
GEN showed ameliorative effects on glucose and lipid dysmetabolism and hepatic and colonic dysfunction; most importantly, GEN could ameliorate inflammation and insulin resistance through modulation of gut microbiota composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus, commonly referred to as diabetes, is a cluster of chronic metabolic abnormalities, comprising type 1 diabetes, type 2 diabetes (T2D), gestational diabetes, and other rare forms of diabetes [1]. Of these, T2D accounts for around 90% of all cases and is associated with high rates of mortality due to the presence of many serious complications, such as nephropathy, retinopathy, and cardiovascular disease [2, 3]. Although the pathogenesis of T2D is very complicated, it is clear that insulin secretion deficiency of pancreatic beta cells and insulin resistance (IR) are the two major causes [4, 5].
Emerging data suggest that gut microbiota–host interactions play an important role in the pathophysiology of T2D and regulate glucose homeostasis and IR [6]. Dysbiosis of gut microbiota in T2D may cause systemic low-grade chronic inflammation, which is an important cause in the pathogenesis of IR [7, 8]. Furthermore, one of the mechanisms by which the microbiota affects host health and disease is its ability to produce either beneficial metabolites, to prevent disease, or harmful metabolites related to disease development [9]. On the one hand, some carbohydrates and proteins, which escape digestion in the upper gut, can be metabolized by the microbiota in the colon. The major of beneficial metabolites produced by microbial fermentation are short-chain fatty acids (SCFAs), including mainly acetate, propionate, and butyrate, which are tightly related in maintaining intestinal integrity and health [10]. However, lipopolysaccharide (LPS), which is an important component of cell walls of Gram-negative bacteria, plays a crucial role in the progression of inflammation and IR [11]. Intestine-derived LPS, also considered as endotoxin, may enter the blood through the damaged intestinal mucosa and then cause systemic inflammation [12]. There is evidence that subjects with T2D have elevated LPS levels, inflammation, and IR [13]. Thus, the change in gut microbiota composition is tightly implicated in the development of T2D [14].
In the recent years, some naturally occurring substances, which have a variety of advantages, including diverse biological activities and low toxicity, have been considered to be valuable resources for the development of new therapeutic agents [15]. Genistein (GEN: 4′,5,7-trihydroxyisoflavone) is an active isoflavone, abundantly present in soy products [16]. Due to its structural similarity to 17β-estradiol, it is also known as a phytoestrogen [17]. Accumulating evidence has shown that GEN has diverse biological properties, including antioxidant, hypolipidemic, antiapoptotic, and anticancer effects [18,19,20]. A recent study showed that GEN administration ameliorated the inflammation and IR in rats fed with fructose [21]. Furthermore, a separate study found that long-term GEN treatment, regulated the gut microbiota and decreased the neuroinflammatory response in obese mice [22]. However, it remains unclear whether GEN exerts ameliorative effects on inflammation and IR through regulation of gut microbial composition in T2D. Hence, this research aimed to determine the beneficial effects of GEN in mice with T2D and explore the underlying mechanisms of action.
Materials and methods
Animals
A total of 32 healthy male C57BL/6J mice (age, 5–6 weeks; weight, 18–22 g) were provided by the Animal Center of Anhui Medical University (Hefei, China). The animals were kept under environmentally controlled conditions at a temperature of 22–24 °C, a relative humidity of 50–60%, and an alternating 12-h light/dark cycle circumstance. All the mice were fed with water and food ad libitum. The animal protocols were approved by the Laboratory Animal Ethics Committee of Bengbu Medical College and carried out as per ethical standards.
Experimental design
The mice were acclimatized to laboratory conditions for seven days before the onset of the experiments. A mouse model of T2D was established based on previous research with some modifies [23]. All the healthy mice were assigned randomly into a normal (NC) group (standard laboratory rodent diet, 8 mice) and an HFD group (high-fat diet: 10% lard, 20% white sugar, 0.2% pig bile salt, 3% cholesterol, and 66.8% standard laboratory rodent diet, the specifications of the diet were listed in Table 1; 24 mice). After respective diets feeding for 4 weeks, mice in the HFD group were deprived of food for 14 h and intraperitoneally injected with 40 mg/kg streptozotocin (STZ, Sigma-Aldrich, USA; freshly dissolved in pre-cooling citrate buffer at pH 4.4) for 5 consecutive days, while mice in the NC group were treated with the equal amount of citrate buffer in parallel. Three days after the last injection, the fasting blood glucose (FBG) level from the tail vein was measured using a portable glucometer (Roche, Basel, Switzerland). A mouse with FBG over 11.1 mmol/L was considered diabetic and used for the next experiment.
