Abstract
Purpose
In Malaysia, hip fracture incidence is higher in Chinese women than other ethnic groups. This study compared the effects of a high-calcium vitamin D fortified milk with added FOS-inulin versus regular milk over 1 year on aspects of bone health in Chinese postmenopausal women in Malaysia.
Methods
One-hundred and twenty-one women (mean age 59 (± 4) years) were randomized into two groups: control (n = 60; regular milk, 428 mg calcium per day) or intervention (n = 61; fortified milk at 1200 mg calcium, 96 mg magnesium, 2.4 mg zinc, 15 μg vitamin D and 4 g FOS-inulin per day). At baseline, weeks 12, 24, 36 and 52, parathyroid hormone (PTH), C-Telopeptide of Type I Collagen (CTx-1), Procollagen I Intact N-Terminal propeptide (PINP) and vitamin D levels were assessed. Bone density (BMD) was measured at baseline and week 52 using a GE Lunar iDXA.
Results
Body mass index, lumbar spine and femoral neck BMD did not differ between groups at baseline. Over 52 weeks, mean plasma 25 (OH) D3 levels increased to 74.8 nmol/L (intervention group) or remained at 63.1 nmol/L (control group) (p < 0.001 between groups). PTH levels increased in the control group (p = 0.001). The intervention resulted in a significant suppression of CTx-1 and PINP at p = 0.018 and p = 0.004. Femoral neck BMD remained stable in the intervention group but decreased significantly in the controls, with a borderline treatment effect (p = 0.07).
Conclusion
Compared with regular milk, the fortified milk suppressed bone turnover markers and tended to increase femoral neck BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of osteoporosis and its associated fracture risk is a worldwide concern as many countries are experiencing large-scale demographic shifts to ageing populations [1]. The burden of osteoporosis, as an age-related disease, is anticipated to disproportionately affect Asian countries due to their large population sizes, high rates of economic development and urbanisation, and projected large elderly populations. A more than twofold increase in total population and life expectancy for the elderly in the developing world is anticipated over the next 25 years, as reported in Hernlund et al. [2]. Over two decades ago [3], it was estimated that Asia would experience 50% of all hip fractures worldwide by 2050 [4, 5]. The IOF Asian audit of 2009 cites a two to threefold rise in hip fracture incidence over the past 30 years for most Asian countries [6]. Malaysia is a multi-ethnic country and hip fracture incidence appears to be higher for Chinese women than other races [7], with Lee et al. [8] reporting an incidence of 220 hip fractures per 100,000 among Chinese females in Malaysia.
Both low calcium intakes and insufficient 25 (OH) vitamin D3 (25(OH)D3) levels have been reported as risk factors for osteoporosis in Asian women [5]. While recommendations for minimum calcium intake are similar for Asian and Caucasian women, at 1000 mg/day [9], actual intakes in Asian women are reported to fall significantly short of this minimum [7, 10,11,12,13]. As in most countries, the Malaysian Dietary Guidelines recommend an adequate intake of dairy products such as low fat milk and other foods to enable an adequate intake of calcium [14].
Most of the more recent studies in populations of Asian women investigating vitamin D status describe sub-optimal 25(OH)D3 levels, with some studies reporting negative relationships between 25(OH)D3 and PTH, and some also demonstrating further associations with bone turnover markers and bone mass [10, 12, 15,16,17,18,19]. The general incidence of low levels of vitamin D and low habitual dietary calcium intakes indicates that many Asian women are likely to experience high bone turnover with a concomitant increase in fracture risk. Daily consumption of milk fortified with 800 mg calcium was shown to reduce bone loss in a study of Chinese women living in Hong Kong [20, 21]. An intervention with Chinese women in Malaysia supplying a higher dose of calcium, 1200 mg, in fortified milk, significantly reduced the percentage total body, femoral neck and total hip bone loss over 24 months, compared to the control group [22]. The fortified milk also contained vitamin D 10 µg/day, which resulted in improved vitamin D status even though the average serum 25 (OH)D3 for these women was 69 nmol/L at baseline. Their status improved to 87 nmol/L over 12 months, demonstrating maintenance of a sufficient vitamin D status [23, 27].
In addition to providing additional dietary calcium and vitamin D, further micronutrients can be provided to the diet that may affect calcium absorption and bone health in Asian women. Oligofructose-enriched inulin (FOS-inulin) has been shown to increase intestinal calcium and magnesium absorption in older women [24], and reduce bone resorption [24]. Magnesium has been shown to reduce hydroxyapatite crystal size, thereby affecting bone quality [25] and increasing bone density in older women.
