Abstract
Summary
Optimal levels of 25-hydroxyvitamin D [25(OH)D] were investigated in premenopausal Chinese women. Parathyroid hormone (PTH) change at 3 months was associated with change in 25(OH)D but not with baseline levels, and PTH fell even when starting levels of 25(OH)D were >40 nmol/L, consistent with optimal values for 25(OH)D of ≥40 nmol/l.
Introduction
The upper level of 25-hydroxyvitamin D [25(OH)D] which constitutes a long-term bone health risk by causing elevated PTH levels is uncertain. Although many studies have addressed this question using cross-sectional data, the present study is one of few employing a prospective approach to determine 25(OH)D levels required to minimize PTH.
Methods
Relationships among baseline values and 3-month changes (Δ) in PTH and 25(OH)D were assessed in 221 Chinese women, aged 28.0 ± 4.4 years (mean ± SD), taking part in a placebo-controlled dairy product intervention delivering 200 IU vitamin D3/day.
Results
Baseline 25(OH)D was 34 ± 11 nmol/L and was inversely related to baseline PTH (r = −0.18, P = 0.007), with a plateau in PTH levels when 25(OH)D was >40 nmol/L. After 3 months intervention, PTH fell 11% and neither Δ25(OH)D nor ΔPTH differed between treatment and control groups. ΔPTH was inversely related to Δ25(OH)D (P < 0.001) but not to baseline 25(OH)D. Similarly, ΔPTH differed between quartiles of Δ25(OH)D (P < 0.001), but not between quartiles of baseline 25(OH)D and no interaction was observed between quartiles of baseline 25(OH)D and Δ25(OH)D. Even in the highest quartile of baseline 25(OH)D (>40 nmol/L), PTH fell 0.4 ± 0.1 pmol/L (mean ± SEM; P = 0.008).
Conclusions
We conclude that vitamin D deficiency is common in young women in Hong Kong. The cross-sectional analysis indicates that optimal 25(OH)D is >40 nmol/L, and the longitudinal data is consistent with a higher optimal value which is not defined in this study’s results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D insufficiency, indicated by low levels of 25-hydroxyvitamin D [25(OH)D] results in elevated parathyroid hormone (PTH) levels [1]. Elevated levels of PTH are likely to result in lowered bone mineral density and increased fracture risk [1]. Vitamin D supplementation of elderly people with low vitamin D status lowers PTH, reduces bone loss and may prevent hip fractures [2, 3]. Despite this, the optimal level of 25(OH)D is unclear [4] and a systematic review of the literature investigating thresholds in the relationship between PTH and 25(OH)D has reported estimates which have ranged from 25 to 122 nmol/L [5].
A frequently employed method for determining optimal vitamin D status uses the relationship between 25(OH)D and PTH in cross-sectional databases to identify a threshold level of 25(OH)D above which a plateau in PTH occurs [4, 6, 7]. An alternative approach for determining optimal 25(OH)D levels is to use a prospective study design to look for a level of baseline 25(OH)D that is not associated with reduction in PTH following vitamin D supplementation. Utilizing longitudinal data in this way may be superior to a cross-sectional approach since it eliminates bias from confounding effects such as age, health status or calcium intake that may be associated with 25(OH)D levels. To date, only four studies have investigated a 25(OH)D threshold longitudinally [5, 8–10], and have reported 25(OH)D thresholds at the lower end of the range determined from cross-sectional studies (between 40 and 50 nmol/L). Longitudinal data from a further study providing supplemental vitamin D found an association between PTH reductions and initial levels of 25(OH)D [11], but did not report a threshold.
Older adults have higher PTH levels at the same level of 25(OH)D compared to their younger counterparts [12, 13]. This is likely to result from age-related reductions in intestinal calcium absorption [14] and renal function [15]. The vast majority of published cross-sectional studies are of adults older than 50 years, although there are some studies in children [16–20], and younger adults [21–26]. Additionally, most studies of younger people have either not reported a threshold-based optimal 25(OH)D level, or have not observed an association between 25(OH)D and PTH at all [16–23, 25, 26]. Furthermore, since longitudinal investigations have only been undertaken in older groups (mean ages between 47 and 67 years) the effect of age in modulating PTH response to changes in 25(OH)D is unknown.
