Abstract
Objectives
To investigate time course of bladder dysfunction and concurrent changes in number and affinity of the muscarinic and P2X receptor in the early stage of streptozotocin (STZ)-induced diabetic rats.
Materials and methods
Diabetic rats were prepared by the intraperitoneal injection of 50 mg/kg of STZ to 7-week-old female Wistar rats. We performed recording of 24-h voiding behavior and cystometry at 1, 4, 8, and 12 weeks after the induction of diabetes. A muscle strip experiments with electrical field stimulation (EFS), carbachol, and α,β-methylene adenosine 5′-triphosphate (α,β-MeATP) were also performed at the same time-points. Additionally, concurrent changes in number and affinity of bladder muscarinic and P2X receptor were measured by a radioreceptor assay using [N-methyl-3H] scopolamine methyl chloride ([3H]NMS) and α,β-methylene-ATP (2,8-3H) tetrasodium salt ([3H]α,β-MeATP).
Results
In STZ-induced diabetic rats, polydipsic polyuric pollakiuria were noted on recording of 24-h voiding behavior from early stage. Also, the residual urine volume markedly increased in diabetic rats on cystometry. In the muscle strip experiment, the detrusor contractions induced by EFS, carbachol, and α,β-MeATP were enhanced in STZ-induced diabetic rats. Based on the radioreceptor assay, the maximum number of sites (Bmax) for the specific binding of [3H]NMS and [3H]α,β-MeATP was concurrently increased in the bladder from diabetic rats.
Conclusion
Increased bladder contractility is found in early stage of diabetic rats. Then, bladder dysfunction is associated with increased number of muscarinic and P2X receptors in STZ-induced diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder dysfunction is one of the most frequent complications of diabetes. Diabetic patients suffer from underactive bladder characterized by a bladder hypesthesia on urodynamic testing and reduced detrusor contractility that lead to a voiding dysfunction [1, 2]. However, a storage dysfunction, such as overactive bladder, has also been reported in diabetic patients [3, 4]. In fact, we often experience in the daily clinical practice that diabetic patients in early stage have complains of overactive bladder symptoms, such as urinary urgency, pollakiuria, and nocturia. Although the report suggests that bladder dysfunctions progress from overactive to underactive in diabetic condition, the detailed mechanisms are poorly understood especially in the number and affinity of receptors in concern. Streptozotocin (STZ)-induced diabetic rats have been widely used in basic studies on diabetes. Based on previous studies, the early stage of diabetes in rats is considered to be up to 4–12 weeks after the induction of diabetes [5,6,7,8,9,10,11].
Among studies on bladder dysfunction in the diabetic rats, observations of the maximum voiding pressure and detrusor overactivity were particularly interesting [5]. According to reports of Tong et al. [6] and Cheng et al. [7], in rats of the early stage of diabetes, the expressions of proteins and mRNA of muscarinic receptors (M2, M3) were enhanced in the urothelium and muscle layer. Saito et al. [8] also reported elevated maximum voiding pressure and the enhancement of muscarinic receptors (M2, M3) mRNA expression in the bladder [6,7,8]. These reports suggested that alterations of muscarinic receptor expression contribute to bladder dysfunction, such as increased bladder contractility, in the early stage of diabetic rats.
Additionally, a lot of studies currently noted that non-adrenergic non-cholinergic (NANC) pathway, has an important role in a regulation of bladder function [12,13,14]. NANC pathway is mostly mediated by purinergic receptor (such as P2X receptor) which induces bladder phasic contractions and hypersensitivity [15, 16]. However, few reports are addressed concerning purinergic receptors that affect diabetic bladder dysfunction especially on their number of sites (Bmax) or pharmacological affinity (Kd) [11]. Therefore, we investigated whether muscarinic receptor, in addition to P2X receptor, has an effect on diabetic bladder dysfunction using cystometry, ex vivo study, and receptor binding assay in early stage of STZ-induced diabetic rats. In this study, we suppose that the alterations of these two receptors act mutually to induce bladder dysfunction, such as increased bladder contractility, in early stage of diabetes.
