Abstract
Purpose
We explored the pathophysiologic mechanisms of long-term fructose-induced lower urinary tract symptoms (LUTS) in rats.
Methods
Male Wistar rats were fed with fructose for 3 or 6 months. Biochemical and transcystometric parameters were compared between fructose-fed and age-matched normal-diet rats. Pelvic nerve and external urethral sphincter-electromyogram activity recordings were performed to investigate fructose effects on neural control of bladders. Mitochondrial structure, ATP and acetylcholine content and purinergic and muscarinic cholinergic receptors were examined. Cytosolic cytochrome C staining by Western blot and immunocytochemistry for mitochondrial injury and PGP 9.5 stain for nerve density were also determined.
Results
The fructose-fed rats with higher plasma triglyceride, LDL and fasting glucose levels displayed LUTS with increased frequency and suppressed voiding contractile amplitude in phase 1 and phase 2 duration versus normal-diet control. Fructose feeding altered the firing types in pelvic afferent and efferent nerves and external urethral sphincter-electromyogram activity. Increased mast cell number, disrupted and swollen mitochondria, increased cytosolic cytochrome C stain and expression and decreased nerve density in bladder smooth muscle layers appeared in the fructose-fed rats. Fructose feeding also significantly reduced ATP and acetylcholine content and enhanced protein expression of postsynaptic P2X1, P2X2 and P2X3 purinergic receptors and M2 and M3 muscarinic cholinergic receptors expression in the smooth muscles of urinary bladder.
Conclusion
Long-term fructose feeding induced neuropathy and myopathy in the urinary bladders. Impaired mitochondrial integrity, reduced nerve density, ATP and acetylcholine content and upregulation of purinergic and muscarinic cholinergic receptors expression may contribute to the bladder dysfunction of fructose-fed animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome and diabetes are known risk factors for the development of lower urinary tract symptoms (LUTS) including a high prevalence of overactive bladder [1–4]. The increased high-fructose consumption results in obesity and metabolic syndrome [5, 6], which may contribute to vesicular neuropathy. In patients, there were linear associations between storage/voiding LUTS (frequency and incomplete emptying) and increased abdominal fat mass, plasma glucose and low-density lipoprotein cholesterol (components of the metabolic syndrome) [7]. Alterations in these biochemical parameters may lead to oxidative stress and mitochondria injury in neural, vascular and muscular dysfunction [8–10]. In the urinary bladder, ATP-purinergic and acetylcholine-muscarinic cholinergic signaling associated with coordinated external urethral sphincter activity are required for performing an efficient voiding contraction [11–13]. Several studies have demonstrated that the muscarinic cholinergic and purinergic signaling were altered in detrusor muscle of variable urologic diseases [14, 15].
The fructose-fed rat model has been well developed with a very similar metabolic profile to the human condition [16]. Furthermore, a bladder dysfunction with bladder overactivity in vivo and an alteration in contractile activity to KCl, muscarinic cholinergic agonist or ATP in vitro was noted in the urinary bladder after long-term fructose feeding [16]. Therapeutic potential for using muscarinic cholinergic and/or purinergic antagonist has been frequently discussed in the diseases of the urinary tract [17–19]. Because up to 40 % of the neural responses were purinergic in the pathological situations including interstitial cystitis, outflow obstruction, idiopathic detrusor instability and neurogenic bladder, P2X receptor antagonists are being explored as therapeutic agents [17]. The therapeutic potential of the cholinergic system is also well established in the urinary tract diseases and using the muscarinic antagonists being the primary treatment for patients with the overactive bladder [18, 19].
Therefore, in this study, we attempt to explore the neural mechanisms underlying bladder dysfunction by the long-term effects of metabolic syndrome on male rat urinary bladder function. The bladder in male rat fed with high-fructose-containing diet for 3 and 6 months will be compared to age-matched normal controls. In addition to biochemical, physiologic, pathologic and transcystometric parameters, we investigated and analyzed the neurophysiologic recordings of pelvic afferent and efferent nerve activity and external urethral sphincter-electromyogram, mitochondrial structure, ATP and acetylcholine content, purinergic (P2X1, P2X2 and P2X3) and muscarinic cholinergic receptor (M2 and M3) expression of the urinary bladder and immunostaining with PGP 9.5 for examination of the nerve density in the urinary bladder.
Methods and procedures
Experimental animals
Twenty-eight male Wistar rats were housed at the Experimental Animal Center, National Taiwan University, at a constant temperature and with a consistent light cycle (light from 07:00 to 18:00 o’clock). Fructose-fed animals were fed a fructose-rich diet (60 % fructose diet, Harlan Teklad), whereas control animals received standard rat chow for 3 or 6 months, respectively. The rats were divided into four groups as following characterization: 3 months of fructose-fed rats with LUTS (n = 8); 6 months of fructose-fed rats with LUTS (n = 8), and age-matched control groups for 3 or 6 months of standard rat chow (n = 6 each). All surgical and experimental procedures were approved by National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee and are in accordance with the guidelines of the National Science Council of Republic of China (NSC 1997). All efforts were made to minimize animal suffering and the number of animals throughout the experiment.
