Abstract
Objective
The aim of this study was to explore whether vitamin D receptor (VDR) polymorphisms are associated with susceptibility to rheumatoid arthritis (RA).
Methods
Meta-analyses were conducted on the associations between the VDR FokI, BsmI, and TaqI polymorphisms and RA.
Results
A total of seven studies were considered in the meta-analysis, involving a total of 923 patients and 912 controls. Meta-analysis of the VDR FokI polymorphism showed no association between RA and the F allele in the entire studied cohort (odds ratio, OR = 1.1740, 95 % confidence interval, CI = 0.994–1.387, p = 0.059). However, stratification by ethnicity revealed a significant association between the F allele and RA in Europeans (OR = 1.402, 95 % CI = 1.126–1.746, p = 0.003). Furthermore, an association was found between RA and the VDR FokI polymorphism using both the dominant model and homozygote contrast. Meta-analysis revealed no association between RA and the VDR BsmI B and TaqI T polymorphisms in Europeans (OR for the B allele = 1.065, 95 % CI = 0.911–1.245, p = 0.427; OR for the T allele = 1.065, 95 % CI = 0.834–1.361, p = 0.613).
Conclusion
This meta-analysis suggests that the VDR FokI polymorphism is associated with susceptibility to RA in European populations.
Zusammenfassung
Ziel
Ziel der vorliegenden Studie war es zu untersuchen, ob Vitamin-D-Rezeptor(VDR)-Polymorphismen mit der Anfälligkeit für rheumatoide Arthritis (RA) in Zusammenhang stehen.
Methoden
Es wurden Metaanalysen zu den Assoziationen zwischen den FokI-, BsmI- und TaqI-Polymorphismen des VDR und RA durchgeführt.
Ergebnisse
In der Metaanalyse wurden 7 Studien berücksichtigt, die 923 Patienten und 912 Kontrollen umfassten. Die Metaanalyse des VDR-FokI-Polymorphismus ergab keine Assoziation zwischen RA und dem F-Allel in der gesamten untersuchten Kohorte (Odds Ratio, OR = 1,1740; 95 %-Konfidenzintervall, 95%-KI = 0,994–1,387; p = 0,059). Jedoch zeigte die Stratifizierung nach Ethnizität eine signifikante Assoziation zwischen dem F-Allel und RA bei Europäern (OR = 1,402; 95 %-KI = 1,126–1,746; p = 0,003). Darüber hinaus wurde eine Assoziation zwischen RA und dem VDR-FokI-Polymorphismus anhand des dominanten Modells und des homozygoten Kontrasts festgestellt. Wie die Metaanalyse zeigte, bestand keine Assoziation zwischen RA und den VDR-BsmI-B- und -TaqI-T-Polymorphismen bei Europäern (OR für das B-Allel = 1,065; 95 %-KI = 0,911–1,245; p = 0,427; OR für das T-Allel = 1,065; 95 %-KI = 0,834–1,361; p = 0,613).
Schlussfolgerung
Die vorliegende Metaanalyse liefert Hinweise darauf, dass der VDR-FokI-Polymorphismus mit der Anfälligkeit für RA in der europäischen Bevölkerung assoziiert ist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rheumatoid arthritis (RA) is a chronic inflammatory disease predominantly affecting the synovial joints and suffered by up to 1 % of adults worldwide. Human leukocyte antigen (HLA) class II molecules have been shown to be strongly associated with RA, but family studies suggest that this association accounts for only one third of genetic susceptibility and that non-HLA genes are also involved [1, 2].
Although the primary function of vitamin D is regulation of bone mineral homeostasis, it is also involved in interleukin (IL)-2 inhibition, antibody production, and lymphocyte proliferation; therefore, vitamin D is considered to be a regulator of the immune system [3]. It is known that 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) inhibits interferon secretion and negatively regulates IL-12 production by downregulating nuclear factor-kappa B [4]. Furthermore, in one in vitro study, 1,25(OH)2D3 was found to have a preventative effect on autoimmune diseases [5].