Materials and methods
Animals
All animal experiments were performed following the guidelines for animal experiments of Nihon University School of Medicine. Seven-week-old female Wistar rats (body weight: 160–190 g, Charles River Japan) were divided into control and STZ-induced diabetes groups, and experiments were performed at 1, 4, 8, and 12 weeks after STZ administration.
Induction of diabetes
In the STZ-induced diabetes group, STZ (Sigma-Aldrich, USA) dissolved in 0.05 M citrate buffer (pH 4.5) was intraperitoneally injected at 50 mg/kg after 24-h fasting. Blood samples were collected from the tail vein after 24 h, and rats with a serum glucose level of 300 mg/dl or higher [5,6,7,8,9,10] were regarded as having diabetes and appropriate for the study. The control rats underwent the same procedure, except injection with vehicle instead of STZ.
Recording of 24-h voiding behavior
An electronic balance (GX-400, A&D, Japan) was set up at the urine collection unit of a metabolic cage (Shinano, Japan). Animals were maintained in the metabolic cages under a 12-h dark/12-h light condition for 3 days prior to recording to obtain sufficient acclimation and were given free access to food and water during the experiment. The recordings of voided urine weight were started at 8:00 p.m. and had been sampling every 15 s for 24 h using PowerLab system (AD Instruments, Australia). Total volume of water intake, total and tidal voided volume, and frequency of voiding were evaluated.
Cystometry
Cystometry was performed under awake condition. A polyethylene catheter (PE50, Becton–Dickinson Co., Ltd., USA) was implanted into the bladder dome of rat by laparotomy under anesthesia with pentobarbital (30 mg/kg, i.p.). Three days after the implant surgery, the intravesical catheter was connected via a three-way stopcock to pressure transducer (DX-100, Nihon Kohden, Japan) for recording intravesical pressure and syringes pump for infusing saline into the bladder. An electronic balance (GX-400, A&D, Japan) was placed under the cage for simultaneous measurement of the tidal volume. The measurement of intravesical pressure was initiated at the infusion rate of 10 ml/h. All changes in intravesical pressure and tidal volume were recorded and analyzed by PowerLab system and LabChart 5 (AD Instruments, Australia). For measurement of the residual urine, we discontinued intravesical infusion and collected urine using a 1-ml syringe immediately after urination [17].
Muscle strip experiment using isolated bladder
Rats were euthanized by exsanguination, and the urinary bladder was excised. Detrusor strips (width 1 mm, length 10 mm) were prepared (3 strips per bladder), and fixed to an FD pick-up transducer (TB-611T, Nihon Kohden, Japan) in an organ bath filled with Krebs–Henseleit solution (pH 7.4). Resting tension (1 g) was loaded, and 40 mM KCl-induced detrusor contraction was checked after equilibrium for about 90 min. EFS-induced detrusor contraction was induced by loading field stimulation (voltage 50 V, duration 0.5 ms, frequency 0.5–32 Hz, train 3 s). For agonist-induced detrusor contraction, carbachol (Wako, Japan) or α,β-methylene adenosine 5′-triphosphate (α,β-MeATP) (Sigma-Aldrich, USA) was cumulatively added to the organ bath to the final concentration of 1 × 10−9–1 × 10−4 M. The contractile force of detrusor strip was recorded and analyzed by PowerLab system and LabChart 5.
Bladder muscarinic and P2X receptor measurement by radioreceptor assay
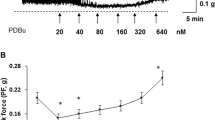
The radioreceptor assays for muscarinic and P2X receptors were performed using [N-methyl-3H] scopolamine methyl chloride ([3H]NMS) and α,β-methylene-ATP (2,8-3H) tetrasodium salt ([3H]α,β-MeATP) (PerkinElmer Life Sciences, USA) according to the method reported by Nasrin et al. [18]. Briefly, the tissue membrane samples for the assay were prepared by disintegrating bladders removed from rats and centrifuge separation. Subsequently, the tissue membrane samples were incubated with various concentrations of [3H]NMS (0.06–1.5 nM) for 60 min at 25 °C for muscarinic receptors or [3H]α,β-MeATP (0.3–10 nM) for 60 min at 4 °C for P2X receptors, respectively. The reaction was terminated by rapid filtration (Cell harvester; Brandel Co, Gaithersburg, MD, USA) through Whatman GF/B glass filters. The tissue-bound radioactivity was extracted from the filters overnight in the scintillation fluid, and the radioactivity was determined by a liquid scintillation counter. The specific bindings of [3H]NMS and [3H]α,β-MeATP were determined experimentally from the difference between counts in the absence and presence of 1 μM atropine and 3 μM α,β-MeATP, respectively.