Baseline parameters
After 3 or 6 months of treatment, the metabolic characteristics and baseline of micturition parameters were measured in all animals. All animals were anesthetized with subcutaneous urethane (1.2 g/kg), which is known to anesthetize the animals permit full reflex bladder contractions [12]. Body temperature of the animal was kept at 36.5–37 °C by an infrared light and evaluated by a rectal thermometer. At the end of each experiment, the animals were killed by an intravenous KCl injection. Blood samples were collected immediately to measure blood glucose (One Touch II; LIFESCAN, Milpitas, CA, USA), cholesterols, low-density lipoprotein and triglycerides [16]. Hepatic steatosis, an index of metabolic syndrome, was assessed using oil red O staining. Oil red O staining was performed to evaluate the accumulation of fat droplets in hepatocytes in frozen liver sections from the fructose-feeding rats. The bladder was removed and one part of the bladder was fixed in 4 % buffered formalin for morphological staining. Part of the samples was stored at −70 °C for postsynaptic receptor protein expression by Western blot analysis. Part of samples was OCT embedded for nerve distribution by PGP 9.5 stain.
Cystometric findings
We applied a transcystometric model to evaluate the micturition response in the bladder after fructose feeding [16]. The method has been well established in our laboratory [12, 13]. The bladder catheter was connected via a T-tube to a pressure transducer (P23 1D, Gould-Statham, Quincy, USA), and the intravesical pressure (IVP) was recorded continuously in an ADI system (Power-Lab/16S, ADI Instruments, Pty., Ltd., Castle Hill, Australia). The following parameters of bladder responsiveness were measured: intercontraction interval (ICI, the time lag between two micturition cycles identified with active contractions (>15 mmHg), baseline bladder pressure (BP), threshold pressure (TP) and peak intravesical pressure (pIVP).
Recording of pelvic afferent and efferent nerve activity
The electrophysiological technique used to record pelvic afferent and efferent nerve activity was described previously [12, 13]. In brief, with the help of a stereoscopic dissecting microscope (Olympus, SZ-STU2), pelvic afferent and efferent nerve activities were recorded simultaneously from two pelvic nerves dissected from the surface of the bladder. Nerve fiber with pelvic afferent activity was confirmed by its ability to show increased activity in response to small increments of IVP by saline infusion via T-tube. Nerve fiber with pelvic efferent nerve activity had minimal activity until a threshold volume/pressure in the bladder was reached, which produced a bursting discharge causing a micturition contraction.
Recording of extraurethral sphincter-electromyogram activity
We used epoxy-coated stainless steel wire (50 μm, M. T. Giken Co., Tokyo, Japan) as an electrode for determining extraurethral sphincter-electromyogram activity [12]. In brief, a 30-gauge needle with a hooked extraurethral sphincter-electromyogram electrode was inserted into the sphincter approximately 5–10 mm lateral to the urethra. Extraurethral sphincter-electromyogram signals were amplified and passed through a discriminator/rate meter. Pelvic afferent and efferent nerve activities, extraurethral sphincter-electromyogram activity and cystrometrogram were simultaneously recorded by an ADI system (Power-Lab/16S, ADI Instruments, Pty., Ltd., Castle Hill, Australia).
ATP and acetylcholine content assay
The portion of the bladder above the ureteral orifices was harvested as the bladder body. Under a dissecting microscope (Olympus SZ61), we obtained the muscle layers from the bladder body wall by stripping off the urothelium and mucosa with sharp tweezers (DUMONT, Switzerland). To avoid cross-contamination by these two layers, the proficiency of separation was assessed histologically as described previously [11]. We further analyzed the ATP and acetylcholine content and postsynaptic receptor expression in the isolated smooth muscle layers. The smooth muscle layers were collected and stored at −70 °C for ATP and acetylcholine content analysis. The method for measurement of bladder acetylcholine content was used by a commercial kit (Choline/Acetylcholine Quantification Kit, K615-100, BioVision) as described previously [20]. The kit can detect 10 pmol–5 nmol of acetylcholine. In brief, the isolated smooth muscle tissue was homogenized in Choline Assay Buffer and centrifuged at 600×g for 30 min to remove debris. The supernatant was tested by mixing enough reagents in each well. A total of 50 μl reaction mixture contains 44 μl Choline Assay Buffer, 2 μl choline probe, 2 μl acetylcholinesterase and 2 μl enzyme mixture. All the samples were incubated at room temperature for 30 min in dark room and measured OD at 570 nm for the colorimetric assay in a micro-plate reader (Wallac 1420 vector2, PerkinElmer, Inc., San Jose, CA, USA). We subtracted the background value (the 0 choline control) from all standard and sample readings.