The aims of the current study were to compare the effects of a high-calcium vitamin D fortified milk with added FOS-inulin and magnesium versus regular milk on vitamin D status, bone biomarkers and serum parathyroid hormone (PTH) as well as bone density in Chinese postmenopausal (PM) women living in Malaysia, over 52 weeks.
Methods
The study was approved by the Ethics Committee for Research Involving Human Subjects, Universiti Putra Malaysia.
Study population, inclusion and exclusion criteria
Women over 55 years of age, at least 5 years post-menopause, and with a body mass index (BMI) between 17 and 32 kg/m2 were recruited. Exclusion criteria included a history of metabolic bone disease; abnormal liver or kidney function tests; diagnosed diabetes mellitus or insulin resistance; lactose intolerance/milk allergy; regular use of calcium and/or vitamin D supplements; regular use of anti-acids containing calcium; more than two units of alcohol per day; smoking; regular use of medication that may influence bone mass; rheumatoid arthritis/autoimmune disease and fractures in the last 6 months.
Randomisation criteria, procedures and intervention
Recruitment
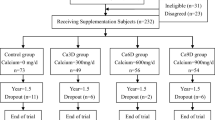
A total of 975 postmenopausal community dwelling women were initially screened for eligibility using a structured questionnaire, and finger-prick cholesterol and glucose tests. From these, 416 women passed the initial screening exercise and were invited to a second stage screening (Fig. 1).
Screening visit
A screening questionnaire on general health was administered at an initial visit. A blood sample was taken between 8 and 10 a.m., after an overnight fast, and haematology, blood minerals and metabolic markers were assessed. If the blood test results were normal, study subjects were asked to have a bone mineral density (BMD) measurement of whole body, femoral neck and spine (L1–L4) (using a GE Lunar iDXA, GE Healthcare, Madison, WI, USA). Body weight was measured to the nearest 0.1 kg using SECA scales and standing height was measured to the nearest 0.1 cm using a stadiometer. The medical history of each subject was recorded at this visit.
Intervention
One-hundred and twenty-one postmenopausal Malaysian Chinese women were successfully screened and provided written informed consent for the trial. The women were randomized into two groups with 60 in the control group and 61 in the intervention (Int) group. The control group was assigned to receive two servings per day of regular milk powder (~ 428 mg calcium) and the Int group was assigned to receive 2 servings per day of fortified milk powder (1200 mg calcium, plus 96 mg magnesium, 2.4 mg zinc, 15 µg vitamin D and 4 g FOS-inulin) (Anlene™, Fonterra Brands (Singapore Pte Ltd.)) for 52 weeks (Table 1).
Baseline
Blood sampling
Blood samples were taken between 8 and 10 a.m. (after an overnight fast) for the baseline measurements. Samples were taken to measure plasma markers of bone metabolism [C-Telopeptide of Type I Collagen (CTx-1) and Procollagen I Intact N-Terminal propeptide (PINP)] as well as serum calcium, magnesium, phosphorus, lipid profile, glucose, insulin, parathyroid hormone (PTH) and 25 (OH) vitamin D3. The samples for measuring minerals and metabolic health were collected, processed and analysed immediately by local diagnostic laboratories. Plasma samples for CTx-1, PINP, PTH and 25 (OH) vitamin D3 were snap frozen and stored at −80°C before being analysed by Canterbury Health Laboratories, Christchurch, New Zealand.
Anthropometry
Methodology as per screening visit.
Questionnaires completed at baseline
A 3-day diet record, food frequency questionnaire, demographics, physical activity level, sun exposure and a list of all medication taken in the last 6 months were recorded at baseline.
Follow-up measurements
At weeks 12, 24, 36 and 52, blood samples were taken between 8 and 10 a.m. (after an overnight fast) for 25 (OH) vitamin D3 and bone marker measurements. In addition, blood samples at weeks 12, 24 and 52 blood samples were also analysed for blood minerals, lipid profile and glucose. Collection and analysis of the samples were conducted as per the baseline visit. At week 52, anthropometry, DXA, and questionnaires were completed as at baseline. Retrospectively, a number of DXA scans were of insufficient quality/consistency to be analysed resulting in low final numbers for DXA data.
Blood and bone marker analyses
PTH, CTx-1 and total PINP were analysed by electrochemiluminescence immunoassay using the Roche COBAS® e411 system (Roche Diagnostics, Indianapolis, IN, USA). 25 (OH)D3 was analysed using isotope-dilution liquid chromatography–tandem mass spectrometry (ID-LC–MS–MS) [26].