Statistical analysis
Each experiment was repeated in three times. All analysis of the experiments was performed using GraphPad Prism 4.01(GraphPad Software, Inc., USA). All experimental values are presented as the mean ± SEM. The significance of differences was analyzed using unpaired Student’s t test, and a p value < 5% was regarded as significant.
Results
Body weight, bladder weight, and serum glucose level of the experimental animals
In the STZ-induced diabetes group, the body weight significantly decreased at all time-points compared to those in the age-matched control group. The bladder weight significantly increased, and the blood glucose level also significantly rose in the STZ-induced diabetes group than in the age-matched control group (P < 0.01) (Table 1).
24-h voiding behavior
In the STZ-induced diabetes group, the volume of water intake, the voided volume, the frequency of voiding, and the tidal voided volume significantly increased at all time-points compared to those in the age-matched control group (P < 0.001) (Fig. 1).
Time courses of 24-h voiding behavior in STZ-induced diabetic rats. a Total volume of water intake, b total voided volume, c frequency of voiding, d tidal voided volume. Data are shown as mean ± SEM of 10 rats in each group. ***P < 0.001 versus control group at each time-point (unpaired Student’s t test)
Cystometric parameters
In the STZ-induced diabetes group, the maximum voiding pressure, the tidal volume, the residual urine volume, and the bladder capacity significantly increased at all time-points compared to those in the age-matched control group (maximum voiding pressure at 4 and 12 weeks: P < 0.01, all others: P < 0.001). In addition, the voiding efficiency significantly decreased from 4 weeks in the STZ-induced diabetes group, compared to the control group (4 and 8 weeks: P < 0.01, 12 weeks: P < 0.001) (Fig. 2).
Time courses of cystometric parameters in STZ-induced diabetic rats. a Maximum voiding pressure, b tidal voided volume, c residual urine volume, d bladder capacity, e voiding efficiency. Data are shown as mean ± SEM of 8 rats in each group. **P < 0.01 versus control group at each time-point (unpaired Student’s t test). ***P < 0.001 versus control group at each time-point (unpaired Student’s t test)
Analysis of detrusor contractility using the isolated bladder
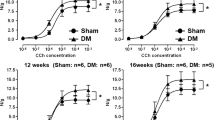
EFS-induced detrusor contraction was significantly enhanced from 4 weeks in the STZ-induced diabetes group, compared to the control group. This enhanced contraction persisted until 12 weeks. In the detrusor muscle strip experiment using carbachol, the significant enhancement of the contraction was observed from 4 weeks in the STZ-induced diabetes group, compared to the control group, and this enhanced contraction also persisted until 12 weeks. In the detrusor muscle strip experiment using α,β-MeATP, a significant enhancement of contraction was observed at 4 and 8 weeks in the STZ-induced diabetes group, compared to the control group. However, no difference was noted in α,β-MeATP-induced detrusor contraction between both groups at 12 weeks (Fig. 3).
Muscarinic and P2X receptor in the bladder
The maximum number of sites (Bmax) for [3H]NMS was significantly increased at 4, 8, and 12 weeks after STZ administration compared to those in the age-matched control group (P < 0.05), but no significant change was noted at 1 week after STZ administration. The dissociation constant (Kd) was not significantly different between the control and STZ-induced diabetes groups at any time-point (Table 2).