The bladder ATP content was measured using a luciferin-luciferase assay kit (Roche, Penzberg, Germany) according to the manufacturer’s instructions and methods published previously [21]. The method was used to measure the ATP dependency of the light-emitting luciferase-catalyzed oxidation of luciferin. The isolated smooth muscle tissue was homogenized using liquid nitrogen and diluted in a buffer containing 100 mM Tris and 4 mM EDTA (pH 7.75) and mixed immediately with equal amounts of the luciferase reagent. The light emitted from the luciferase was measured using a Chemiluminescence Analyzing System (CLD-110, Tohoku Electronic In. Co., Sendai, Japan), and the values were calibrated against a standard ATP curve. Furthermore, we used BioVision’s ATP Colorimetric/Fluorometric Assay Kit (Catalog #K354-100) to further determine ATP content in the muscle layer of the urinary bladder. In brief, for the colorimetric assay, the ATP standard was prepared to obtain 0, 2, 4, 6, 8, 10 nmol/well of ATP standard. The bladder tissue was homogenized using perchloric acid (BioVision, Cat. # K808-200) and centrifuge ice cold at 15,000×g for 2 min to collect supernatant. The supernatant was mixed with 44 μl ATP Assay Buffer, 2 μl ATP probe, 2 μl ATP converter and 2 μl developer mix. All the samples were incubated at room temperature for 30 min in dark room and measured OD at 570 nm for the colorimetric assay in a micro-plate reader (Wallac 1420 vector2). We subtracted background value (the 0 ATP control) from all standard and sample readings.
Electron microscopic analysis
Bladder tissues from 4 groups of rats were performed electron microscopic observation. The tissue for electron microscopy was fixed in half-strength Karnovsky’s solution (1 % paraformaldehyde and 1.25 % glutaraldehyde in 0.1 M Na cacodylate buffer, pH 7.0), postfixed in osmium tetroxide, dehydrated in graded ethanol and embedded in epoxy resin. These thin tissue sections were stained with uranyl acetate and lead citrate and examined with a camera system (Advanced Microscopy Techniques, Danvers, MA, USA). The ultrastructure especially in mitochondria of the bladder specimen was photographically documented as described previously [16].
Mast cell, cytochrome C and PGP 9.5 staining
We performed toluidine blue staining for specific mast cell labeling [22], cytochrome C immunostaining to evaluate mitochondrial injury, and an antiubiquitin carboxyl terminal hydrolase antibody, PGP 9.5, to evaluate the nerve distribution in urinary bladder of four groups of rats (n = 3 each) [12]. In brief, the urinary bladder was perfused with Tris-buffered saline and fixed with a cold mixture of 2 % paraformaldehyde, lysine and sodium periodate for two hours. Tissues were OCT embedded, and frozen sections were obtained. After drying for two hours, sections were rehydrated and washed with Tris-buffered saline. We incubated rehydrated sections with toluidine blue solution (Polysciences, No. 1234, 1 g/100 ml of 70 % ethanol stock) diluted 1:10 with 1 g/ml aqueous NaCl for 2 min followed by 3 rinses with deionized water. Specific mast cell stain was demonstrated in blue color under microscopic observation.
The frozen sections of OCT-embedded tissues were incubated overnight at 4 °C with polyclonal cytochrome C antibody (Santa Cruz Biotechnology, Inc.) and antiubiquitin carboxyl terminal hydrolase antibody (CEDARLANE Laboratories Limited, Burlington, ON, Canada, 1:1,000). The reaction was visualized by the avidin–biotin–peroxidase complex method. These stains were taken by a microscopy (Leica CM1900). The value of mast cells and PGP 9.5 stain/total section area was counted by Adobe Photoshop 7.0.1 image software analysis.
Western blot
We evaluated the expression of M2- and M3-muscarinic cholinergic receptors, P2X1, P2X2, and P2X3 receptors of bladder smooth muscle layer tissues by a Western blotting technique as described previously [23]. In brief, the muscle samples were homogenized with a prechilled mortar and pestle in extraction buffer and kept at 4 °C for 30 min. The antibodies against M2 (AB5166, Chemicon International, CA), M3 (Alomone Labs, Israel), P2X1 (Santa Cruz Biotechnology, Germany), P2X2 (Neuromics, Northfield, MN), P2X3 receptor (Santa Cruz Biotechnology, Germany) and β-actin (Sigma, Saint Louis, MO) were indicated. The density of the band with the appropriate molecular mass was determined semi-quantitatively by densitometry using an image analyzing system (Alpha Innotech, San Leandro, CA).
Statistical analysis
Values are displayed as means ± standard error mean (SEM). Data were subjected to analysis of variance, followed by Duncan’s multiple-range test for assessment of the differences among groups. Student’s paired t test was used to detect differences between control and drug treatment. P < 0.05 was considered to indicate statistical significance.
Results
Baseline parameters
The baseline physiologic and biochemical data of four groups of male Wistar rats are indicated in Table 1. There were no significant differences of body weights of the fructose-fed rats and control animals in both 3- and 6-month groups. However, the level of triglycerides, low-density lipoprotein (LDL) and fasting glucose was significantly elevated in 3- or 6-month fructose-fed rats versus age-matched control rats. We further confirmed that a marked lipid accumulation by oil red O staining in the livers of 3-month (Fig. 1b) and 6-month fructose-fed rats (Fig. 1d). The mean changes of hepatic oil red stain in these rats were demonstrated in Fig. 1e. The mean arterial pressure showed a tendency to be increased but was not significantly increased in the 3-month fructose-fed rats. However, mean arterial blood pressure was significantly increased after 6-month fructose feeding. The significantly elevated levels in plasma triglyceride, LDL, fasting glucose and mean arterial blood pressure were found in our fructose-fed male rats indicating the successful induction of metabolic syndrome.