After successfully establishing models, mice in the HFD group were randomly subdivided into three groups (8 mice each): (i) NC and T2D groups were daily gavaged with 0.5% carboxymethylcellulose sodium (CMC-Na, Sangon Biotechnology, Shanghai, China) solution; (ii) LGEN group was daily gavaged with 20 mg/kg GEN (Sigma-Aldrich, USA; freshly prepared in 0.5% CMC-Na solution); (iii) HGEN group was daily gavaged with 40 mg/kg GEN [24]. These mice were fed with respective diets throughout the whole experimental period. Body weight and FBG were monitored weekly. After treatment for 8 weeks, the overnight fast mice were weighed and anesthetized with isoflurane. The blood was gathered by cardiac puncture to collect serum. After the mice were sacrificed, their livers and colons were excised immediately and weighed. The ratio of liver weight (mg)/body weight (g) (hepatic index) was calculated.
Oral glucose tolerance test (OGTT)
One day before the end of the experiment, the OGTT was conducted to evaluate glucose tolerance in 12 h-fasted mice. These mice were gavaged with 2 g/kg d-glucose. The blood samples were obtained from the tail vein of the mice at 0 min (just before glucose load), 15, 30, 60, 90, and 120 min after oral glucose administration. Blood glucose concentration was measured using the glucometer and then the area under the curve (AUC) was reckoned [25].
Detection of serum biochemical indices
Serum FBG, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured using the assay kits (Jiancheng Biotechnology, Nanjing, China). Serum insulin, LPS, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 were measured using corresponding commercially enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotechnology, Wuhan, China) as per manufacturer's protocols. The serum glucagon level was measured using an ELISA kit (Jiancheng Biotechnology). Homeostasis model assessment (HOMA)-IR was calculated [25].
Morphological examination
The fresh liver and colon were fixed in 4% formaldehyde, mounted in paraffin, and subsequently processed into 4-μm-thickness paraffin slices. These slices were dewaxed and stained with hematoxylin and eosin (H&E) for histological examination. The hepatic slices were also stained with Masson's trichrome and periodic acid-Schiff (PAS) reagents.
The hepatic tissues soaked in 4% paraformaldehyde were processed for frozen analysis. These tissues were cut into 9 μm-thickness slices and then stained with oil red O (ORO) reagent. All the images were captured using a NanoZoomer digital pathology slide scanner (DPSS).
Immunohistochemical (IHC) assay
The paraffin-embedded hepatic slices were dewaxed in xylene, rehydrated with a series of gradient ethanol, and treated with hydrogen peroxide to eliminate peroxidase activities. After treating with bovine serum albumin, these slices were probed with rabbit antinuclear factor (NF)-κB p65 (Proteintech Group, Wuhan, China) and nucleotide-binding oligomerization domain-like receptor protein (NLRP) 3 (Boster Biotechnology, Wuhan, China) primary antibodies. Next, these slices were probed with a secondary antirabbit antibody (Boster Biotechnology), and stained with diaminobenzidine and hematoxylin. IHC results were imaged using DPSS. Five fields of each slice were selected randomly and the number of positively stained cells was reckoned using Image-pro Plus (IPP) software.
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assessment
TUNEL staining was assessed to evaluate hepatocyte apoptosis using an in situ cell death assay kit (Roche) as per manufacturer's instruction. In general, the liver sections mounted in the wax were dewaxed, rehydrated, and incubated with proteinase K. After receiving three washes with phosphate buffer saline, these sections were covered with the reaction mixture and subsequently treated with converter-POD, diaminobenzidine, and hematoxylin. The average number of brown-labeled positive cells in every section was reckoned using IPP software.
Determination of tissue inflammatory factors
Hepatic and colonic tissues were homogenized in a nine-fold volume of chilled saline solution. After centrifugation, the supernatant fluids were collected to measure the protein concentrations using a bicinchoninic acid assay kit (Jiancheng Biotechnology). TNF-α, IL-1β, and IL-18 levels were determined using the corresponding ELISA kits (Cusabio Biotechnology).
Western blot analysis
Colonic tissues were homogenized with a radioimmunoprecipitation assay buffer containing 1 mmol/L phenylmethanesulfonyl fluoride (Beyotime Biotechnology, Shanghai, China). After centrifugation, the supernatant was collected to detect the protein content. Equal amounts of protein were electrophoresed onto 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and thereafter transferred onto polyvinylidene fluoride membranes (Millipore; Burlington, MA, USA). After immersing with 5% skim milk powder diluted in tris-buffered saline Tween-20 (TBST), the membranes were probed overnight with primary antibodies directly against occludin, zonula occludens-1 (ZO-1), and GAPDH (all purchased from Boster Biotechnology) at 4 °C. After three washes with TBST, membranes were incubated with horseradish peroxidase-conjugated IgG secondary antibody. The protein bands were visualized with enhanced chemiluminescence reagents (Millipore) and captured with a gel-imaging system (Tanon Technology, Shanghai, China). The relative band density was normalized to that of the GAPDH loading control and analyzed using Quantity One software.