Compliance
Milk powder was dispensed to each subject at baseline and on a monthly basis thereafter. In addition, phone calls were made to monitor milk consumption of the subjects. Each subject was provided with a monthly diary and asked to record their milk powder intake each day.
Statistical analyses
Four subjects (one from the Int and three from the control group) were retrospectively found to meet exclusion criteria and were excluded from the analyses [regular use of vitamin D supplements (n = 1) or signs of metabolic bone disease (n = 3)]. Hence, a total of one-hundred and seventeen subjects (n = 60 for Int and n = 57 for control) were included in the analyses.
Based on published data, the within-subject standard deviation for one of the primary outcome variables, CTx-1 was estimated to be 0.045 ng/mL and a representative mean CTx-1 value was taken to be 0.23 ng/mL. The corresponding standard deviation of the treatment difference was calculated as 0.064 ng/mL. To detect a difference of 20% (i.e. 0.046 ng/mL) with a power of 90% and an alpha of 5%, we required 42 subjects per group. To allow for dropouts, and/or potentially a lower difference (16.5%), the number of volunteers required was increased to 60 per group.
SAS (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Outcome measures: the primary outcome variables were difference from baseline for Vitamin D and CTx-1, secondary outcomes were difference from baseline for P1NP and PTH, vitamin D status, as well as absolute values of vitamin D, CTx-1, P1NP, PTH, BMD and T scores, and serum calcium, magnesium, phosphorous, glucose, insulin, and total cholesterol. Mixed models approach or repeated measures analysis of variance was used and the reported p values for the effects of treatment group, time, and their interaction were based on a compound symmetry covariance pattern model. Data were analysed as (a) raw data, (b) difference from baseline, and (c) percentage change from baseline. For (b) and (c), the baseline results (week 0) were included in the model as a covariate and the repeated measures analysis was based on the results from during the intervention (i.e. weeks 12–52). ANOVA was followed by post hoc comparisons of treatment means using the Tukey–Kramer test. Data were log10 transformed if required to achieve homogeneity of variance but all results are shown in the original units. Measurements were considered to be significantly different if p < 0.05.
Results
Baseline characteristics
Table 2 summarises the characteristics of the women. The mean ages for the control and the Int group were 59 and 60 years, respectively, and the mean BMI for the control group was 24.5 versus 23.4 kg/m2 for the Int group (p = 0.363). Lumbar spine BMDs were slightly lower in the Int group with T scores of − 0.28 for the control and − 0.75 for the Int groups (p = 0.070). For the femoral neck, BMDs were normal with T scores at − 0.48 for the control and − 0.92 for the Int group. Mean calcium intake from the food frequency questionnaire for these groups was 547 mg (control) and 527 mg/day (Int). Baseline 25 (OH)D3 was 64.8 nmol/L for the control group versus 62.3 nmol/L for the Int group. No significant differences in age, BMI, femoral neck BMD, calcium intake or 25 (OH)D3 levels were detected.
Impact of intervention
Compliance, general health and blood minerals
The women’s compliance with taking the products varied between 86 and 90% over the 52 weeks. General measures of health including fasting blood glucose and fasting lipid profiles were within normal ranges at weeks 0 and 52 (Table 3) and all blood minerals including calcium, magnesium, and phosphorus were within normal ranges. No significant changes were observed for the blood minerals over time. There was an overall treatment effect (p = 0.012) for the change in total cholesterol, with a reduction in total cholesterol in the Int group and no change in the control group. There was a treatment by time effect on the change in LDL levels (p = 0.033) where levels reduced in the Int group compared to baseline but remained stable in the control group (Table 3).
Vitamin D status
For assessment of the vitamin D status of the women, the Institute of Medicine Guidelines (IOM) were used where a level of ≥ 50 nmol/L is considered adequate, and < 50 nmol/L is inadequate [27]. For the control group, 28% had inadequate levels at baseline and over the 52 weeks of supplementation the percentage remained at 21%. In the Int group, 32% had inadequate 25 (OH)D3 levels at baseline and after 52 weeks of supplementation all the women in the Int group were sufficient.
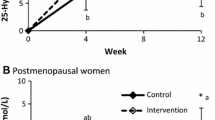
Mean serum 25 (OH) vitamin D3 levels at baseline were sufficient (equal to or > 50 nmol/L) [27] in the control as well as the Int groups (Table 4). Over the 52 weeks, serum 25 (OH)D3 levels remained constant in the control group (64.8–63.1 nmol/L) but increased significantly in the Int group (62.3–74.8 nmol/L, p = 0.002) (Fig. 2). The change over time was significantly different between the groups (p < 0.001).