Altered cystometric data after fructose feeding
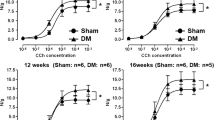
In the present study, we selected 3- or 6-month fructose-fed male rats (n = 8 each) with LUTS to examine its possibly pathophysiologic mechanisms in comparison with respective age-matched control rats. We have demonstrated the original diagrams in Fig. 2 and showed that several cytometric parameters are altered after long-term fructose feeding. As listed in Table 1, the LUTS characterized by decreased ICI and pIVP together with increased BP and TP are present in 3- or 6-month groups of fructose-fed rats, but not in the age-matched control groups.
Alterations in micturition parameters in 3- and 6-month fructose-fed groups. Cystometric parameters are indicated in 3-month control group (a, c), 3-month fructose-fed group (b, d), 6-month control group (E&G) and 6-month fructose-fed group (f, h). c, d, g, h are the graphs (marked with asterisk from a, b, e, f) with fast chart speed. Phase 1 and 2 contractile responses are well reserved in 3-month (c) and 6-month control group (g), whereas phase 1 and 2 contractile responses are depressed in 3-month (d) and 6-month fructose feeding (h). BP baseline bladder pressure, TP threshold pressure, ICI intercontraction interval, pIVP peak value of intravesical pressure
More specifically, we found that the contractile components of phase 1 and duration in phase 2 in fast chart speed are changed after 3- (Fig. 2d) or 6-month (Fig. 2h) fructose-feeding versus respective control groups (Fig. 2c, g). Depressed phase 1 contractile amplitude and phase 2 contractile duration in the urinary bladder are markedly indicated in the 3- or 6-month fructose-feeding rats. The phase 2 contraction almost disappeared in the voiding contraction after 6-month fructose feeding (Fig. 2h).
The original recordings of pelvic afferent and efferent nerve activities, extraurethral sphincter-electromyogram activity and IVP in the 3-month control rat, 3- and 6-month fructose-feeding rats were displayed in Fig. 3a. In the control rats, saline infusion gradually enhanced pelvic afferent nerve activity and extraurethral sphincter-electromyogram activity and increased IVP, but did not activate pelvic efferent nerve activity during the filling. At phase 1 for voiding contraction, pelvic afferent nerve activity increased sharply in parallel with the rising IVP and enhanced tonic pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity. During phase 2 contractions of voiding function, pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity fired in burst discharges and IVP displayed high frequency oscillations. After phase 2 contractions, the increased pelvic afferent and efferent nerve activity, extraurethral sphincter-electromyogram activity and IVP were reduced and returned to their baseline levels. After 3-month fructose feeding, there was a decrease in IVP level in phase 1 contraction, a decrease in voiding duration in phase 2 contraction and a longer enhancement in pelvic afferent nerve activity. After 6-month fructose feeding, there was a decreased IVP, a shortened phase 2 contractile duration, and an alteration in pelvic afferent nerve activity, pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity. The recordings of pelvic afferent nerve activity, pelvic efferent nerve activity, extraurethral sphincter-electromyogram activity and IVP showed that 3- or 6-month fructose feeding markedly altered neural and myogenic activity during a voiding contraction when compared to control group.
a An integrated graph of simultaneous recordings of PANA, PENA, EUS-EMG and IVP during a continuous cystometrogram (0.10 ml/min infusion) in three groups of rats is demonstrated. Abnormal pattern of PANA, PENA, EUS and IVP (indicated by a) is found in fructose-fed rats when compared to the control rat. The urinary bladder ATP content measured by luciferin-luciferase assay (b) and by ATP colorimetric assay (c) indicated that the ATP content in smooth muscle of urinary bladder is significantly decreased after 3-month (3 M) or 6-month (6 M) fructose feeding when compared to respective normal control group. The acetylcholine content in smooth muscle of urinary bladder is also significantly depressed after 3-month (3 M) or 6-month (6 M) fructose feeding when compared to normal controls (d). *P < 0.05 when compared to respective control group
We further evaluated the content of ATP and acetylcholine, two major neurotransmitters contributing to bladder contractions, in the normal and fructose-feeding rats. The level of ATP in 3- and 6-month normal controls was 8.42 ± 0.27 and 8.09 ± 0.28 μg/mg tissue by luciferin-luciferase assay (Fig. 3b) and 3.27 ± 0.87 and 3.17 ± 0.78 nmol/mg bladder tissue by ATP colorimetric assay (Fig. 3c), respectively. The ATP level of 3- and 6-month fructose feeding was 6.68 ± 0.29 and 6.12 ± 0.42 μg/mg tissue by luciferin-luciferase assay and 1.58 ± 0.0.31 and 1.29 ± 0.24 nmol/mg bladder tissue by ATP colorimetric assay. The level of acetylcholine of the urinary bladder in 3- and 6-month normal controls was 76 ± 3 and 74 ± 9 nM/mg tissue (Fig. 3d) and that was significantly decreased to 34 ± 3 and 35 ± 6 nM/mg tissue after 3- and 6-month fructose feeding.