The action of vitamin D is dependent on the vitamin D receptor (VDR), a member of the nuclear hormone receptor superfamily, and the VDR gene is one of the most frequently studied genes in the context of RA. This gene is located on chromosome 12q13.11 [6], and three polymorphisms—BsmI (rs1544410) and ApaI (rs7975232), both in intron 8; and TaqI (rs731236) in exon 9—have been identified at the 3’-end of the gene and been shown to be in strong linkage disequilibrium (LD; [7]). The other polymorphic region, FokI (rs10735810), is located in the start codon [6]. Although the functional significances of these four VDR polymorphisms remains unknown, it is believed that LD and one or more functional polymorphism(s) elsewhere in the VDR gene explain the observed associations between VDR gene polymorphisms and autoimmune diseases. These VDR polymorphisms have been associated with RA in some reports, but by no means in all [8, 9, 10, 11, 12]. The reasons for this disparity may be small sample sizes, low statistical power, and/or clinical heterogeneity [13, 14, 15]. Therefore, in order to overcome the limitations of individual studies, resolve inconsistencies, and reduce the likelihood that random errors are responsible for false-positive or false-negative associations, we turned to meta-analysis. The aim of the present study was to determine using meta-analysis whether the VDR FokI, BsmI, and TaqI polymorphisms are associated with susceptibility to RA.
Methods
Identification of eligible studies and data extraction
Literature search
We performed a search for studies that examined associations between VDR polymorphisms and RA. The literature was searched using the MEDLINE, EMBASE, and Cochrane citation databases to identify available articles in which VDR polymorphisms were analyzed in RA patients. Combinations of keywords, such as, ‘vitamin D,’ ‘VDR,’ ‘polymorphism,’ ‘rheumatoid arthritis,’ and ‘RA’ were entered as Medical Subject Headings (MeSH) or text words. References in identified studies were also investigated to identify additional studies not indexed by the electronic databases. No language or country restrictions were applied.
Inclusion criteria
Studies were included if: (1) they were case–control studies; (2) the data were original (independence among studies); (3) they provided enough data to calculate an odds ratio (OR); (4) they had a distribution of the VDR polymorphism in normal controls consistent with the Hardy–Weinberg equilibrium (HWE).
Exclusion criteria
The following studies were excluded: (1) studies that contained overlapping data; (2) studies in which the number of null and wild type genotypes could not be ascertained; and (3) studies in which family members were studied, because these analyses are based on linkage considerations.
Data extraction
We conducted a systematic review and meta-analysis in accordance with the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Regarding the methods and results of meta-analysis, data were extracted from original studies by two independent reviewers. Discrepancy between the reviewers was resolved by consensus or a third reviewer. The following information was extracted from each study: author, year of publication, ethnicity of the study population, demographics, and numbers of cases and controls for the FokI, BsmI, and TaqI genotypes. Frequencies of alleles were calculated from corresponding genotype distributions.
Evaluations of statistical associations
We performed meta-analyses using: (1) allelic contrast, (2) contrast of homozygotes, (3) recessive, and (4) dominant models. Point estimates of risk, ORs, and 95 % confidence intervals (CI) were estimated for each study. In addition, intra- and interstudy variations or heterogeneities were assessed using Cochran’s Q-statistic. This heterogeneity test assesses the null hypothesis that all studies evaluated the same effect. The effect of heterogeneity was quantified using I2, which ranges between 0 and 100 % and represents the proportion of interstudy variability that can be attributed to heterogeneity as opposed to chance [16]. I2 values of 25, 50, and 75 % were nominally assigned as low, moderate, and high estimates, respectively. The fixed effects model assumes that genetic factors have similar effects on RA susceptibility across all investigated studies, and that observed variations among studies are caused by chance alone [17]. The random effects model assumes that different studies show substantial diversity and assesses both intrastudy sampling error and interstudy variance [18]. We used the fixed effects model in this meta-analysis. Statistical manipulations were undertaken using the Comprehensive Meta-Analysis computer program (Biosta, Englewood, NJ, USA).
Evaluation of quality score assessment, sensitivity test, and publication bias
The included studies were scored by the two reviewers, based on criteria selected from published recommendations on the evaluation of the quality of genetic association studies [19]; any disagreement was adjudicated by a third author. Total scores ranged from 0 to 10. Sensitivity analysis was also performed; firstly to assess the influence of each study on the pooled OR (by omitting each individual study) and secondly, to evaluate the statistical robustness of the results. Funnel plots are often used to detect publication bias. However, due to the limitations of funnel plotting, which requires a range of studies of varying sizes involving subjective judgments, publication bias was evaluated using Egger’s linear regression test [20], which measures funnel plot asymmetry using a natural logarithm scale of OR.