Changes in PTH
Figure 3 shows the changes from baseline in PTH levels in the two groups of women over time, while Table 4 shows the actual PTH levels for the different time points. PTH levels in the control group increased from 3.56 to 4.10 pmol/L over the 52 weeks (p < 0.001). In the Int group, however, PTH decreased from 3.76 to 3.61 pmol/L but then increased again to 3.98 (p > 0.05) at week 52. The change from baseline in PTH in the Int group was significantly different from the change from baseline in the control group (p = 0.001 between groups, Fig. 3)
Change in CTx-1 and PINP
Figures 4 and 5 show the changes from baseline in CTx-1 and PINP over the 52 weeks of supplementation and Table 4 shows CTx-1 and PINP levels for the different time points. Both the control as well as the Int group of women exhibited bone turnover within the typical range for postmenopausal women with mean plasma CTx-1 values at 0.44–0.45 μg/L at baseline. In the control group, plasma CTx-1 reduced significantly between baseline and week 52 (0.44–0.39 µg/L, p = 0.006) and in the Int group, CTx-1 reduced from 0.45 to 0.35 µg/L (p < 0.001) over the first 12 weeks and remained at this level till week 52 (0.37 µg/L). The changes from baseline in CTx-1 for the Int group were significantly different from the control group (p = 0.018). P1NP decreased in both groups, but the decrease was greater in the fortified than the regular milk group (p = 0.004 for fortified vs regular). PINP was significantly reduced in the Int group from 48 to 42 µg/L (p = 0.001) over the 52 weeks.
Change in bone density
Lumbar spine bone density (BMD) and the T scores remained constant, with no significant changes in both groups of women over 52 weeks. Femoral neck BMD decreased in the control group over the 52 weeks and was significantly different from baseline (p = 0.009). The femoral neck BMD remained stable in the Int group with a borderline treatment effect (p = 0.07). The T scores remained stable in the Int group but reduced significantly in the control group (p = 0.610 for Int and p = 0.009 for control for difference from baseline) and 0.084 for Int versus control. The Z score for the control group decreased significantly over the 52 weeks (p = 0.015), and the difference from baseline was significantly different between the two groups at p = 0.046. The Z scores for whole body and lumbar spine increased throughout the study period, when compared to baseline in both groups indicating a beneficial effect of milk on overall bone density (Table 5).
Linear regression analyses
Using linear regression analysis, we found that the greater the decrease in CTx-1 between baseline and week 36, the lower the loss of BMD in the femoral neck and the lumbar spine over the intervention period (p = 0.010 and p < 0.001, respectively). Changes in Vitamin D status also showed some relationship to femoral neck BMD but it was not significant. Regression analyses using baseline calcium intake and the outcome measures did not indicate any significant relationships.
Discussion
Supplementation of healthy postmenopausal Chinese women with calcium, vitamin D and FOS-inulin fortified milk for 52 weeks significantly reduced bone resorption and improved vitamin D status.
The habitual mean calcium intake for the cohorts of women in the present study was just over 500 mg/day. In a short communication, Haines et al. [28] reported calcium intake of 390 mg/day in older women attending a clinic in Hong Kong which was below the recommended intake for women in Asia. The current recommended intake for Chinese women in Hong Kong is 900 mg [29] and according to Heaney [9] should be closer to 1000 mg for Asian women in general. Chee et al. [7] recorded a calcium intake of 470 mg/day in their cohort of Chinese women in Malaysia and Kruger et al. [30, 31] reported that postmenopausal women living in Beijing had a calcium intake of less than 500 mg/day. Calcium intakes in several Asian countries are, therefore, significantly lower overall than the daily requirement for Asian women and the current recommended nutrient reference value of 800 mg/day for Malaysia (Table 2).