Increased mast cells in the bladder after fructose feeding
An increased mast cell number located in the interstitium or around the blood vessels was found in the urinary bladder after 3-month (Fig. 4b, f) or 6-month (Fig. 4d, h) fructose-fed rats, but not in 3-month (Fig. 4a, e) or 6-month control rats (Fig. 4c, g) by toluidine blue and H&E stain, respectively. The quantitative data of the mast cells were shown in the Fig. 5a. A significant increase in mast cells by toluidine blue stain was found in the 3- and 6-month fructose-fed rats versus 3- and 6-month normal-diet rats.
Effects of long-term fructose feeding on morphological alterations and bladder nerve density in the urinary bladder. a–d Specific mast cell staining (indicated by arrows) by toluidine blue dye. e–h Morphologic changes and increased mast cells in the fructose-feeding bladder (marked with arrows) by H&E stain. i–l Disrupted, swollen and bloated mitochondria (marked with arrows) found in the smooth muscles of 3-month (j) and 6-month (l) fructose-treated bladder by electron micrography. m–p Marked cytochrome C stain (brown color) in the muscle of 3- (n) and 6-month (p) fructose-fed groups when compared to 3- (m) and 6-month (o) controls. q–t A decrease in nerve innervations in the smooth muscles of 3-month (r) and 6-month (t) fructose-treated urinary bladder by PGP 9.5 stain (brown color) when compared to 3- (q) and 6-month (s) controls
Quantitative data of mast cell numbers, PGP 9.5 denisty and cytosolic cytochrome C (C cyt c) stain by Western blot in urinary bladders of the normal controls and fructose-feeding rats. a Statistic data of toluidine blue positive cells in the urinary bladders. b Quantitative data of PGP 9.5 density in the smooth muscles of urinary bladders. c Original graph and semi-quantitative data of the C cyt c from the normal control and fructose-feeding rats. *P < 0.05 when compared to respective control group
Ultrastructure analysis by electron micrography
We found that the mitochondrial structure is markedly altered with the disrupted and swollen characteristics (Fig. 4j, l) in the smooth muscles of fructose feeding versus mitochondria in controls (Fig. 4i, k). Cytochrome C, a marker for mitochondrial injury, is markedly stained in the 3-month (Fig. 4n) and 6-month (Fig. 4p) fructose-fed bladder smooth muscles when compared to 3- and 6-month controls (Fig. 4m, o). The semi-quantitative analysis of cytosolic cytochrome C by Western blot showed that the cytosolic cytochrome C expressions were significantly enhanced in the 3- and 6-month fructose-fed bladders than those in the 3- and 6-month control bladders (Fig. 5c).
Reduced nerve density in smooth muscle layer
To address whether alterations in nerve density of bladder smooth muscles after fructose feeding, we used antibody PGP 9.5 to stain nerve fibers. Noted that the stained fibers were found in the muscles and vessels of the 3-month (Fig. 4q) or 6-month control urinary bladder (Fig. 4s). A decreased density of PGP 9.5 stained fibers was seen in the smooth muscle layer of urinary bladder after 3-month (Fig. 4r) or 6-month fructose feeding (Fig. 4t). The quantitative data of PGP 9.5 density was displayed in the Fig. 5b. A significantly decreased PGP 9.5 stain was noted in 3- and 6-month fructose-fed bladders than that in 3- and 6-month controls.
Upregulation of purinergic and muscarinic cholinergic receptors after fructose feeding
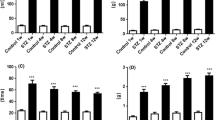
Figure 6 demonstrates representative Western blotting results and statistic comparisons for M2 and M3 muscarinic cholinergic receptors protein expression in the smooth muscles between controls and fructose-fed groups. Significant upregulation of M2 (Fig. 6a) and M3 muscarinic cholinergic receptors (Fig. 6b) was implicated in the 3- and 6-month fructose-fed groups in comparison with respective control groups. As shown in Fig. 7, significant upregulation in P2X1 (Fig. 7a), P2X2 (Fig. 7b) and P2X3 (Fig. 7c) receptor protein expression is also noted in the fructose-fed rats when compared to respective control group.
Western blot of M2 (a) and M3 receptor (b) protein expression in the smooth muscle of urinary bladders from control and fructose-fed rats. After 3 or 6 months of fructose feeding, the M2 and M3 receptor protein expression in the urinary bladder is significantly enhanced when compared to respective control group. *P < 0.05 when compared to respective control group
Western blots of P2X1 (a), P2X2 (b) and P2X3 receptor (c) in the smooth muscles of urinary bladders of control and fructose-fed animals. After 3 or 6 months of fructose feeding, the P2X1, P2X2 and P2X3 receptor protein expression in the urinary bladder is significantly enhanced when compared to respective control group. *P < 0.05 when compared to respective control group
Discussion
The consumption of fructose has been reported to increase the metabolic syndrome, because fructose triggers hypertriacylglycerolemia, hyperglycemia and hypertension [6, 11, 16, 24], which are consistently found in our animals. The body weight gain was not different after 3- or 6-month fructose feeding. These results are in accordance with previous reports, which demonstrated that fructose intake did not further increase the body weight [25–27].