Results
Studies included in the meta-analysis
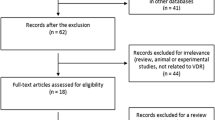
In total, 621 studies were identified by electronic and manual searches, and 10 were selected for a full-text review based on title and abstract details [8, 9, 10, 11, 12, 21, 22, 23, 24, 25]. Five of these 10 studies were excluded; four because they had a genotype distribution in controls that was inconsistent with the HWE [21, 22, 23, 24] and one because it did not include the FokI, BsmI, and TaqI polymorphisms [25]. Five studies thus met the inclusion criteria [8, 9, 10, 11, 12]. Two of the eligible studies contained data on two different groups [9, 12] and these groups were treated independently. The study selection protocol is illustrated in Fig. 1. Therefore, a total of seven separate comparisons were considered in the meta-analysis, comprising 923 patients and 912 controls in total (Tab. 1). These seven studies included one Asian, one Tunisian, and five European populations. An ethnicity-specific meta-analysis was thus conducted for European populations. Six studies examined the VDR FokI polymorphism, six the VDR BsmI polymorphism, and three the VDR TaqI polymorphism. Selected characteristics of these studies with respect to associations between the VDR polymorphisms and RA are summarized in Tab. 1.
Meta-analysis of relationships between VDR polymorphisms and rheumatoid arthritis
A summary of the meta-analysis findings regarding the association between the different VDR polymorphisms and RA is provided in Tab. 2 and Tab. 3.
FokI polymorphism
Meta-analysis of the VDR FokI polymorphism showed no association between RA and the F allele in all study subjects (OR = 1.1740, 95 % CI = 0.994–1.387, p = 0.059; Tab. 2, Fig. 2). However, stratification by ethnicity revealed a significant association between the F allele and RA in Europeans (OR = 1.402, 95 % CI = 1.126–1.746, p = 0.003; Tab. 2, Fig. 3). Furthermore, an association was found between RA and the VDR FokI polymorphism using both the dominant model and the homozygote contrast (Tab. 2).
BsmI polymorphism
Meta-analysis revealed no association between RA and the BsmI B allele in all study subjects (OR = 1.065, 95 % CI = 0.911–1.245, p = 0.427; Tab. 2, Fig. 2). Stratification by ethnicity indicated an association between the VDR BsmI allele and RA in Europeans (OR = 1.474, 95 % CI = 1.067–2.036, p = 0.019; Tab. 2). However, there was a high heterogeneity in the meta-analysis and the random effects model showed no association (OR = 0.801, 95 % CI = 0.251–2.562, p = 0.709). The recessive and dominant models, as well as homozygote contrast failed to reveal an association between the BsmI polymorphism and RA in all subjects (Tab. 2).
TaqI polymorphism
Meta-analysis indicated no association between RA and the TaqI T allele in the European populations (OR = 1.065, 95 % CI = 0.834–1.361, p = 0.613; Tab. 3; Fig. 2). The recessive and dominant models, as well as homozygote contrast failed to reveal an association between the TaqI polymorphism and RA (Tab. 3).
Heterogeneity, sensitivity test, and publication bias
Some interstudy heterogeneity was found during the meta-analysis of the VDR BsmI polymorphism. However, no interstudy heterogeneity was found during the meta-analyses of the VDR FokI and TaqI polymorphisms.
Sensitivity analysis was performed to assess the influence of each individual study on the pooled OR by removing each study in turn and recalculating the pooled OR. This analysis showed that no individual study significantly affected the pooled OR of the VDR TaqI polymorphism, indicating statistically robust results from this meta-analysis. After the study of Karray et al. or Ghelani-2 et al. was excluded, the results of the VDR FokI polymorphism were changed (OR = 1.228, 95 % CI = 1.021–1.476, p = 0.029; OR = 1.281, 95 % CI = 1.058–1.550, p = 0.011; Fig. 4). When the study of Maalej et al. or Ghelanin-1 et al. was excluded, the results of the VDR BsmI polymorphism changed (OR = 1.268, 95 % CI = 1.071–1.500, p = 0.006; OR = 0.786, 95 % CI = 0.661–0.934, p = 0.006; Fig. 4). However, when two studies [9, 10] at one side of the vertical line and one study [12] at the opposite side were excluded, the results of the VDR BsmI polymorphism did not change and heterogeneity disappeared (OR = 1.038, 95 % CI = 0.850–1.267, p = 0.714; I 2 = 0, heterogeneity p = 0.661).
It was difficult to correlate the funnel plot, which is usually used to detect publication bias, as the number of studies included in the analysis was relatively small. However, Egger’s regression test showed no evidence of publication bias (Tab. 2, Tab. 3, Fig. 5).