The Bmax for specific binding of [3H]α,β-MeATP significantly increased at 4 and 8 weeks after STZ administration compared to those in the age-matched control group (P < 0.05), but no significant change was noted at 12 weeks after STZ administration. No significant difference was noted in Kd at any time-point between the control and STZ-induced diabetes groups (Table 3).
Discussion
It is traditionally described that diabetic cystopathy mainly include voiding problems such as underactive bladder rather than storage [1, 2]. However, a lot of clinical and experimental reports also indicated that diabetic dysfunction often had storage problems such as urgency and urge incontinence [4, 5]. Generally, the multifariousness of bladder dysfunction in diabetes is ascribed to length of the disease.
In this study, we attempted examining whether the alteration of purinergic and muscarinic receptors, which played a very important role for bladder functions, is associated with bladder dysfunctions in the early stage of diabetes.
In the STZ-induced diabetes group, recording of 24-h voiding behavior showed polydipsic polyuric pollakiuria from 1 week after the induction of diabetes, which was consistent with the report of Liu and Daneshgari [19]. Voiding dysfunction, such as an increase in the residual urine volume, was also noted from the early stage of diabetes on cystometry. However, maximum voiding pressure was increased in diabetic rats. Many researchers reported that the maximum voiding pressure was elevated at early stage after the induction of diabetes [5, 9] and following decompensatory state from the compensatory state, i.e., progression of diabetic cystopathy, and that it may decrease at later stage [5, 10]. The results of this study and these reports suggest that detrusor contractility increased rather than decreased during the early stage after the induction of diabetes.
Bladder contraction is regulated through the cholinergic and non-adrenergic non-cholinergic nervous (NANC) systems [13]. Cholinergic nerves release acetylcholine from the nerve endings and induce detrusor contraction through muscarinic receptor (mainly M3 receptor) [20]. NANC nerves mainly release ATP from the nerve endings and induce rapid detrusor contraction through the purine receptor (P2X1 receptor) [21,22,23]. In addition, it has recently been suggested that urothelium plays an important role in afferent signal transmission and that acetylcholine and/or ATP produced by the urothelium in response to bladder extension regulate an excitement of submucosal afferent nerves [24,25,26].
We focused to examine whether purinergic and muscarinic pathways ascribed to the increased detrusor contraction in the early stage of the diabetes. In the present study, EFS-induced enhancements were noted in the muscle strip experiment from 4 weeks after STZ administration and persisted until 12 weeks, indicating that detrusor contraction was increased in the early stage of disease. Also, carbachol and α,β-MeATP-induced enhancements were noted in the muscle strip experiment from 4 weeks after STZ administration. Carbachol-induced enhancements persisted until 12 weeks, but α,β-MeATP-induced enhancement decreased toward the control level at 12 weeks.
Kd for the specific [3H]NMS binding in the bladder did not change in STZ-induced diabetic rats, and Bmax significantly increased at 4, 8, and 12 weeks after STZ administration. Similarly, Kd for the specific [3H]α,β-MeATP binding did not change, and Bmax significantly increased at 1, 4, and 8 weeks in the bladder after STZ administration. As we described before, muscarinic receptor may be mainly consisted of M3 receptor [20,21,22,23] and purine receptor P2X1 receptor; however, we could not specify the subtype of receptors by our experiments. These results indicated that the number of purinergic and muscarinic receptors concomitantly increased with no change in the receptor affinity. It is interesting to note that the alteration of purinergic receptor precedes that of muscarinic receptor. Our findings suggested that increased detrusor contraction is affected by the number of purinergic and muscarinic receptors in the bladder.
Though we observed increased numbers of both muscarinic and P2X receptors, and their association of bladder contraction in diabetic bladder, we could not determine whether their increases were dependent on each other or irrelevant. However, Munoz et al. [11] observed P2X-mediated contractions are more sensitive to desensitization induced by cholinergic activation in diabetic bladders at week 4 than control and concluded diabetes has a plasticity effect for bladder contraction. These differences may be derived from different observation periods and different methods, for instance, inhibitory intervention by atropine.