A clinical finding indicated the storage/voiding symptoms including frequency, urgency, weak stream, intermittency, and incomplete emptying occurred in the LUTs patients with metabolic syndrome [7]. Martin et al. [7] indicated that the functional decline of the lower urinary tract is related with the factors including abdominal fat mass, plasma glucose, HDL cholesterol and energy intake. These factors positively associated with storage LUTS were increased abdominal fat mass, plasma glucose and low HDL cholesterol. Overconsumption of sugar-sweetened beverages, in particular carbonated soft drinks, promotes the development of overweight and obesity and is associated with metabolic disturbances, including intrahepatic fat accumulation and metabolic syndrome [28]. Hyperlipidemia induced by a high-fat diet is associated with increased urinary frequency, and decreased bladder blood vessel and nerve density in rats [29]. The alterations in the bladder smooth muscle, neuronal degeneration and urothelial dysfunction have also been observed in a type II diabetes mellitus rat model induced by a high-fat diet and low-dose streptozotocin [30]. Our serial reports have found that fructose-induced metabolic syndrome induces LUTS in our animal models. These reports and our data evidence that metabolic syndrome characterized with hyperlipidemia and hyperglycemia is associated with LUTS.
Our results were the first to determine the long-term fructose effects on the neural elements contributing to voiding function by simultaneously recording cystometrogram, pelvic afferent and efferent nerve activity and extraurethral sphincter-electromyogram activity in the urethane-anesthetized rats. According to our data and other report [13, 31], voiding function is activated by distension-induced pelvic afferent nerve activity from the bladder receptors, triggered the neurons located in the sacral spinal cord and elicited the early central reflex of tonic firing of pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity in phase 1. Tonic pelvic efferent nerve activity may release ATP and acetylcholine and leads to contraction of the detrusor muscle, whereas tonic excitation of the extraurethral sphincter-electromyogram activity contributes to the tight closure of the sphincter, thus increasing the IVP. Fluid emission was confined almost completely in phase 2, when a series of high frequent oscillations accompanied the acetylcholine-dependently bursting pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity [13]. Alterations in the firing types of pelvic afferent nerve activity, pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity have been demonstrated in bladder dysfunction like substance P-induced hyperactive bladder [12] and bladder outlet obstruction [13]. In the fructose-induced LUTS, we recognized the suppression in phase 1 contractile amplitude, phase 2 voiding duration and the alteration in pelvic afferent nerve activity, pelvic efferent nerve activity and extraurethral sphincter-electromyogram activity. ATP is responsible for mediating phase 1 detrusor contraction, whereas acetylcholine is required for regulating phase 2 detrusor contraction [13]. According to our findings, the decreased ATP and acetylcholine content in the smooth muscle of the urinary bladder are found after long-term fructose feeding. The reduction in ATP and acetylcholine may participate in the alteration of pelvic nerve activity and result in metabolic syndrome-induced LUTS.
How fructose feeding reduces ATP and acetylcholine content in the urinary bladder? Fructose produces deleterious metabolic effects in animal models. There are some possible mechanisms for the reduction in these neurotransmitters. In the urinary bladder, a decrease in ATP content could be due to a metabolic effect or resulted from a consequence of metabolic syndrome. ATP can be depleted either in primary hepatocytes or in the liver in vivo by fructose treatment directly because of its metabolic effect [32]. Another reason is that the metabolic syndrome with hyperlipidemia and hyperglycemia impair mitochondrial structure and function for incapability to producing ATP in the long-term fructose-fed urinary bladder smooth muscles. Nevertheless, it requires further experiments to explore the mechanisms for fructose feeding on reducing bladder ATP and acetylcholine concentration.
ATP contributes to excitatory neurotransmission in the urinary bladder through P2X receptors in detrusor smooth muscle [33, 34]. P2X2 and P2X3 receptors play unique and tissue-specific roles in micturition reflex pathways. Upregulation of P2X2 receptors in detrusor smooth muscle from patients with idiopathic detrusor instability has been indicated [33]. Our data and other reports have found the changes in postsynaptic P2X1 and P2X3 receptors [16, 24] expression in the urinary bladders of female rats after long-term fructose feeding. P2X3 receptor knockout mice exhibit bladder hyporeflexia on cystometry with decreased voiding frequency and increased bladder capacity and voided volume but normal bladder pressures [34]. On the other hand, acetylcholine, another important neurotransmitter, via M2 and M3 receptors regulates micturition. Some reports [11, 16] indicated fructose feeding increased postsynaptic M2 and M3 muscarinic cholinergic receptors in the smooth muscle layer of the female rats. In the present study, we further found upregulation in P2X1, P2X2, P2X3 and M2 and M3 muscarinic cholinergic receptors in the male rats with long-term fructose feeding. Longer fructose feeding has a tendency to further enhance these postsynaptic receptors expression. In vitro study shows that 6-month fructose treatment significantly reduced the contractile amplitude to filed stimulation, KCl and carbachol, but increased the contractile response to high [ATP] (>5×10−4 M) in the isolated detrusor from Wistar female rats [16]. Alterations in purinergic and cholinergic pathways have found in some pathophysiological states of bladder, such as aging, diabetes or spinal cord injury [35–37]. We implicate the alterations in purinergic and muscarinic cholinergic receptors in the urinary bladder smooth muscles possibly contribute to fructose feeding–induced neurogenic and myogenic changes.