In addition to age, calcium intake is likely to affect the relationship between PTH and 25(OH)D. Low calcium intake is independently associated with elevated PTH in the elderly [11, 12] and in younger adults [27]. A 25(OH)D threshold has not previously been investigated either cross-sectionally or longitudinally in Chinese or other East Asian populations. Their lower calcium intake [28, 29] may change any relationships between vitamin D status and PTH levels.
The purpose of this study is to investigate whether an optimal level of 25(OH)D could be determined in a population of young, healthy Chinese women through the identification of a threshold level of 25(OH)D above which there is no change in PTH levels following vitamin D supplementation.
Methods
Participant recruitment
A randomized controlled trial of the effect of a calciferol-containing dairy product on bone health, carried out in Hong Kong, presented an opportunity to investigate the relationship between 25(OH)D and PTH changes in younger women. Healthy Chinese volunteers aged between 20 and 35 years were recruited between February and June 2002 through poster advertisements in public places and mass emailing as previously described [30]. An initial interview excluded women who had a medical history of metabolic bone, liver, endocrine, connective tissue, and respiratory diseases, cancer or previous operations or who were taking calcium or vitamin D supplements or medications (other than oral contraceptives) likely to affect bone metabolism. Women who were amenorrheic, lactating, or intending to become pregnant during the next 2 years were also excluded from the study. A total of 327 women were screened, of whom 65 were ineligible, and a further 41 did not attend the first visit. The remaining 221 were randomized to receive either two daily sachets of fortified milk powder containing a total of 1,000 mg calcium and 200 IU (5 µg) cholecalciferol (Milk group), or no dairy product (Control group). Randomization was balanced between two age groups, 20–27 years and 28–35 years: a total of 111 and 110 women, from each age group, respectively, took part in the study. Compliance was checked by counting returned empty sachets. Approval to conduct the study was provided by The Chinese University of Hong Kong Ethics Committee.
Baseline assessment
All participants completed a questionnaire containing demographic and health information. They also completed baseline assessments of physical activity and dietary intake. Physical activity was measured using a modified version of the Physical Activity Scale for the Elderly [31], with examples of types of activity in the different categories of exercise intensity altered to be more appropriate for the population group. Dietary intake was assessed using a 5-day diet record completed prior to their visit. Records were checked during the visit by a nutritionist, who clarified uncertain food item portions using actual size food models and food pictures. Nutritional analysis was undertaken using Food Processor, version 8.0 software (ESHA Research, OR, USA) with nutrient composition of local Chinese foods added to the database using data from manufacturers and from the Institute of Nutrition and Food Safety of China, China Food Composition [32]. Height and body mass were measured in light indoor clothing without shoes. Percent body fat was determined from total body DXA measurements made using a Hologic Delphi A scanner (Hologic Inc, Bedford, MA, USA).
Blood collection and analysis
Fasting blood samples were collected at baseline and 3 months for measurement of serum calcium, albumin, phosphate, 25(OH)D, and PTH. Calcium, albumin, and phosphate were measured using Roche Modular DP Colorimetry. DiaSorin radioimmunoassay (DiaSorin Inc., Stillwater, MN, USA) was used to quantify 25(OH)D (sensitivity 3.7 nmol/L; coefficient of variation (CV) 9.1% at 82 nmol/L) and Immulite 1000 chemiluminescence immunoassay (Diagnostic Products Corporation, Los Angeles, CA, USA) was used to quantify PTH (sensitivity 0.1 pmol/L; CV 8.6% at 6.2 pmol/L). All assays were carried out at the Chinese University of Hong Kong Pathology Laboratory which is accredited by the Australian National Association of Testing Authorities and the Royal College of Pathologists of Australasia.