SCFAs analysis
One day before the end of the experiment, the feces from each mouse were collected and frozen at − 70 °C. Acetate, propionate, and butyrate in fecal samples were analyzed using gas chromatography–mass spectrometry (GC–MS) [26]. Briefly, the feces were homogenized with a saturated sodium chloride solution and acidified with 10% sulfuric acid. Next, diethyl ether was used to extract SCFAs. After centrifugation, the supernatants were harvested for GC–MS.
Gut microbiota analysis
Fecal DNA was isolated using an E.Z.N.A. Stool DNA Kit (Omega, USA) as per manufacturer's instruction. The variable regions 3–4 of the 16S rRNA gene were amplified using a forward primer 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR products were purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA) and quantified by Qubit (Invitrogen). The amplicon pools were prepared for sequencing and the size and the quantity of the amplicon library were assessed on Agilent 2100 Bioanalyzer (Agilent, CA, USA) and with the Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA). 16S rRNA gene sequences were analyzed on an Illumina MiSeq platform. Paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Paired-end reads were merged using FLASH. Quality filtering on the raw tags was performed under specific filtering conditions to obtain high-quality clean tags according to the Fqtrim (Version 0.94). Chimeric sequences were filtered and the sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs) using Vsearch software (Version 2.3.4). Representative sequences were chosen for each OTU, and taxonomic data were then assigned to each representative sequence using the ribosomal database project classifier. The differences of the dominant species in different groups and multiple sequence alignments were conducted using the Mafft software (Version 7.310) to study the phylogenetic relationship of different OTUs. OTUs abundance information was normalized using a standard of sequence number corresponding to the sample with the least sequences. Alpha diversity including Chao1 and Shannon indices and beta diversity including principal coordinates analysis (PCoA) were calculated using QIIME (Version 1.8.0).
Statistical analysis
Experimental results were expressed as the mean ± standard deviation (SD). The data were analyzed using an one-way analysis of variance following the Newman–Keuls test among multiple groups. Student's t test was performed to assess the differences between the two groups. P < 0.05 was considered to suggest a statistically significant difference.
Results
Effects of GEN on OGTT and serum biochemical indices
The results of the OGTT (Fig. 1a, b) showed that the blood glucose concentration reached the highest level within 15–30 min, and subsequently gradually declined. Normal mice were more tolerant to the glucose overload and thus the glucose concentration reduced rapidly to the initial state, whereas the glucose content in diabetic mice decreased very slowly, indicating that glucose tolerance was impaired in T2D mice. The value of the AUC showed that the glucose tolerance was improved in diabetic mice administered the low and high doses of GEN.
In contrast to normal mice, the levels of FBG, insulin, HOMA-IR, and glucagon were increased in the T2D group. When compared with the T2D group, although there was no statistical difference in FBG in the LGEN group, the levels of insulin, HOMA-IR, and glucagon were decreased; in the HGEN group, the levels of FBG, insulin, HOMA-IR, and glucagon were significantly decreased (Fig. 1c–f). These data indicated that GEN treatment ameliorated hyperglycemia, IR, and hyperglucagonemia.
Compared with the NC group, the levels of serum pro-inflammatory factors including LPS, TNF-α, IL-1β, and IL-6 were increased in the T2D group. GEN treatment reduced the levels of pro-inflammatory factors in the LGEN and HGEN groups compared with the T2D group (Fig. 1g, h), indicating that GEN alleviated the inflammation in T2D mice.
Effects of GEN on serum lipid profiles
In contrast to the NC group, the levels of TG, TC, and LDL-C were elevated, whereas HDL-C was reduced in the T2D group. In contrast to the T2D group, GEN treatment ameliorated the aforementioned indices, especially in the HGEN group, which indicated that GEN could ameliorate dyslipidemia in T2D mice (Fig. 2).
Effects of GEN on the histological alterations of the liver
As shown in Fig. 3a, before the STZ injection, the body weight of the HFD-fed mice increased significantly when compared with the NC group. However, STZ injection resulted in body weight loss in T2D mice. GEN treatment inhibited a decline in the body weight in diabetic mice.
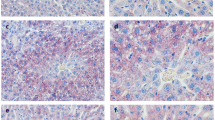
Effects of GEN on hepatic function and histopathological alterations in the different groups. a Body weight, b hepatic index, c ALT, d AST, e H&E staining, f Masson's trichrome staining, g PAS staining, h oil red O staining. Values are mean ± SD (n = 8). **P < 0.01 vs. NC group; #P < 0.05, ##P < 0.01 vs. T2D group
As shown in Fig. 3b–d, diabetes increased the levels of the hepatic index, ALT, and AST. Compared with the T2D group, although there was no statistical difference in the hepatic index in the LGEN group, the levels of ALT and AST were decreased; in the HGEN group, the levels of the hepatic index, ALT, and AST were significantly decreased, indicating that GEN treatment alleviated hepatic hypertrophy and dysfunction.