Mitochondria are one of major sources of reactive oxygen species (ROS) in cells, and mitochondrial dysfunction increases the generation of ROS from the mitochondrial respiratory chain. Hyperglycemia and hyperlipidemia increased the expression of NADPH oxidase and ROS release [8]. Increased oxidative stress causes intramural bladder nerve dysfunction, nerve fiber injury, mitochondrial injury and detrusor muscle cell damage [38]. In substance P-induced bladder hyperactivity [12], increased superoxide anion evokes alterations in pelvic afferent nerve activity, pelvic efferent nerve activity and IVP responses, and the use of superoxide dismutase attenuates these alterations indicating oxidative stress contributing to bladder hyperactivity. Mitochondrial oxidative stress resulting from hyperlipidemia or hyperglycemia is additive and directly injures dorsal root ganglion neurons via interaction with the receptor lectin-like oxLDL receptor-1 and NAD(P)H oxidase activity [9]. Oxidative stress increased the susceptibility of muscle to the accumulation of mitochondrial damage [39]. Metabolic syndrome in our model characterized with hyperglycemia, hyperlipidemia and hypertension could increase oxidative stress and impair mitochondrial structure [24] and function in axons and smooth muscles, which may block axonal transport and neurotransmitter production/release in the damaged pelvic nerves and depress detrusor cell contractility. This injury could also possibly result in the conspicuous degenerative in axons, neuromuscular junction and the reduction in the synaptic vesicles for production/release of ATP and acetylcholine. Our data found a decrease in PGP 9.5 nerve density in fructose-treated bladder. The alterations in pelvic nerve activity and density may also contribute to urinary bladder dysfunction.
On the other hand, the death of smooth muscle cells is associated with markedly decreased mitochondrial transmembrane potential as well as upregulation of cellular ROS and cytosolic cytochrome C release [10]. Our results have demonstrated the abnormally structural mitochondria and observed the increased cytosolic cytochrome C stain and expression in the fructose-treated smooth muscles. In myogenic injury, our previous study has shown that a down-regulation of anti-apoptotic Bcl-2 protein and an increased Bax/Bcl-2 ratio/caspase 3 signaling enhances an intrinsic apoptotic pathway in the smooth muscles of fructose-fed rats [24]. The increased apoptosis formation promotes the loss of bladder smooth muscle cells, which have been recognized by the decreased smoothelin expression, a novel cytoskeletal protein specific to smooth muscle cells, in the bladder smooth muscles of fructose-fed rats [24]. Furthermore, the decreased in pIVP of the urinary bladder smooth muscles in phase 1 and loss of phase 2 found in our fructose-fed rats may indicate fructose-induced injury in the smooth muscles.
Increased mast cell number and histamine release in the kidney impaired neural afferent signaling after diabetes [40]. After fructose feeding, we also found increased mast cells in the urinary bladder. We suggest the increased mast cells possibly via the action of increased histamine release, inflammatory cytokines or oxidative stress to impair neuronal and muscular function in the LUTS. The rat bladder contains approximately 16,000 axons of which at least half are sensory and approximately 3/4 of the mitochondria of a nerve are primarily located in axons [41]. Our data found significantly reduced nerve density in smooth muscle layer after 3 and 6 months of fructose feeding. Decreased sensory and sympathetic innervation may affect filling capacity resulting in micturition frequency, whereas reduced parasympathetic innervation could decrease micturition pressure for incomplete emptying. This will be further determined in future.
In conclusion, long-term fructose feeding induced LUTS associated with the alterations in firing type of pelvic nerve and external urethral sphincter activity. Increased mast cell numbers, impaired mitochondrial integrity, reduced nerve distribution, ATP and acetylcholine content and upregulation of postsynaptic purinergic and muscarinic cholinergic receptors expression may play a role in long-term fructose feeding–induced bladder dysfunction.