Interestingly, the purinergic receptor increased from the first week of diabetes and kept increasing at week 4 and 8 to the plateau at week 12. The muscarinic receptor increased from the week 4 and kept increasing until the week 8, then decreased at week 12. These results correlate with the results of muscle strip contractility. Since voiding dysfunction of clinical diabetes progresses following OAB → DHIC (detrusor hyperreflexia/impaired contractility) → UAB (underactive bladder) [27], the results of present study may reflect this process. OAB symptoms of early diabetic patients may not be improved only by anticholinergic drugs. It suggests that the upregulation of purinergic receptors might have a role in bladder dysfunction in human diabetes.
Further investigation is required concerning urethral function concurrent with bladder function of diabetic rats, because it is possible that these changes of bladder function were caused by urethral dysfunction (urethral relaxation failure) associated with diabetic neuropathy [28,29,30]. With a limitation of this issue, we obtained results suggesting the cause of overactive bladder symptoms which occur in the early stage of diabetes.
In conclusion, concomitant alteration of P2X and muscarinic receptors contributes to bladder dysfunction, such as increased bladder contractility, in early stage of STZ-induced diabetic rats.
Abbreviations
- STZ:
-
Streptozotocin
- EFS:
-
Electrical field stimulation
- α,β-MeATP:
-
α,β-methylene adenosine 5′-triphosphate
- [3H]NMS:
-
[N-methyl-3H] scopolamine methyl chloride
- [3H]α,β-MeATP:
-
α,β-methylene-ATP (2,8-3H) tetrasodium salt
- Kd:
-
Dissociation constant
- Bmax:
-
Maximum number of sites
- NANC:
-
Non-adrenergic non-cholinergic
References
Frimodt-Moller C (1978) Diabetic cystopathy. A review of the urodynamic and clinical features of neurogenic bladder dysfunction in diabetes mellitus. Dan Med Bull 25(2):49–60
Ellenberg M (1980) Development of urinary bladder dysfunction in diabetes mellitus. Ann Intern Med 92(2 Pt 2):321–323
Kaplan SA, Te AE, Blaivas JG (1995) Urodynamic findings in patients with diabetic cystopathy. J Urol 153(2):342–344
Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C (2007) Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn 26(6):814–819. https://doi.org/10.1002/nau.20422
Christ GJ, Hsieh Y, Zhao WX, Schenk G, Venkateswarlu K, Wang HZ, Tar MT, Melman A (2006) Effects of streptozotocin-induced diabetes on bladder and erectile (dys)function in the same rat in vivo. BJU Int 97(5):1076–1082. https://doi.org/10.1111/j.1464-410X.2006.06058.x
Tong YC, Cheng JT, Hsu CT (2006) Alterations of M(2)-muscarinic receptor protein and mRNA expression in the urothelium and muscle layer of the streptozotocin-induced diabetic rat urinary bladder. Neurosci Lett 406(3):216–221. https://doi.org/10.1016/j.neulet.2006.07.065
Cheng JT, Yu BC, Tong YC (2007) Changes of M3-muscarinic receptor protein and mRNA expressions in the bladder urothelium and muscle layer of streptozotocin-induced diabetic rats. Neurosci Lett 423(1):1–5. https://doi.org/10.1016/j.neulet.2007.05.062
Saito M, Kinoshita Y, Satoh I, Shinbori C, Suzuki H, Yamada M, Watanabe T, Satoh K (2007) Ability of cyclohexenonic long-chain fatty alcohol to reverse diabetes-induced cystopathy in the rat. Eur Urol 51(2):479–487. https://doi.org/10.1016/j.eururo.2006.06.024 (discussion 487–478)
Malmgren A, Andersson PO, Uvelius B (1989) Bladder function in rats with short- and long-term diabetes; effects of age and muscarinic blockade. J Urol 142(6):1608–1614
Daneshgari F, Liu G, Imrey PB (2006) Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol 176(1):380–386. https://doi.org/10.1016/S0022-5347(06)00582-9
Munoz A, Boone TB, Smith CP, Somogyi GT (2013) Diabetic plasticity of non-adrenergic non-cholinergic and P2X-mediated rat bladder contractions. Brain Res Bull 95:40–45. https://doi.org/10.1016/j.brainresbull.2013.03.006