The women who participated in the study were mostly vitamin D sufficient. This is in contrast to a study which reported increased incidence of vitamin D inadequacy in Eastern Asia [33]. The minimum level of serum 25 (OH)D3 estimated by some researchers to be optimal for fracture prevention varies between 50 and 80 nmol/L [32, 34], while the IOM concluded that 40 nmol/L seem to be a threshold for optimal bone health [27]. Twenty-eight percent of the control group and 32% of the Int group had inadequate levels at baseline (based on sufficiency = 50 nmol/L [27]). The percentage of women with inadequate levels of 25 (OH)D3 remained at 21% over the 52 weeks of supplementation in the control group, but at week 52 all women in the Int group were sufficient. The improvement in vitamin D status in the Int group could be an indication of study adherence and results and further relationships should be interpreted with caution. The levels we measured in the women compare well with those reported for Chinese women living in Malaysia [7, 22], where both studies reported a mean of 68.8 nmol/L and as well as a significant inverse relationship between vitamin D status and PTH levels. In our study, we did not observe a significant inverse relationship between 25(OH)D3 levels and PTH. Shibli-Rahhal and Paturi [35] assessed variations in PTH levels in patients with low vitamin D status. They concluded that less than half of patients with low vitamin D status will have an elevated PTH and improving vitamin D status does not immediately result in changes in PTH. In their study, only 30% of the patients with 25 (OH)D3 levels below 75 nmol/L had an elevated PTH. The association between vitamin D status and PTH levels is influenced by dietary calcium intake and in some of our participant’s intake may have been sufficient to perturb the association between 25(OH)D3 levels and PTH [35, 36].
Huang et al. [37] reported a reduction in PTH levels, bone resorption and bone loss in postmenopausal Chinese women in response to 1000 mg calcium and 10 µg of vitamin D for 12 months. Chee et al. [22] reported significantly less bone loss at the femoral neck as well as total hip over 2 years of supplementation of postmenopausal Chinese women with fortified milk. Femoral neck BMD indeed increased in the intervention group at months 18 and 24 of supplementation. Similarly, Lau et al. [20, 21] reported increases in femoral neck BMD at months 18 and 24 of supplementation with fortified milk. In our Int group, bone density and the T score of the femoral neck remained stable with no significant changes over 52 weeks, while BMD as well as the T score reduced significantly in the control group.
The effects of the fortified milk on BMD are supported by the significant reduction in bone resorption represented by the changes in CTx-1 over time. CTx-1 levels reduced in both groups of women, demonstrating the beneficial effects of milk on bone health, but the change induced by the fortified milk was more pronounced and was significantly different from controls at week 12 (p < 0.05) while the change in CTx-1 in the control group was significant at week 52 (p < 0.006). Increasing calcium intake has significant effects on CTx-1 levels as has been shown by several researchers over time. [20, 21, 29,30,31,32]. High-calcium milk supplementation reduced CTX-1 levels more than 30% over 4 weeks in South East Asian women [38], and by 25% in Chinese women living in Beijing, China [30]. Aloia et al. illustrated in 2010 that supplementation with up to 100 µg vitamin D per day had no effect on bone turnover or PTH levels [39]. In a more recent study, this group ran an intervention study in 159 postmenopausal women for 28 weeks. The women were supplemented with 100 µg vitamin D, with or without 1200 mg calcium, calcium alone or with a placebo. CTx-1 was significant reduced by the calcium alone or in combination with vitamin D while vitamin D alone had no effect [39].
The significant decrease in CTx-1 between baseline and week 52 was also associated with less bone loss at the lumbar spine (p < 0.001). Four prospective studies have shown that increased levels of bone resorption markers are associated with an increased risk of fracture [40]. CTx-1 can be predictive of future bone loss [41, 42] and future risk of fracture [43, 44] as well as predict long-term changes in BMD [43]. Tamaki et al. [45] demonstrated that increased levels of bone resorption markers in women more than 5 years past menopause were associated with increased risk of vertebral fractures independent of BMD. While lumbar spine BMD did not change significantly in both control and Int groups, femoral neck BMD remained stable in the Int group but was reduced in the control group, with significant changes in the T scores as well. Femoral neck bone density is a better predictor for hip fracture than any other site in the skeleton; thus, the maintenance of femoral neck BMD in the Int group should indicate some protection from future fractures [44,45,46].
Our findings are in agreement with the study reported by Chee et al. [22] where milk supplementation of older Chinese women in Malaysia resulted in a reduction of bone loss at several sites; a follow-up of the same women 18 months later indicated that in the group that had previously consumed fortified milk, the beneficial effects were maintained while bone loss was increased in the control group [47]. In a meta-analysis on the effects of milk intake on bone biomarkers as well as bone density, milk supplementation reduced bone resorption significantly even in interventions less than 6 months in duration [48]. In addition, the analyses indicated that total body bone mineral content increased significantly in the intervention groups compared to controls while total body BMD increased by 0.01 g/cm2 with borderline significance. Whether these relatively small changes could result in a reduction of fracture risk is not known, and an earlier meta-analysis reported no reduction in risk for hip fracture due to milk intake [49].