Change history
23 March 2024
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s00394-024-03373-3
References
Kahn R, Buse J, Ferrannini E, Stern M (2005) The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28:2289–2304
Rohrmann S, Smit E, Giovannucci E, Platz EA (2005) Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond) 29:310–316
Yu HJ, Lee WC, Liu SP et al (2004) Unrecognized voiding difficulty in female type 2 diabetic patients in the diabetes clinic: a prospective case-control study. Diabetes Care 27:988–989
Lee WC, Wu CC, Wu HP, Tai TY (2007) Lower urinary tract symptoms and uroflowmetry in women with type 2 diabetes mellitus with and without bladder dysfunction. Urology 69:685–690
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543
Le KA, Tappy L (2006) Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care 9:469–475
Martin SA, Haren MT, Marshall VR et al (2011) Members of the Florey Adelaide Male Ageing Study. Prevalence and factors associated with uncomplicated storage and voiding lower urinary tract symptoms in community-dwelling Australian men. World J Urol 29:179–184
Shen GX (2010) Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol 88:241–248
Vincent AM, Hayes JM, McLean LL et al (2009) Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 58:2376–2385
Kedi X, Ming Y, Yongping W, Yi Y, Xiaoxiang Z (2009) Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis 207:123–130
Tong YC, Cheng JT (2007) Alterations of M2,3-muscarinic receptor protein and mRNA expression in the bladder of the fructose fed obese rat. J Urol 178:1537–1542
Chien CT, Yu HJ, Lin TB et al (2003) Substance P via NK1 receptor facilitates hyperactive bladder afferent signaling via action of ROS. Am J Physiol Renal Physiol 284:F840–F851
Chien CT, Yu HJ, Lin TB, Chen CF (2000) Neural mechanisms of impaired micturition reflex in rats with acute partial bladder outlet obstruction. Neuroscience 96:221–230
Palea S, Artibani W, Ostardo E et al (1993) Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol 150:2007–2012
Yokota T, Yamaguchi O (1996) Changes in cholinergic and purinergic neurotransmission in pathologic bladder of chronic spinal rabbit. J Urol 156:1862–1866
Lee WC, Chien CT, Yu HJ, Lee SW (2008) Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J Urol 179:2470–2476
Burnstock G (2011) Therapeutic potential of purinergic signalling for diseases of the urinary tract. BJU Int 107:192–204
Lawrence GW, Aoki KR, Dolly JO (2010) Excitatory cholinergic and purinergic signaling in bladder are equally susceptible to botulinum neurotoxin a consistent with co-release of transmitters from efferent fibers. J Pharmacol Exp Ther 334:1080–1086
Yoshimura N, Kaiho Y, Miyazato M et al (2008) Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol 377:437–448
Al-Zi’abi MO, Bowolaksono A, Okuda K (2009) Survival role of locally produced acetylcholine in the bovine corpus luteum. Biol Reprod 80:823–832
Yeh CH, Chiang HS, Chien CT (2010) Hyaluronic acid ameliorates bladder hyperactivity via the inhibition of H2O2-enhanced purinergic and muscarinic signaling in the rat. Neurourol Urodyn 29:765–770
Chen MC, Blunt LW, Pins MR, Klumpp DJ (2006) Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol 175:754–759
Yu HJ, Chien CT, Lai YJ et al (2004) Hypoxia preconditioning attenuates bladder overdistension-induced oxidative injury by up-regulation of Bcl-2 in the rat. J Physiol 554:815–828
Lee WC, Chuang YC, Chiang PH et al (2011) Pathophysiological studies of overactive bladder and bladder motor dysfunction in a rat model of metabolic syndrome. J Urol 186:318–325
Girard A, Madani S, Boustani ESE et al (2005) Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats a diet enriched with fructose and saturated fatty acids. Nutrition 21:240–248
Messier C, Whately K, Liang J et al (2007) The effects of high fat, high fructose, and combination diet on learning, weight, and glucose regulation in 57BL/6 mice. Behav Brain Res 178:139–145
Moura RF, Ribeiro C, Oliveira JA et al (2009) Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols. Br J Nutr 101:1178–1184
Sheludiakova A, Rooney K, Boakes RA (2011) Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur J Nutr [Epub ahead of print]
Huang YC, Shindel AW, Ning H et al (2010) Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol 183:1232–1240
Zhang H, Qiu X, Shindel AW et al. (2011) Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev [Epub ahead of print]
Sadananda P, Drake MJ, Paton JF, Pickering AE (2011) An exploration of the control of micturition using a novel in situ arterially perfused rat preparation. Front Neurosci 5:62
Latta M, Künstle G, Leist M, Wendel A (2000) Metabolic depletion of ATP by fructose inversely controls CD95- and tumor necrosis factor receptor 1-mediated hepatic apoptosis. J Exp Med 191:1975–1985
O’Reilly BA, Kosaka AH, Knight GF et al (2002) P2X receptors and their role in female idiopathic detrusor instability. J Urol 167:157–164
Cockayne DA, Hamilton SG, Zhu QM et al (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X(3)-deficient mice. Nature 407:1011–1015
Liu G, Daneshgari F (2005) Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol 288:F1220–F1226
Yoshida M, Miyamae K, Iwashita H et al (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23
Hegde SS, Mandel DA, Wilford MR et al (1998) Evidence for purinergic neurotransmission in the urinary bladder of pithed rats. Eur J Pharmacol 349:75–82
Juan YS, Chuang SM, Kogan BA et al (2009) Effect of ischemia/reperfusion on bladder nerve and detrusor cell damage. Int Urol Nephrol 41:513–521
Fukagawa NK, Li M, Liang P et al (1999) Aging and high concentrations of glucose potentiate injury to mitochondrial DNA. Free Radic Biol Med 27:1437–1443
Chien CT, Chien HF, Cheng YJ et al (2000) Renal afferent signaling diuretic response is impaired in streptozotocin-induced diabetic rats. Kidney Int 57:203–214
Gabella G (1999) Structure of the intramural nerves of the rat bladder. J Neurocytol 28:615–637
Acknowledgments
This work was supported by the National Science Council of the Republic of China (NSC99-2314-B418-002-MY3 and NSC99-2628-B-002-058-MY3) and research fund from Far-Eastern Memorial Hospital (FEMH-99-D-006).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, SD., Chien, CT. & Yu, HJ. Alterations in peripheral purinergic and muscarinic signaling of rat bladder after long-term fructose-induced metabolic syndrome. Eur J Nutr 52, 347–359 (2013). https://doi.org/10.1007/s00394-012-0342-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0342-4