Statistical analyses
Statistical analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Both cross-sectional and longitudinal relationships between 25(OH)D, and PTH were examined. Linear relationships were reported as Pearson’s correlation coefficients. One-phase exponential decay curves were fitted to identify possible thresholds in the data. Stepwise, multiple linear regression models (P in = 0.05, P out = 0.10) were used to identify determinants of baseline 25(OH)D and PTH. We included demographic, nutritional intake, physical activity, adiposity, and clinical biochemical variables in the models. We applied a two-way general linear analysis of variance (ANOVA) to determine whether change (Δ) in PTH differed between quartiles of baseline 25(OH)D or with quartile of 3-month change in 25(OH)D [Δ25(OH)D]. Data are presented as mean ± standard error of the mean (SEM) unless otherwise stated.
Results
Participants
Prior to the intervention, 25(OH)D levels were in the range 11–70 nmol/L. They were <25 nmol/L in 18%, <30 nmol/L in 36%, and <50 nmol/L in 93% of the women. PTH was elevated above the upper reference level (7.3 pmol/L) in only five of the 221 individuals. Baseline characteristics are shown in Table 1.
Baseline analyses
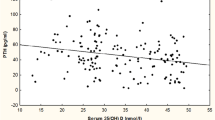
Baseline PTH and 25(OH)D were negatively correlated, with PTH showing a gradual curvilinear decline with increasing 25(OH)D (Fig. 1). There was no further reduction in PTH above a 25(OH)D of 40 nmol/L.
The relationship between baseline parathyroid hormone (PTH) and 25-hydroxyvitamin D [25(OH)D] (r = -0.18, P = 0.007 for linear relationship). 25(OH)D levels were grouped in intervals of 5 nmol/L. The curve represents the best fit to the data of one-phase exponential decay to a PTH plateau of 2.9 pmol/L (r 2 = 0.04) Numbers in brackets indicate the sample size for each data point
Physical activity index was positively associated with baseline 25(OH)D while baseline PTH was negatively associated with serum albumin and calcium and with dietary intakes of protein, fat, and energy (Table 2). Multiple regression models retained physical activity index (positive) and baseline PTH (negative) as significant predictors of 25(OH)D, explaining 7.4% of its variance (P < 0.001). Determinants of PTH on multiple regression analysis were serum calcium, fat intake and 25(OH)D (all negative), which collectively explained 13% of its variance (P < 0.001).
Changes in 25(OH)D and PTH
Mean levels of 25(OH)D rose by 9 ± 1 nmol/L at 3 months and those of PTH fell by 0.3 ± 0.1 pmol/L (P < 0.001 for both). There was no significant difference in either Δ25(OH)D or ΔPTH between the milk and control groups, although there was a trend for Δ25(OH)D to be greater in the milk group (11 ± 1 nmol/L compared to 8 ± 1 nmol/L in the control group, P = 0.06). Data from both groups were combined for longitudinal analyses. Δ25(OH)D was 14 ± 1 nmol/L in those with baseline values below the median of 33 nmol/L compared with 5 ± 1 nmol/L in those above the median (P < 0.001).
Δ25(OH)D was negatively correlated with ΔPTH (Fig. 2) and explained 5% of its variance in a multiple regression model which included both baseline and Δ25(OH)D (P < 0.001). Baseline 25(OH)D was not related to ΔPTH (Fig. 3). Neither baseline 25(OH)D nor treatment group, when it was included, entered the ΔPTH regression model. Of the 13 women who had baseline 25(OH)D levels ≥50 nmol/L, only three experienced increases ≥10 nmol/L in this metabolite.
To further clarify the relationships between baseline 25(OH)D, and the changes in 25(OH)D and PTH, the 25(OH)D variables were expressed as quartiles (Fig. 4). A two-way fixed factorial ANOVA with ΔPTH as the dependent variable demonstrated an effect of quartiles of Δ25(OH)D (P = 0.006), confirming the data shown in Fig. 2. Bonferroni post-hoc testing showed that the lowest quartile of Δ25(OH)D was significantly different from the two highest quartiles, with respect to ΔPTH values (P < 0.05). ΔPTH was not different between quartiles of baseline 25(OH)D, confirming the data shown in Fig. 3, and there was no significant interaction between baseline and Δ25(OH)D. Thus, all quartile groups with an increase in 25(OH)D of 10 nmol/L or more showed a mean fall in PTH, and there was no threshold for this effect, with respect to baseline 25(OH)D. Across the highest quartile of baseline 25(OH)D (>40 nmol/L), PTH fell 0.4 ± 0.1 pmol/L (P = 0.008, for a one-sample t test of ΔPTH difference from 0).