Discussion
Although the multifactorial natures of autoimmune diseases are well recognized, genetic factors are considered to be strong determinants of these diseases, and researchers have thus been encouraged to search for the genes responsible. Many genes have been studied in this context; the VDR gene being an example of those studied in the context of RA [26]. Vitamin D plays a key role in calcium homeostasis and also contributes to regulation of the immune system [3]. Given the immunosuppressive effects of vitamin D and the potential link between vitamin D deficiency and autoimmune diseases, VDR polymorphisms, which may influence VDR activity, have been studied as potential causes of autoimmune diseases, including RA [27, 28].
In this meta-analysis, we combined data from published studies to evaluate genetic associations between the most commonly studied polymorphisms of the VDR gene, namely the FokI, BsmI, and TaqI polymorphisms, and RA. Our meta-analysis of the VDR BsmI and TaqI polymorphisms showed no association with RA; neither in all study subjects nor in Europeans. In contrast, meta-analysis of the FokI polymorphism showed a significant association with RA in European populations (OR = 1.402, 95 % CI = 1.126–1.746, p = 0.003) without heterogeneity, suggesting that the VDR FokI F allele may be a risk factor for RA in Europeans.
However, our results should be interpreted with caution because of the limited number of studies included in this meta-analysis, which also restricted further subgroup analyses. High heterogeneity was observed in the VDR polymorphism meta-analysis, and sensitivity analysis performed by removing each study in turn, revealed that the results of meta-analysis of the VDR FokI and BsmI polymorphisms were not statistically robust. Furthermore, the relative importance of the VDR polymorphisms during the development of RA may be dependent on ethnicity. We were able to perform ethnicity-specific meta-analysis of the FokI polymorphism only in the European patients.
The FokI and BsmI polymorphisms have a functional role. The FokI polymorphism located in a start codon creates an alternative start site, resulting in a protein of different length [29]. The short protein (F allele) is more active than the long variant (f allele) with respect to its transactivation activity [30]. Vitamin D concentration was significantly increased in patients with the ff genotype compared to individuals carrying the FF genotype [30]. VDR mRNA level was significantly decreased in patients with the VDR B allele compared to those not having the B allele [31]. However, our results are not consistent with these functional studies of the VDR BsmI polymorphism. Epidemiologic results often do not coincide with functional studies, because RA is a complex disease; with contributions from multiple genes, different genetic backgrounds, and environmental factors. The VDR BsmI polymorphism might not be associated with RA susceptibility, but rather with RA severity or clinical findings. Additionally, our results on the VDR BsmI polymorphism might be due to type II error.
The present study has some limitations that require consideration. Firstly, heterogeneity and confounding factors may have distorted the analysis. Secondly, ethnicity-specific analysis included data from European patients; therefore, the results are only applicable to that particular ethnic group. Thirdly, haplotype analysis may have provided more information and would have been more powerful than single polymorphism analysis. Furthermore, LD was found for the BsmI, TaqI, and ApaI polymorphisms [7]. However, no meta-analysis of haplotypes was possible due to inadequate haplotype data. Fourthly, the VDR polymorphisms may be associated with RA severity and clinical features, but the small amount of available data did not allow us to investigate these associations.
Conclusion
This meta-analysis demonstrates that the VDR FokI polymorphism is associated with susceptibility to RA in European populations. However, an association was not found between the VDR BsmI and TaqI polymorphisms and RA. Larger-scale studies in populations with different ethnicities are necessary to explore the roles of VDR gene polymorphisms in the pathogeneses of RA.