H&E staining (Fig. 3e) showed that the hepatocytes were arranged neatly, with no steatosis and minimal inflammatory cell infiltration in the NC group. The hepatocytes were swollen and hepatic steatosis and inflammatory cell infiltration were aggravated in the T2D group. GEN treatment alleviated hepatic pathological changes, especially in the HGEN group.
Masson's trichrome staining (Fig. 3f) exhibited normal hepatocytes with less collagen deposition in the NC group. Lots of collagen fibers were deposited in the liver of T2D mice. GEN treatment alleviated collagen deposition compared with the T2D group.
PAS-stained hepatic tissue (Fig. 3g) showed that compared with the NC group, the level of purple-stained glycogen was reduced in the T2D group. After treatment with GEN, the contents of hepatic glycogen were elevated in the LGEN and HGEN groups.
ORO staining displayed normal hepatocytes with minimal lipid accumulation in the NC group. In the T2D group, a large number of lipids were accumulated in the liver. GEN treatment reduced the accumulation of lipids, especially in the HGEN group (Fig. 3h). These results indicated that GEN alleviated pathological changes in the liver of diabetic mice.
Effects of GEN on hepatic inflammation and apoptosis
When compared with the NC group, the protein expression levels of NF-κB and NLRP3 and the number of apoptotic cells were notably elevated in the T2D group. GEN treatment effectively reduced the expression levels of NF-κB and NLRP3 and the number of apoptotic cells in the LGEN and HGEN groups (Fig. 4a–c).
Effects of GEN on inflammation-related protein expression in hepatic tissue of each group. a Representative IHC result for NF-κB p65, b representative IHC result for NLRP3, c representative TUNEL result, d TNF-α, e IL-1β, f IL-18. Values are mean ± SD (n = 8). **P < 0.01 vs. NC group; #P < 0.05, ##P < 0.01 vs. T2D group
In contrast to the NC group, the levels of hepatic pro-inflammatory cytokines including TNF-α, IL-1β, and IL-18 were markedly elevated in the T2D group. GEN treatment distinctly decreased the levels of pro-inflammatory cytokines in diabetic mice (Fig. 4d–f). These findings revealed that GEN alleviated the inflammation and apoptosis in the diabetic liver.
GEN improved colonic barrier function
As shown in Fig. 5a, colonic histology showed a large number of goblet cells in the crypts and minimal inflammatory cell infiltration in the NC group. Conversely, the intestinal structure was disordered with a large amount of inflammatory cell infiltration in the T2D group. When compared with the T2D group, the infiltration of inflammatory cells was ameliorated obviously in the GEN treatment groups, especially in the HGEN group.
Effects of GEN on pathological alterations, tight junction proteins, and pro-inflammatory cytokines in colonic tissue of each group. a Colonic H&E staining, b the protein expression levels of occludin and ZO-1 in the colonic tissue, c TNF-α, d IL-6. Values are mean ± SD (n = 8). **P < 0.01 vs. NC group; ##P < 0.01 vs. T2D group
As shown in Fig. 5b, compared with the NC group, the protein expression levels of colonic occludin and ZO-1 were significantly decreased in the T2D group. Compared with the T2D group, GEN treatment elevated the expression levels of occludin and ZO-1 in the LGEN and HGEN groups.
Furthermore, diabetes elevated the levels of colonic TNF-α and IL-6 in the T2D group compared with the NC group. GEN treatment distinctly decreased the levels of TNF-α and IL-6 in the diabetic colon (Fig. 5c, d). These findings suggested that GEN could effectively improve the tight junctions of epithelial cells in colonic tissue through inhibition of the inflammatory response.
Effects of GEN on SCFAs
In contrast to the NC group, the levels of fecal SCFAs including acetate, propionate, and butyrate were markedly declined in the T2D group. GEN treatment distinctly increased the levels of SCFAs in the HGEN group (Fig. 6).
GEN-modulated gut microbial composition
To evaluate the impacts of GEN on gut microbiota, we analyzed fecal microbial composition using the 16S rRNA gene pyrosequencing. After the high-throughput pyrosequencing, the 671,268 clean sequences were generated, which were clustered into OTUs based on a 97% similarity degree. First, we analyzed the alpha diversity of the microbiota, which represented the richness and diversity of the species. As shown in Fig. 7a, b, when compared with the NC group, the levels of Chao1 and Shannon indices were decreased in the T2D group, indicating that diabetes reduced the richness and diversity of the species in mice. There were no significant differences in Chao1 and Shannon indices between the T2D and HGEN groups, suggesting that GEN treatment had no significant improved effects on the richness and diversity of the species. Subsequently, PCoA was used to analyze beta diversity, which reflected the species differences among the three groups. The results showed that GEN treatment could reshape the intestinal microbial community of diabetic mice (Fig. 7c).