Our study had some limitations: Firstly, the fortified milk delivered 4 g of FOS-inulin, 96 mg magnesium and 2.4 mg zinc per day. FOS-inulin has been shown to increase calcium and magnesium absorption but with no significant effects on bone markers [24 and the FOS-inulin could have contributed to the positive effects observed in the Int group by supporting calcium bioavailability and bone mineralisation [50]. Some epidemiological studies indicate that magnesium intake is associated with increased bone density in older women, and limited supplementation studies in adults indicate a correlation between magnesium intake and bone density [51]. Zinc supplementation trials reported increased bone density after up to 18 months of supplementation [52, 53] but at much higher levels of 24–40 mg/day. It is not possible to ascertain which of the additional ingredients in the fortified milk contributed to the effect on markers and bone density, thus the effects shown relate to the complete formulation rather than to specific ingredients. Secondly, up to 90% of Chinese adults are lactose intolerant [54]. As intolerance was one of our exclusion criteria, our population and findings may not be representative of Chinese postmenopausal women.
In summary, in our study, both regular and fortified milk reduced bone turnover but the effect of the fortified milk was greater. No significant effects were observed for PTH levels of both groups. While BMD did not increase in any of the groups, the fortified milk maintained BMD of the femoral neck with a borderline treatment effect. Osteoporosis is a significant health problem resulting in loss of independence due to fractures resulting in morbidities. The incidence of osteoporosis is increasing yearly and has a large impact on individuals, society and escalating healthcare costs. Consumption of milks fortified with additional calcium and vitamin D provides a means to reduce poor bone health and the subsequent risk of fractures.
References
Liu J, Ning G, Chen J (2007) Osteoporotic fractures in Asia: risk factors and strategies for prevention. J Bone Min Metab 25(1):1–5
Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey E, Jönsson B, Kanis J (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1–2):136
Cooper C, Campion G, Melton L III (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2(6):285–289
Lau EMC, Suriwongpaisal P, Lee JK, De Das S, Festin MR, Saw SM, Khir A, Torralba T, Sham A, Sambrook P (2001) Risk factors for hip fracture in Asian Men and Women: the Asian Osteoporosis Study. J Bone Mineral Res 16:572–580
Suzuki T (2001) Risk factors for osteoporosis in Asia. J Bone Min Metab 19:133–141
Mithal V, Dhingra V, Lau E (2009) The Asian Audit. Epidemiology, costs and burden of osteoporosis in Asia. International Osteoporosis Foundation. www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf. Accessed 11 Oct 2016
Chee WS, Suriah AR, Chan SP, Yap SL, Chan YM (2002) Dietary calcium intake in postmenopausal Malaysian women: comparison between the food frequency questionnaire and three day food records. Asia Pac J Clin Nutr 11(2):142–146
Lee J-K, Khir ASM (2007) The incidence of hip fracture in Malaysians above 50 years of age: variation in different ethnic groups. Int J Rheum Dis 10(4):300–305
Heaney RP (2002) Ethnicity, bone status and the calcium requirement. Nutr Res 22:153–178
Green TJ, Skeaff CM, Rockell JEP, Venn BJ, Lambert A, Todd J, Khor GL, Loh SP, Muslimatun S, Agustina R, Whiting SJ (2008) Vitamin D status and its association with parathyroid hormone concentrations in women of child-bearing age living in Jakarta and Kuala Lumpur. Eur J Clin Nutr 62:373–378
Lau EMC, Cooper C (1996) The epidemiology of osteoporosis: the oriental perspective in a world context. Clin Orthop 323:65–74
Rahman SA, Chee WSS, Yassin Z, Chan SP (2004) Vitamin D status among postmenopausal Malaysian women. Asia Pacific J CLin Nutr 13(3):255–260
Sirichakwal PP, Kamchansuppasin A, Akoh CC, Kriengsinyos W, Charoenkaitkhul S, O’Brien KO (2015) Vitamin D status is positively associated with calcium absorption among postmenopausal Thai women with low calcium intakes. J Nutr 145:990–995