References
MacGregor AJ, Snieder H, Rigby AS et al (2000) Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43(1):30–37
Kim HR, Park MK, Cho ML et al (2010) Induction of macrophage migration inhibitory factor in ConA-stimulated rheumatoid arthritis synovial fibroblasts through the P38 map kinase-dependent signaling pathway. Korean J Intern Med 25(3):317–326
Maruotti N, Cantatore FP (2010) Vitamin D and the immune system. J Rheumatol 37(3):491–495
Boonstra A, Barrat FJ, Crain C et al (2001) 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167(9):4974–4980
Koizumi T, Nakao Y, Matsui T et al (1985) Effects of corticosteroid and 1,24R-dihydroxy-vitamin D3 administration on lymphoproliferation and autoimmune disease in MRL/MP-lpr/lpr mice. Int Arch Allergy Appl Immunol 77(4):396–404
Miyamoto K, Kesterson RA, Yamamoto H et al (1997) Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol 11(8):1165–1179
Morrison NA, Yeoman R, Kelly PJ, Eisman JA (1992) Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor gene polymorphism and circulating osteocalcin. Proc Natl Acad Sci U S A 89(15):6665–6669
Karray EF, Ben Dhifallah I, Ben Abdelghani K et al (2012) Associations of vitamin D receptor gene polymorphisms FokI and BsmI with susceptibility to rheumatoid arthritis and Behcet’s disease in Tunisians. Joint Bone Spine 79(2):144–148
Maalej A, Petit-Teixeira E, Michou L et al (2005) Association study of VDR gene with rheumatoid arthritis in the French population. Genes Immun 6(8):707–711
Goertz B, Fassbender WJ, Williams JC et al (2003) Vitamin D receptor genotypes are not associated with rheumatoid arthritis or biochemical parameters of bone turnover in German RA patients. Clin Exp Rheumatol 21(3):333–339
Garcia-Lozano JR, Gonzalez-Escribano MF, Valenzuela A et al (2001) Association of vitamin D receptor genotypes with early onset rheumatoid arthritis. Eur J Immunogenet 28(1):89–93
Ranganathan P (2009) Genetics of bone loss in rheumatoid arthritis—role of vitamin D receptor polymorphisms. Rheumatology (Oxford) 48(4):342–346
Nath SK, Harley JB, Lee YH (2005) Polymorphisms of complement receptor 1 and interleukin-10 genes and systemic lupus erythematosus: a meta-analysis. Hum Genet 118(2):225–234
Lee YH, Witte T, Momot T et al (2005) The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus: two case-control studies and a meta-analysis. Arthritis Rheum 52(12):3966–3974
Lee YH, Ji JD, Song GG (2007) Tumor necrosis factor-alpha promoter − 308 A/G polymorphism and rheumatoid arthritis susceptibility: a metaanalysis. J Rheumatol 34(1):43–49
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Egger M, Smith GD, Phillips AN (1997) Meta-analysis: principles and procedures. BMJ 315(7121):1533–1537
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Clark MF, Baudouin SV (2006) A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Med 32(11):1706–1712
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Hitchon CA, Sun Y, Robinson DB et al (2012) Vitamin D receptor polymorphism rs2228570 (Fok1) is associated with rheumatoid arthritis in North American natives. J Rheumatol 39(9):1792–1797
Hussien YM, Shehata A, Karam RA et al (2013) Polymorphism in vitamin D receptor and osteoprotegerin genes in Egyptian rheumatoid arthritis patients with and without osteoporosis. Mol Biol Rep 40(5):3675–3680
Rass P, Pakozdi A, Lakatos P et al (2006) Vitamin D receptor gene polymorphism in rheumatoid arthritis and associated osteoporosis. Rheumatol Int 26(11):964–971
Lee CK, Hong JS, Cho YS et al (2001) Lack of relationship between vitamin D receptor polymorphism and bone erosion in rheumatoid arthritis. J Korean Med Sci 16(2):188–192
Milchert M (2010) Association between BsmI vitamin D receptor gene polymorphism and serum concentration of vitamin D with progression of rheumatoid arthritis. Ann Acad Med Stetin 56(1):45–56
Raychaudhuri S (2010) Recent advances in the genetics of rheumatoid arthritis. Curr Opin Rheumatol 22(2):109–118
Lee YH, Bae SC, Choi SJ et al (2011) Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 38(6):3643–3651
Lee YH, Choi SJ, Ji JD, Song GG (2012) Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: a meta-analysis. Mol Biol Rep 39(6):6471–6478
Arai H, Miyamoto K, Taketani Y et al (1997) A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 12(6):915–921
Monticielo OA, Brenol JC, Chies JA et al (2012) The role of BsmI and FokI vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D in Brazilian patients with systemic lupus erythematosus. Lupus 21(1):43–52
Luo XY, Yang MH, Wu FX et al (2012) Vitamin D receptor gene BsmI polymorphism B allele, but not BB genotype, is associated with systemic lupus erythematosus in a Han Chinese population. Lupus 21(1):53–59
Acknowledgements
This study was supported in part by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI13C2124).
Compliance with ethical guidelines
Conflict of interest. G.G. Song, S.-C. Bae, and Y.H. Lee state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, G., Bae, SC. & Lee, Y. Vitamin D receptor FokI, BsmI, and TaqI polymorphisms and susceptibility to rheumatoid arthritis. Z Rheumatol 75, 322–329 (2016). https://doi.org/10.1007/s00393-015-1581-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-015-1581-6