Subsequently, we analyzed the composition of gut microbiota in each group. As shown in Fig. 8, a significant difference in gut microbial composition was found among the three groups. The microbial community consisted mainly of Firmicutes, Bacteroidetes, and Proteobacteria at the phylum level. Compared with the NC group, the Firmicutes/Bacteroidetes ratio and the relative abundance of Proteobacteria were increased in the T2D group. GEN treatment decreased the Firmicutes/Bacteroidetes ratio and the relative abundance of Proteobacteria in the HGEN group.
Effects of GEN on the relative abundance of gut microbiota at the phylum level. a The relative abundance of gut microbiota at the phylum level, b the ratio of Firmicutes/Bacteroidetes, c the relative abundance of Proteobacteria. Values are mean ± SD (n = 5). **P < 0.01 vs. NC group; #P < 0.05, ##P < 0.01 vs. T2D group
Finally, we analyzed the gut microbiota at the genus level. As shown in Fig. 9, compared with the NC group, the relative abundances of Bacteroides and Prevotella were decreased, whereas the relative abundances of Ruminococcus and Helicobacter were increased in the T2D group. GEN treatment ameliorated the above-mentioned indices in the HGEN group. These results indicated that GEN could modulate the gut microbial composition in diabetic mice.
Effects of GEN on the relative abundance of gut microbiota at the genus level. a The relative abundance of gut microbiota at the genus level, b the relative abundance of Bacteroides, c the relative abundance of Prevotella, d the relative abundance of Ruminococcus, e the relative abundance of Helicobacter. Values are mean ± SD (n = 5). **P < 0.01 vs. NC group; #P < 0.05, ##P < 0.01 vs. T2D group
Discussion
T2D is a complex chronic metabolic disease, belonging to the noninsulin-dependent class of diabetes [27]. Insulin is the only hormone that lowers blood glucose in vivo, and therefore, IR not only causes high blood glucose but also leads to metabolic syndrome and T2D. An increasing number of studies have suggested that GEN, a bioactive isoflavone, found extensively in soy products, has diverse beneficial bioactivities, including antiinflammatory, antidiabetic, and hypolipidemic effects [28, 29]. However, it remains unclear whether GEN has protective effects against IR through regulation of the gut microbiota in T2D. In the current study, after supplementation with GEN, there were obvious improvements in glucose and lipid metabolism, hepatic morphology, and function, including colonic barrier function, and intestinal microbiota reshaping in T2D mice.
T2D is commonly accompanied by hyperglycemia, hyperlipidemia, hyperinsulinemia, and hyperglucagonemia. In this study, we combined an HFD and STZ to establish a T2D mouse model. The results showed that compared with normal mice, the levels of FBG, insulin, and glucagon were elevated, and lipid metabolism was dysregulated in diabetic mice, indicating that the T2D model was successfully established. The OGTT is a key test for evaluating glucose tolerance in T2D, while the HOMA-IR is an important indicator reflecting the degree of IR. These experimental results indicated that glucose tolerance was impaired and the degree of IR was aggravated in T2D mice. After supplementation with GEN, the aforementioned indices were effectively ameliorated, especially in the HGEN group, indicating that GEN could ameliorate the glucose and lipid metabolism dysfunction and IR in T2D mice.
Chronic low-grade inflammation is an important cause of IR [30] and in our study, the results showed that serum pro-inflammatory factors including LPS, TNF-α, IL-1β, and IL-6 levels were increased in T2D mice, demonstrating that this inflammation was implicated in the development of IR and T2D. Furthermore, hepatic IR, which is the primary event in systemic IR, often causes adipose tissue and skeletal muscle IR in metabolic syndrome [31].
The liver is an important organ mainly characterized by metabolic function and plays a salient role in maintaining glucose and lipid homeostasis [32]. Hepatic dysfunction is strongly associated with IR and serum ALT and AST levels are indicators of liver injury [23]. Furthermore, elevated glucagon secretion can act on the liver by enhancing glycogen degradation and suppressing glycogen synthesis, thereby stimulating glucose production [33]. The results of PAS staining and glucagon levels showed that diabetes decreased hepatic glycogen content. Whereas, ORO staining showed excessive accumulation of lipids in the liver of T2D mice. These results indicated a disturbance in liver function and glucose and lipid metabolism in T2D mice. GEN treatment ameliorated liver injury and glucose and lipid metabolism dysregulation in diabetic mice, indicating that this compound could alleviate hepatic IR.
Inflammation is closely associated with the progression of hepatic IR [34] and NF-κB is a pro-inflammatory factor that produces numerous pro-inflammatory cytokines, such as TNF-α and IL-1β [35]. NLRP3 is a crucial component of the NLRP3 inflammasome, which produces the activation of pro-inflammatory cytokines such as IL-1β and IL-18 and further aggravates inflammation and tissue damage [36]. Furthermore, increased apoptosis reduces the sensitivity of the liver to insulin. Our study showed that the levels of NF-κB and NLRP3 expression, pro-inflammatory cytokines, and hepatocyte apoptosis were increased, demonstrating that this inflammation exacerbated hepatic IR in diabetic mice. Following treatment with low and high doses of GEN, the aforementioned indices were ameliorated, especially in the HGEN group. These results suggested that GEN could alleviate hepatic IR by inhibiting inflammation.