Change in PTH as a function of both change and baseline 25(OH)D. Across the entire lowest quartile of Δ25(OH)D, ΔPTH was 0.3 ± 0.2 pmol/L, which was significantly different from ΔPTH in the highest two quartiles (−0.7 ± 0.2 pmol/L and −0.7 ± 0.2 pmol/L for quartiles 3 and 4, respectively). Note that for the entire highest quartile of baseline 25(OH)D (>40 nmol/L), ΔPTH was −0.4 ± 0.1 pmol/L, so a reduction in PTH was still evident
Discussion
These data demonstrate low vitamin D status in healthy premenopausal Chinese women living in Hong Kong (latitude 22°N). The mean serum 25(OH)D level of 34 nmol/L found here during spring is considerably lower than winter levels in young women in the USA (63 nmol/L) [33], Australia (64 nmol/L) [34] or year-round levels of young women in the UK or New Zealand (49 nmol/L) [35, 36]. Surprisingly, our results indicate lower 25(OH)D levels in the present cohort than in young women of a similar age living in urban Shenyang (North East China: 42°N) [23], where 19% had levels <30 nmol/L compared with 36% in the present study. Levels in Hong Kong young women also appear to be somewhat lower than results from middle-aged and older Chinese women living in Hong Kong [37] and Malaysia [38], who have mean levels of around 70 nmol/L.
Although the reasons for such low levels of 25(OH)D were not fully examined, it is possible that they may be related to the long working hours, indoor lifestyle, and commuting patterns of this cohort. Low ultraviolet radiation levels due to pollution may also be a factor explaining the reduced 25(OH)D. Sun avoidance for cosmetic reasons is also likely to play a part. Frequent sunscreen and parasol use and a dislike of going in the sun have been reported amongst older Chinese women [39]. Anecdotal reports suggest that these practices may be even more common in younger women and perhaps adolescent girls. A random sample from a large study of adolescent girls living in Beijing (40°N) found particularly low 25(OH)D levels in both winter and summer (mean ± SD, 13 ± 8 and 27 ± 11 nmol/L, respectively) [40]. In the present study, baseline measurements took place in spring months when sunshine is plentiful in Hong Kong, and prior to the rainy typhoon season. Although there is a lack of data to describe seasonal changes in vitamin D status in tropical areas, the 3-month increases in 25(OH)D we observed are likely to be contributed to by seasonal changes since they were only slightly greater in the milk group, compared with control.
Our results showed physical activity to be positively associated with 25(OH)D in this cohort, probably because of its association with increased time outdoors [41]. There was a borderline association with vitamin D intake—although few foods contain useful quantities of vitamin D, vitamin D intake has been shown to contribute to 25(OH)D levels when sun exposure is minimal and diets are high in fish or fortified foods [42]. Though not observed in this study, inverse relationships between 25(OH)D and indices of body fat have also been reported previously [41, 43–45]. It is likely that in this lean population, the range in adiposity was insufficient to reveal this relationship.
Our cross-sectional data suggest that a 25(OH)D threshold for decreasing PTH may exist around 40 nmol/L. Many previous studies have estimated optimal 25(OH)D using a range of methods based on cross-sectional data [for example, [7, 18, 24, 27] and produced wide-ranging estimates. Difficulties associated with the identification of optimal 25(OH)D levels through the use of statistical estimates of turning points, plateaus or intersections of two-slope spline models have been recently addressed [5]. Nonetheless, our data show distinctly higher PTH levels when baseline 25(OH)D is below 40 nmol/L and no further reduction above this level (Fig. 1). Both age [13] and calcium intake [27] affect the relationship between PTH and 25(OH)D. It is possible that our estimate of optimal 25(OH)D levels based on its cross-sectional relationship with PTH may be lower than that observed in many reports because the present study is of young women with low dietary calcium intakes.