Malaysian Dietary Guidelines (2010) http://www.moh.gov.my/english.php/pages/view/536. Accessed 11 Oct 2016
Ho-Pham L, Nguyen N, Lai T, Eisman J, Nguyen T (2011) Vitamin D status and parathyroid hormone in a urban population in Vietnam. Osteoporos Intl 22(1):241–248
Lu H, Zhang Z, Ke Y, He J, Fu W, Zhang C, Zhang Z (2012) High prevalence of vitamin D insufficiency in China: relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS One 7(11):e7264
Nguyen H, von Schoultz B, Nguyen T, Dzung D, Duc P, Thuy V, Hirschberg A (2012) Vitamin D deficiency in northern Vietnam: prevalence, risk factors and associations with bone mineral density. Bone 51(6):1029–1034
Oemardi M, Horowitz M, Wishart J, Morris H, Need A, O’loughlin P, Nordin B (2007) The effect of menopause on bone mineral density and bone-related biochemical variables in Indonesian women. Clin Endocrinol 67(1):93–100
Zhen D, Liu L, Guan C, Zhao N, Tang X (2015) High prevalence of vitamin D deficiency among middle-aged and elderly individuals in North-Western China: its relationship to osteoporosis and lifestyle factors. Bone 71:1–6
Lau EMC, Woo J, Lam V, Hong A (2001) Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. J Bone Min Res 16:1704–1709
Lau E, Lynn H, Chan Y, Woo J (2002) Milk supplementation prevents bone loss in postmenopausal Chinese women over 3 years. Bone 31(4):536–540
Chee W, Suriah A, Chan S, Zaitun Y, Chan Y (2003) The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int 14(10):828–834
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011) Evaluation, treatment and prevention of vitamin D deficiency and Endocrine Society clinical practice guidelines. J Clin Endocrinol Metab 96(7):1911–1930
Holloway L, Moynihan S, Abrams SA, Kent K, Hsu AR, Friedlander AL (2007) Effects of oligofructose-enriched inulin on intestinal absorption of calcium and magnesium and bone turnover markers in postmenopausal women. Br J Nutr 97:365–372
Palacios C (2006) The role of nutrients in bone health, from a to Z. Crit Rev Food Sci Nutr 46:621–628
Maunzell Z, Wright DJ, Rainbow SJ (2005) Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem 51(9):1683–1690
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Encrinol Metab 96(1):53–58
Haines CJ, Chung TKH, Leung PC, Leung DHY, Wong MY, Lam LL (1994) Dietary calcium intake in postmenopausal Chinese women. Eur J Clin Nutr 48:591–594
Woo JW, Lau W, Ling X, Lam CWK, Zhao X, Yu W, Xing X, Lau E, Kuhn-Sherlock B, Pocock N, Eastell R (2007) Milk supplementation and bone health in young adult Chinese women. J Women’s Health 16(5):695–702
Kruger MC, Ha P, Todd JM, Kuhn-Sherlock B, Schollum LM, Jiliang M, Qin G, Lau E (2012) High-calcium, vitamin D fortified milk is effective in improving bone turnover markers and vitamin D status in healthy post-menopausal Chinese women. Eur J Clin Nutr 66:856–861
Kruger MC, Ha P, Todd JM, Kuhn-Sherlock B, Schollum LM, Jiliang M, Qin G, Lau E (2013) High-calcium, vitamin D fortified milk is effective in improving vitamin D status and reducing bone resorption in healthy post-menopausal Chinese women. Chin J Osteoporos 19(2):107–113
Bacon CJ, Woo J, Lau EMC, Lam CWK, Gamble GD, Reid IR (2010) Effects of 25-hydroxyvitamin D level and its change on parathyroid hormone in premenopausal Chinese women. Osteoporos Int 21(11):1935–1941
Lim SK, Kung AWC, Sompongse S, Soontrapa S, Tsai KS (2008) Vitamin D inadequacy in postmenopausal women in Eastern Asia. Curr Med Res Opinions 4(1):99–106
Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R (2005) Estimates of optimal vitamin D status. Osteoporos Int 16:713–716
Shibli-Rahhal A, Patiru B (2014) Variations in parathyroid hormone concentration in patients with low 25 hydroxyvitamin D. Osteoporos Int 25(7):1931–1936
Kuchuk NO, Van Schoor NM, Pluijm SM, Chines A, Lips P (2009) Vitamin D status, parathyroid function, bone turnover and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Min Res 24(4):693–701
Huang OR, Lu JH, Zhou Q, Liu YJ, Wang QH (2000) Effect of calcium and vitamin D supplementation on bone loss in postmenopausal Chinese women: a comparative study. Osteoporos Int 11(2):S183
Kruger MC, Schollum LM, Kuhn-Sherlock B, Hestiantoro A, Wijanto P, Li-Yu J, Agdeppa I, Todd JM, Eastell R (2010) The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South-East Asia. Bone 46:759–767
Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Islam S, Yeh JK (2013) Calcim and vitamin D supplementation in postmenopausal women. J Clin Endocrinol Metab 98(11):E1702–E1709
Pi Y-Z, Wu X-P, Liu S-P (2006) Age-related changes in bone biochemical markers and their relationship with bone mineral density in normal Chinese women. J Bone Min Res 24(5):380–385
Trento LK, Pietropolli A, Ticconi C, Gravotta E, De Martino MU (2009) Role of type I collagen C telopeptide, bone specific alkaline phosphatase and osteocalcin in the assessment of bone status in postmenopausal women. J Obstet Gynaecol Res 35(1):152–159
Lenora J, Ivaska KK, Obrant KJ, Gerdhem P (2007) Prediction of bone loss using biochemical markers of bone turnover. Osteoporos Int 18:1297–1305
Grados F, Brazier M, Kamel S, Mathieu M, Hurtebize N, Maamer M, Garabedien M, Sebert J-L, Fardellone P (2003) Prediction of bone mass density variation by bone remodelling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. J Clin Endocrin Metab 88(11):5175–5179
Sahni SS, Mangano KM, Tucker KL, Kiel DP, Casey VA, Hannan MT (2014) Protective association of milk intake on the risk of hip fracture results from the Framingham original cohort. J Bone Min Res 29(8):1756–1762
Tamaki J, Iki M, Kadowaki E, Sato Y, Chiba Y, Akiba T, Matsumoto T, Nishino H, Kagamimori S, Kagawa Y, Yoneshima H, for JPOS study group (2013) Biochemical markers of bone turnover predict risk of vertebral fractures in postmenopausal women over 10 years: the Japanese Population-based Osteoporosis Cohort Study. Osteoporos Int 24:887–897
Liao E-Y, Wu X-P, Deng X-G, Huang G, Zhu X-P, Long Z-F, Wang W-B, Tang W-L, Zhang H (2002) Age-related bone density, accumulated bone loss rate and prevalence of osteoporosis at multiple skeletal sites in Chinese women. Osteoporos Int 13:669–676
Ting GP, Tan SY, Chan SP, Karuthan C, Zaitun Y, Suriah AR, Chee WSS (2007) A follow-up study on the effects of a milk supplement on bone mineral density of postmenopausal Chinese women in Malaysia. J Nutr Health Ageing 11(1):69–73
Ma D, Zheng W, Ding M, Zhang Y, Wang P (2014) Milk intake increases bone mineral content through inhibiting bone resorption: meta-analysis of randomized controlled trials. e-SPEN J 8(1):e1–e7
Bischoff-Ferrari HA, Dawson-Huges B, Baron JA, Kanis JA, Orav EJ, Staehelin HB, Kiel DP, Burckhardt P, Henschowski J, Spiegelman D, Li R, Wong JB, Feskanich D, Willett WC (2011) Milk intake and risk of hip fracture in men and women: a meta-analyses of prospective cohort studies. J Bone Miner Res 26(4):833–889
Coxam V (2007) Current data with inulin-type fructans and calcium, targeting bone health in adults. J Nutr 137:25275–25335
Hayhoe RPG, Lentjes MAH, Luben RN, Khaw K-T, Welch AA (2015) Combined dietary magnesium and potassium intake is associated with greater bone density in women in the EPIC-Norfolk cohort. Proc Nutr Soc. 74:OEC1
Fung EB, Kwiatkowski JL, Huang JN, Gildengorin G, King J, Vichinsky EP (2013) Zinc supplementation improves bone density in patients with thalassemia: a double-blind randomised placebo-controlled trial. Am J Clin Nutr 98:960–971
Shiota J, Tagawa H, Izumi N, Higashikawa S, Kasahara H (2015) effect of zinc supplementation on bone formation in hemodialysis patients with normal or low bone turnover. Ren Fail 37:57–60
Asmawi MZ, Seppo L, Vapaatalo H, Korpela R (2006) Hypolactasia and lactose intolerance among three ethnic groups in Malaysia. Indian J Med Res 124:697–704
Acknowledgements
The study was funded by Fonterra Brands Singapore Pte Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.M Schollum and JM Todd are employees of Fonterra Cooperative Ltd. B Kuhn-Sherlock is a consultant statistician engaged by Fonterra Cooperative Group Ltd, New Zealand. All other authors do not have a conflict to declare.
Rights and permissions
About this article
Cite this article
Kruger, M.C., Chan, Y.M., Lau, L.T. et al. Calcium and vitamin D fortified milk reduces bone turnover and improves bone density in postmenopausal women over 1 year. Eur J Nutr 57, 2785–2794 (2018). https://doi.org/10.1007/s00394-017-1544-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1544-6