LPS, mainly derived from intestinal Gram-negative bacteria, is involved in the inflammatory reaction of T2D patients [13]. Growing evidence suggests that the crosstalk between gut microbiota and the host is implicated in diverse diseases, such as obesity, metabolic syndrome, and T2D [37, 38]. Intestinal harmful bacteria produce excessive LPS which reduces the expression of tight junction proteins, including occludin and ZO-1, resulting in a damaged gut barrier [39]. Subsequently, LPS enters the blood through the leaky gut to the liver and causes hepatic inflammation [40]. Conversely, beneficial bacteria can produce SCFAs to enhance the intestinal barrier function. Therefore, dysbiosis of gut microbiota not only decreases the concentrations of SCFAs but also promotes intestinal damage and subsequent systemic inflammation, which in turn accelerates the progression of T2D [41]. In our study, the results showed that diabetes not only aggravated colonic damage and inflammatory responses but also decreased the levels of SCFAs. Thus, we inferred that hepatic inflammation might be caused by gut microbial dysbiosis.
Therefore, we analyzed the composition of gut microbiota in mice and the results showed that diabetes decreased the richness and diversity of gut microbiota in contrast to normal mice. Although diabetes increased the ratio of the phyla Firmicutes/Bacteroidetes and the relative abundance of Proteobacteria that produce large amounts of LPS; this is consistent with previous research [42]. The genus Helicobacter belonging to the Proteobacteria phylum has a pro-inflammatory effect and is tightly associated with IR [43]. The genus Ruminococcus also exerts pro-inflammatory effects [44]. Accumulating evidence has revealed that the genera Bacteroides and Prevotella belonging to the Bacteroidetes phylum are important for producing acetate and propionate [45]. Moreover, Prevotella not only reduces the blood LPS content but also increases hepatic glycogen content [22, 46]. The current study showed that diabetes reduced the abundances of Bacteroides and Prevotella and increased the abundances of Ruminococcus and Helicobacter, demonstrating that diabetes produced excessive LPS and reduced SCFA concentrations by changing the composition of gut microbiota. Following treatment with GEN, although there were no obvious beneficial effects on microbial richness and diversity, the ratio of Firmicutes/Bacteroidetes and the abundances of Proteobacteria, Ruminococcus, and Helicobacter were decreased, whereas the abundances of Bacteroides and Prevotella were increased. These results indicated that high-dose GEN supplementation could effectively improve the composition of gut microbiota by increasing beneficial bacteria and reducing harmful bacteria, thereby improving the intestinal barrier function and alleviating systemic inflammation in diabetic mice.
Conclusions
In summary, the results of this study suggest that oral GEN treatment has ameliorative effects on inflammation and IR by modulating the composition of the gut microbiota in T2D mice. Thus, GEN may be used as a health supplement for the treatment of diabetes.
References
Li BY, Xu XY, Gan RY, Sun QC, Meng JM, Shang A, Mao QQ, Li HB (2019) Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 8(10):440. https://doi.org/10.3390/foods8100440
Laakso M (2019) Biomarkers for type 2 diabetes. Mol Metab 27S:S139–S146. https://doi.org/10.1016/j.molmet.2019.06.016
Wang Q, Zhou J, Xiang Z, Tong Q, Pan J, Wan L, Chen J (2019) Anti-diabetic and renoprotective effects of Cassiae Semen extract in the streptozotocin-induced diabetic rats. J Ethnopharmacol 239:111904. https://doi.org/10.1016/j.jep.2019.111904
Davegardh C, Garcia-Calzon S, Bacos K, Ling C (2018) DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol Metab 14:12–25. https://doi.org/10.1016/j.molmet.2018.01.022
Czech MP (2017) Insulin action and resistance in obesity and type 2 diabetes. Nat Med 23(7):804–814. https://doi.org/10.1038/nm.4350
Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L, Zhang H, Chen W, Sun J, Chen YQ (2017) A.nti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep 7(1):7046. https://doi.org/10.1038/s41598-017-07335-0
Tilg H, Moschen AR (2014) Microbiota and diabetes: an evolving relationship. Gut 63(9):1513–1521. https://doi.org/10.1136/gutjnl-2014-306928