The longitudinal data from the present study do not show a 25(OH)D threshold, above which there is no fall in PTH, in contrast to our cross-sectional analysis. As evident from Fig. 4, young women with baseline 25(OH)D levels in the upper quartile (>40 nmol/L) displayed a potentially beneficial 3-month fall in PTH of 0.4 pmol/L. If 25(OH)D was approaching a threshold, then the reduction in PTH would be expected to be less at higher baseline levels of 25(OH)D, yet we observed no relationship between baseline 25(OH)D and the change in PTH. Of the four studies that have investigated a PTH plateau using a prospective approach, all have reported markedly reduced changes in PTH at baseline 25(OH)D levels of 40–50 nmol/L [5, 8–10]. Since only three subjects in the present study who had increases in 25(OH)D ≥ 10 nmol/L also had a baseline 25(OH)D ≥ 50 nmol/L, we had insufficient numbers to detect a threshold of this level. Therefore, results from our prospective analysis are not inconsistent with past findings.
In conclusion, the present study demonstrates that serum 25(OH)D concentrations are substantially lower in healthy young women in Hong Kong than in comparable Western populations, and also lower than those reported in postmenopausal women in the same region. Cross-sectionally, 25(OH)D values of <40 nmol/L were associated with elevations in PTH. Supplementation with a milk product containing 200 IU/day was inadequate to raise 25(OH)D compared to the control group in this cohort. During follow-up, increases in 25(OH)D in both treatment and control groups were related to declines in PTH, but these did not appear to be related to the baseline 25(OH)D concentration. We observed potentially beneficial falls in PTH even for individuals with baseline 25(OH)D levels in the 40–50 nmol/L range. Thus, if there is a threshold above which further increases in 25(OH)D are not accompanied by suppression of PTH, it is above this range in this cohort. The fall in PTH following improved vitamin D status in these healthy young Chinese women, even those in the highest quartile of baseline 25(OH)D, suggests that this cohort is likely to benefit from vitamin D supplementation.
References
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–1642
Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P (1995) Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab 80:1052–1058
Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R (2005) Estimates of optimal vitamin D status. Osteoporos Int 16:713–716
Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK (2006) Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 84:602–609
Lips P (2004) Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Mol Biol 89–90:611–614
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443
Malabanan A, Veronikis IE, Holick MF (1998) Redefining vitamin D insufficiency. Lancet 351:805–806
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221
Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR (2009) High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 20:1407–1415
Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, Johnston CC (2000) Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab 85:3011–3019
McKane WR, Khosla S, Egan KS, Robins SP, Burritt MF, Riggs BL (1996) Role of calcium intake in modulating age-related increases in parathyroid function and bone resorption. J Clin Endocrinol Metab 81:1699–1703
Vieth R, Ladak Y, Walfish PG (2003) Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 88:185–191
Heaney RP, Recker RR, Stegman MR, Moy AJ (1989) Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res 4:469–475
Aloia JF, Feuerman M, Yeh JK (2006) Reference range for serum parathyroid hormone. Endocr Pract 12:137–144
Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO (2005) Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab 90:5576–5581
Cheng S, Tylavsky F, Kröger H, Kärkkäinen M, Lyytikäinen A, Koistinen A, Mahonen A, Alen M, Halleen J, Väänänen K, Lamberg-Allardt C (2003) Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr 78:485–492
Fuleihan GE-H, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, Tannous R (2001) Hypovitaminosis D in healthy schoolchildren. In Pediatrics. p E53.