Tong YC, Hung YC, Shinozuka K, Kunitomo M, Cheng JT (1997) Evidence of adenosine 5′-triphosphate release from nerve and P2X-purinoceptor mediated contraction during electrical stimulation of rat urinary bladder smooth muscle. J Urol 158(5):1973–1977. https://doi.org/10.1016/S0022-5347(01)64196-X
Andersson KE, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84(3):935–986. https://doi.org/10.1152/physrev.00038.2003
Lai HH, Smith CP, Munoz A, Boone TB, Szigeti GP, Somogyi GT (2008) Activation of cholinergic receptors blocks non-adrenergic non-cholinergic contractions in the rat urinary bladder. Brain Res Bull 77(6):420–426. https://doi.org/10.1016/j.brainresbull.2008.07.011
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407(6807):1011–1015. https://doi.org/10.1038/35039519
Lee WC, Chiang PH, Tain YL, Wu CC, Chuang YC (2012) Sensory dysfunction of bladder mucosa and bladder oversensitivity in a rat model of metabolic syndrome. PLoS ONE 7(9):e45578. https://doi.org/10.1371/journal.pone.0045578
Igawa Y, Persson K, Andersson KE, Uvelius B, Mattiasson A (1993) Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. J Urol 149(4):884–889
Nasrin S, Masuda E, Kugaya H, Ito Y, Yamada S (2013) Improvement by phytotherapeutic agent of detrusor overactivity, down-regulation of pharmacological receptors and urinary cytokines in rats with cyclophosphamide induced cystitis. J Urol 189(3):1123–1129. https://doi.org/10.1016/j.juro.2012.09.054
Liu G, Daneshgari F (2006) Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol 291(3):R837–R843. https://doi.org/10.1152/ajpregu.00917.2005
Longhurst PA, Leggett RE, Briscoe JA (1995) Characterization of the functional muscarinic receptors in the rat urinary bladder. Br J Pharmacol 116(4):2279–2285
Vial C, Evans RJ (2000) P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131(7):1489–1495. https://doi.org/10.1038/sj.bjp.0703720
O’Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, McMahon SB (2001) A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol 165(5):1730–1734. https://doi.org/10.1016/S0022-5347(05)66403-8
Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, Murakami S, Kawabe K, Ueda S (2001) Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol 36(1):99–109. https://doi.org/10.1016/S0531-5565(00)00175-3
Birder LA (2005) More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol 289(3):F489–F495. https://doi.org/10.1152/ajprenal.00467.2004
Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA (2006) Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147(Suppl 2):S132–S143. https://doi.org/10.1038/sj.bjp.0706637
Elbadawi A (1996) Functional anatomy of the organs of micturition. Urol Clin North Am 23(2):177–210
Chancellor MB (2014) The overactive bladder progression to underactive bladder hypothesis. Int Urol Nephrol 46(Suppl 1):S23–S27. https://doi.org/10.1007/s11255-014-0778-y
Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N (2004) Urethral dysfunction in diabetic rats. J Urol 171(5):1959–1964. https://doi.org/10.1097/01.ju.0000121283.92963.05
Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N (2005) alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol 173(3):1027–1032. https://doi.org/10.1097/01.ju.0000146268.45662.36
Yang Z, Dolber PC, Fraser MO (2007) Diabetic urethropathy compounds the effects of diabetic cystopathy. J Urol 178(5):2213–2219. https://doi.org/10.1016/j.juro.2007.06.042
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were approved by the Animal Research and Care Committee at the Nihon University School of Medicine (Approved No. 080139). This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yoshizawa, T., Hayashi, Y., Yoshida, A. et al. Concomitant alteration in number and affinity of P2X and muscarinic receptors are associated with bladder dysfunction in early stage of diabetic rats. Int Urol Nephrol 50, 451–458 (2018). https://doi.org/10.1007/s11255-018-1800-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1800-6