Greenfield JR, Campbell LV (2006) Relationship between inflammation, insulin resistance and type 2 diabetes: “cause or effect”? Curr Diabetes Rev 2(2):195–211. https://doi.org/10.2174/157339906776818532
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165(6):1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Macfarlane GT, Macfarlane S (2012) Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95(1):50–60. https://doi.org/10.5740/jaoacint.sge_macfarlane
Barlow GM, Yu A, Mathur R (2015) Role of the gut microbiome in obesity and diabetes mellitus. Nutr Clin Pract 30(6):787–797. https://doi.org/10.1177/0884533615609896
Xu YH, Gao CL, Guo HL, Zhang WQ, Huang W, Tang SS, Gan WJ, Xu Y, Zhou H, Zhu Q (2018) Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol 238(3):231–244. https://doi.org/10.1530/JOE-18-0137
Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V (2011) Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34(2):392–397. https://doi.org/10.2337/dc10-1676
Qin JJ, Li YR, Cai ZM, Li SH, Zhu JF, Zhang F, Liang SS, Zhang WW, Guan YL, Shen DQ, Peng YQ, Zhang DY, Jie ZY, Wu WX, Qin YW, Xue WB, Li JH, Han LC, Lu DH, Wu PX, Dai YL, Sun XJ, Li ZS, Tang AF, Zhong SL, Li XP, Chen WN, Xu R, Wang MB, Feng Q, Gong MH, Yu J, Zhang YY, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang ZX, Chen H, Yang RF, Zheng WM, Li SG, Yang HM, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490(7418):55–60. https://doi.org/10.1038/nature11450
Liu RM, Dai R, Luo Y, Xiao JH (2019) Glucose-lowering and hypolipidemic activities of polysaccharides from Cordyceps taii in streptozotocin-induced diabetic mice. BMC Complement Altern Med 19(1):230. https://doi.org/10.1186/s12906-019-2646-x
Yang R, Jia Q, Liu XF, Ma SF (2018) Effect of genistein on myocardial fibrosis in diabetic rats and its mechanism. Mol Med Rep 17(2):2929–2936. https://doi.org/10.3892/mmr.2017.8268
Zhou L, Xiao X, Zhang Q, Zheng J, Deng M (2019) Maternal genistein intake mitigates the deleterious effects of high-fat diet on glucose and lipid metabolism and modulates gut microbiota in adult life of male mice. Front Physiol 10:985. https://doi.org/10.3389/fphys.2019.00985
Mukund V, Mukund D, Sharma V, Mannarapu M, Alam A (2017) Genistein: Its role in metabolic diseases and cancer. Crit Rev Oncol Hematol 119:13–22. https://doi.org/10.1016/j.critrevonc.2017.09.004
Shi YN, Zhang XQ, Hu ZY, Zhang CJ, Liao DF, Huang HL, Qin L (2019) Genistein protects H9c2 cardiomyocytes against chemical hypoxia-induced injury via inhibition of apoptosis. Pharmacology 103(5–6):282–290. https://doi.org/10.1159/000497061
Gupta SK, Dongare S, Mathur R, Mohanty IR, Srivastava S, Mathur S, Nag TC (2015) Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol Cell Biochem 408(1–2):63–72. https://doi.org/10.1007/s11010-015-2483-2
Incir S, Bolayirli IM, Inan O, Aydin MS, Bilgin IA, Sayan I, Esrefoglu M, Seven A (2016) The effects of genistein supplementation on fructose induced insulin resistance, oxidative stress and inflammation. Life Sci 158:57–62. https://doi.org/10.1016/j.lfs.2016.06.014
Lopez P, Sanchez M, Perez-Cruz C, Velazquez-Villegas LA, Syeda T, Aguilar-Lopez M, Rocha-Viggiano AK, Del Carmen S-L, Torre-Villalvazo I, Noriega LG, Torres N, Tovar AR (2018) Long-term genistein consumption modifies gut microbiota, improving glucose metabolism, metabolic endotoxemia, and cognitive function in mice fed a high-fat diet. Mol Nutr Food Res 62(16):e1800313. https://doi.org/10.1002/mnfr.201800313
Li S, Huang Q, Zhang LW, Qiao XF, Zhang YY, Tang FS, Li ZB (2019) Effect of CAPE-pNO(2) against type 2 diabetes mellitus via the AMPK/GLUT4/GSK3β/PPARα pathway in HFD/STZ-induced diabetic mice. Eur J Pharmacol 853:1–10. https://doi.org/10.1016/j.ejphar.2019.03.027
Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, Guo TL (2017) Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol Appl Pharmacol 332:138–148. https://doi.org/10.1016/j.taap.2017.04.009
Liu ZQ, Liu T, Chen C, Li MY, Wang ZY, Chen RS, Wei GX, Wang XY, Luo DQ (2015) Fumosorinone, a novel PTP1B inhibitor, activates insulin signaling in insulin-resistance HepG2 cells and shows anti-diabetic effect in diabetic KKAy mice. Toxicol Appl Pharmacol 285(1):61–70. https://doi.org/10.1016/j.taap.2015.03.011
Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J (2015) Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43(2):304–317. https://doi.org/10.1016/j.immuni.2015.07.013
Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX (2016) Anti-diabetic polysaccharides from natural sources: a review. Carbohydr Polym 148:86–97. https://doi.org/10.1016/j.carbpol.2016.02.060
Behloul N, Wu G (2013) Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol 698(1–3):31–38. https://doi.org/10.1016/j.ejphar.2012.11.013
Lee SR, Kwon SW, Lee YH, Kaya P, Kim JM, Ahn C, Jung EM, Lee GS, An BS, Jeung EB, Park BK, Hong EJ (2019) Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 19(1):6. https://doi.org/10.1186/s12885-018-5222-8
Matulewicz N, Karczewska-Kupczewska M (2016) Insulin resistance and chronic inflammation. Postepy Hig Med Dosw (Online) 70:1245–1258
Su Z, Nie Y, Huang X, Zhu Y, Feng B, Tang L, Zheng G (2019) Mitophagy in hepatic insulin resistance: therapeutic potential and concerns. Front Pharmacol 10:1193. https://doi.org/10.3389/fphar.2019.01193
Jones JG (2016) Hepatic glucose and lipid metabolism. Diabetologia 59(6):1098–1103. https://doi.org/10.1007/s00125-016-3940-5
Miller RA, Birnbaum MJ (2016) Glucagon: acute actions on hepatic metabolism. Diabetologia 59(7):1376–1381. https://doi.org/10.1007/s00125-016-3955-y
Gruben N, Shiri-Sverdlov R, Koonen DP, Hofker MH (2014) Nonalcoholic fatty liver disease: a main driver of insulin resistance or a dangerous liaison? Biochim Biophys Acta 1842 11:2329–2343. https://doi.org/10.1016/j.bbadis.2014.08.004
He X, Shu J, Xu L, Lu C, Lu A (2012) Inhibitory effect of Astragalus polysaccharides on lipopolysaccharide-induced TNF-α and IL-1β production in THP-1 cells. Molecules 17(3):3155–3164. https://doi.org/10.3390/molecules17033155
Jia Q, Mehmood S, Liu X, Ma S, Yang R (2020) Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats. Exp Biol Med (Maywood) 245(3):221–230. https://doi.org/10.1177/1535370219899899
Chen X, Devaraj S (2018) Gut microbiome in obesity, metabolic syndrome, and diabetes. Curr Diabetes Rep 18(12):129. https://doi.org/10.1007/s11892-018-1104-3
Upadhyaya S, Banerjee G (2015) Type 2 diabetes and gut microbiome: at the intersection of known and unknown. Gut Microbes 6(2):85–92. https://doi.org/10.1080/19490976.2015.1024918
Bein A, Zilbershtein A, Golosovsky M, Davidov D, Schwartz B (2017) LPS induces hyper-permeability of intestinal epithelial cells. J Cell Physiol 232(2):381–390. https://doi.org/10.1002/jcp.25435
Gabele E, Dostert K, Hofmann C, Wiest R, Scholmerich J, Hellerbrand C, Obermeier F (2011) DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol 55(6):1391–1399. https://doi.org/10.1016/j.jhep.2011.02.035
Wen L, Duffy A (2017) Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr 147(7):1468S-1475S. https://doi.org/10.3945/jn.116.240754
Wu R, Zhao D, An R, Wang Z, Li Y, Shi B, Ni Q (2019) Linggui zhugan formula improves glucose and lipid levels and alters gut microbiota in high-fat diet-induced diabetic mice. Front Physiol 10:918. https://doi.org/10.3389/fphys.2019.00918
Han LH, Li TG, Du M, Chang R, Zhan BY, Mao XY (2019) Beneficial effects of Potentilla discolor bunge water extract on inflammatory cytokines release and gut microbiota in high-fat diet and streptozotocin-induced type 2 diabetic mice. Nutrients 11(3):670. https://doi.org/10.3390/nu11030670
Hall AB, Yassour M, Sauk J, Garner A, Jiang XF, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, Bertha M, Cohen M, Garber J, Khalili H, Gevers D, Ananthakrishnan AN, Kugathasan S, Lander ES, Blainey P, Vlamakis H, Xavier RJ, Huttenhower C (2017) A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med 9(1):103. https://doi.org/10.1186/s13073-017-0490-5
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12(10):661–672. https://doi.org/10.1038/nrmicro3344
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22(6):971–982. https://doi.org/10.1016/j.cmet.2015.10.001
Acknowledgements
This work was supported by the Natural Science Research Project of the Education Commission of Anhui Province (No. KJ2018A0994).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, R., Jia, Q., Mehmood, S. et al. Genistein ameliorates inflammation and insulin resistance through mediation of gut microbiota composition in type 2 diabetic mice. Eur J Nutr 60, 2155–2168 (2021). https://doi.org/10.1007/s00394-020-02403-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02403-0