Guillemant J, Cabrol S, Allemandou A, Peres G, Guillemant S (1995) Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone 17:513–516
Outila TA, Karkkainen MU, Lamberg-Allardt CJ (2001) Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr 74:206–210
Välimäki V-V, Alfthan H, Lehmuskallio E, Löyttyniemi E, Sahi T, Stenman U-H, Suominen H, Välimäki MJ (2004) Vitamin D status as a determinant of peak bone mass in young Finnish men. J Clin Endocrinol Metab 89:76–80
Nakamura K, Ueno K, Nishiwaki T, Okuda Y, Saito T, Tsuchiya Y, Yamamoto M (2005) Nutrition, mild hyperparathyroidism, and bone mineral density in young Japanese women. Am J Clin Nutr 82:1127–1133
Yan L, Prentice A, Zhang H, Wang X, Stirling DM, Golden MM (2000) Vitamin D status and parathyroid hormone concentrations in Chinese women and men from north-east of the People’s Republic of China. Eur J Clin Nutr 54:68–72
Lamberg-Allardt CJ, Outila TA, Kärkkäinen MU, Rita HJ, Valsta LM (2001) Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res 16:2066–2073
Meier DE, Luckey MM, Wallenstein S, Clemens TL, Orwoll ES, Waslien CI (1991) Calcium, vitamin D, and parathyroid hormone status in young white and black women: association with racial differences in bone mass. J Clin Endocrinol Metab 72:703–710
Tangpricha V, Pearce EN, Chen TC, Holick MF (2002) Vitamin D insufficiency among free-living healthy young adults. Am J Med 112:659–662
Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G (2005) Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 294:2336–2341
Chan GM (1991) Dietary calcium and bone mineral status of children and adolescents. Am J Dis Child 145:631–634
Lau EM, Suriwongpaisal P, Lee JK, De Das S, Festin MR, Saw SM, Khir A, Torralba T, Sham A, Sambrook P (2001) Risk factors for hip fracture in Asian men and women: the Asian osteoporosis study. J Bone Miner Res 16:572–580
Woo J, Lau W, Xu L, Lam CWK, Zhao X, Yu W, Xing X, Lau E, Kuhn-Sherlock B, Pocock N, Eastell R (2007) Milk supplementation and bone health in young adult Chinese women. J Womens Health (Larchmt) 16:692–702
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Yang Y, Waing G, Pan X (eds) (2002) Institute of Nutrition and Food Safety, China CDC. China Food Composition 2002. University Medical Press, Peking.
Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR (2002) Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777
Pasco JA, Henry MJ, Nicholson GC, Sanders KM, Kotowicz MA (2001) Vitamin D status of women in the Geelong Osteoporosis Study: association with diet and casual exposure to sunlight. Med J Aust 175:401–405
Rockell JEP, Skeaff CM, Williams SM, Green TJ (2006) Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos Int 17:1382–1389
Ruston D, Hoare J, Henderson L, Gregory J, Bates CJ, Prentice A, Birch M, Swan G, Farron M (2004) The National Diet and Nutrition Survey: adults aged 19 to 64 years. Volume 4: nutritional status (anthropometry and blood analytes), blood pressure and physical activity. In Health Do, Agency FS, Statistics OfN, Research MRCHN (eds). TSO.
Lau EM, Woo J, Lam V, Hong A (2001) Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. J Bone Miner Res 16:1704–1709
Chee WSS, Suriah AR, Chan SP, Zaitun Y, Chan YM (2003) The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int 14:828–834
Kung AWC, Lee K-K (2006) Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: a population survey in Hong Kong. BMC Public Health 6:226
Du X, Greenfield H, Fraser DR, Ge K, Trube A, Wang Y (2001) Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr 74:494–500
Lucas JA, Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR (2005) Determinants of vitamin D status in older women living in a subtropical climate. Osteoporos Int 16:1641–1648
Nakamura K, Nashimoto M, Okuda Y, Ota T, Yamamoto M (2002) Fish as a major source of vitamin D in the Japanese diet. Nutrition 18:415–416
Arunabh S, Pollack S, Yeh J, Aloia JF (2003) Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 88:157–161
Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR (2006) Determinants of vitamin D status in older men living in a subtropical climate. Osteoporos Int 17:1742–1748
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693
Acknowledgment
This study was supported by Fonterra Brands Ltd, Auckland.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bacon, C.J., Woo, J., Lau, E.M.C. et al. Effects of 25-hydroxyvitamin D level and its change on parathyroid hormone in premenopausal Chinese women. Osteoporos Int 21, 1935–1941 (2010). https://doi.org/10.1007/s00198-009-1163-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1163-z