Abstract
This expert opinion paper on atrial fibrillation detection after ischemic stroke includes a statement of the “Heart and Brain” consortium of the German Cardiac Society and the German Stroke Society. This paper was endorsed by the Stroke Unit-Commission of the German Stroke Society and the German Atrial Fibrillation NETwork. In patients with ischemic stroke, detection of atrial fibrillation should usually lead to a change in secondary stroke prevention, since oral anticoagulation is superior to antiplatelet drugs. The detection of previously undiagnosed atrial fibrillation can be improved in patients with ischemic stroke to optimize stroke prevention. This paper summarizes the present knowledge on atrial fibrillation detection after ischemic stroke. We propose an interdisciplinary standard for a “structured analysis of ECG monitoring” on the stroke unit as well as a staged diagnostic scheme for the detection of atrial fibrillation. Since the optimal duration and mode of ECG monitoring has not yet been finally established, this paper is intended to give advice to physicians who are involved in stroke care. In line with the nature of an expert opinion paper, labeling of classes of recommendations is not provided, since many statements are based on the expert opinion, reported case series and clinical experience. Therefore, this paper is not intended as a guideline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is the fourth most common cause of death and the leading cause for comorbidities in Germany. More than 260,000 stroke cases occur annually in Germany alone [1, 2]. About 15–20% of these strokes are due to atrial fibrillation (AF). AF is prevalent in about 2% of the German population and known to increase the risk of ischemic stroke four- to five-fold [3, 4]. Due to a high risk of stroke recurrence and a higher stroke case fatality, patients with AF show a poorer prognosis compared to stroke patients without the rhythm disorder [5]. In a relevant proportion of patients with AF, the rhythm disorder occurs intermittently and often is asymptomatic. This observation also applies to the majority of stroke patients who are diagnosed with AF for the first time in the post stroke course [6]. The detection of AF after ischemic stroke is relevant due to the following reasons:
-
Stroke patients with (undetected) AF have a comparatively high risk of recurrence of ischemic stroke if they remain untreated [5, 7]
-
Strokes that affect specific areas of the brain (e.g., insular cortex) may possibly predict the onset of AF [8, 9] as similarly described for myocardial infarction, infection or certain metabolic disorders [10].
-
AF, which has been detected for the first time in the acute phase of stroke, will also be documented at later stages [11].
-
The diagnosis of AF is relevant for secondary prevention of ischemic stroke [4], even if the documentation of AF does not automatically imply a cardio-embolic cause of stroke [12,13,14]. Oral anticoagulants are effective in the secondary prevention of ischemic stroke in patients with AF and achieve a relative risk reduction of up to 70–80% compared to placebo [15, 16].
-
Effective oral anticoagulation at the time of ischemic stroke is associated with a reduction in stroke severity and mortality in AF patients [17, 18].
Current guidelines and recommendations
In stroke patients the duration of ECG monitoring [19, 20], the quality of the evaluation [21, 22] and patient selection [23, 24] correlate with the frequency of detected AF. ECG monitoring for at least 24 h in patients with ischemic stroke was recommended in 2010 [24]. In addition to a 12-lead resting ECG on admission and continuous ECG monitoring in the stroke unit, the guidelines of the German Neurological Society and European [25, 26] Stroke Organization (ESO) [27] recommend a long-term ECG registration in stroke patients with high suspicion of asymptomatic, paroxysmal AF. Specific information on the duration of ECG monitoring in the acute phase is not given. The US guideline recommends ECG monitoring for 30 days (class IIa, level C) in patients with cryptogenic stroke within the last 6 months [28]. The 2016 AF guidelines by the European Society of Cardiology (ESC) recommend ECG monitoring for at least 72 h for stroke patients and for patients with a transient ischemic attack (TIA) without known AF (Class I, Level B) [4]. In addition to an ECG monitoring with a central monitoring unit, the German Stroke Society (DSG) certification criteria recommend the use of Holter ECGs, a written standard for the application of the Holter monitoring as well as an extended diagnostic concept for patients with cryptogenic stroke [29]. Besides structured rhythm rounds on the stroke unit and the use of specific detection software for monitoring systems, reference is made to the implantation of event recorders.
State of the art for the detection of AF after ischemic stroke
To date, the results of four randomized studies on the efficacy of prolonged ECG monitoring in acute stroke have been presented in patients with ischemic strokes previously classified as cryptogenic (Table 1) [30,31,32,33]. These studies have demonstrated that different monitoring methods can increase the detection rate of AF and increase the number of consecutively anticoagulated stroke patients. The AF detection rate is correlated with the duration of ECG monitoring and decreases over the monitoring period [33]. However, the number of patients or the duration of follow-up of these randomized trials was not powered to demonstrate that prolonged ECG monitoring results in a reduction in clinical endpoints. Currently, the relevance of prolonged inpatient non-invasive ECG monitoring after acute ischemic stroke or TIA is investigated in Germany as part of the multi-center randomized MonDAFIS study [34]. Several prospective cohort studies have shown that the detection of AF after ischemic stroke does not only depend on the duration of the monitoring but also on the time of ECG monitoring relative to the index stroke and patient selection (Table 1). Due to the heterogeneity of the patient cohorts, the ECG monitoring methods, the AF definition used and the duration of the monitoring, published meta-analyses only are of limited value [35]. Nevertheless, they demonstrate that the detection rate of AF is higher in selected compared to unselected stroke cohorts [22]. Optimal AF detection will continue to have relevance in the future, since the first phase III study on systematic anticoagulation after embolic stroke of unknown source (ESUS) was stopped due to futility (NAVIGATE ESUS, NCT02313909). Another phase III study is ongoing (RE-SPECT ESUS, NCT02239120). Interestingly, a relevant subset of ESUS patients demonstrates a first episode of AF during long-term monitoring [35].
Definition of AF and relevance of the type of AF
According to experts, the diagnosis “AF” requires an episode of at least 30 s of atrial arrhythmia with missing P-waves. Shorter episodes (< 30 s) should not be called AF, but “atrial run” or increased supraventricular ectopia. The 30-s duration is also mentioned in the ESC guidelines [4]. However, this definition has not been prospectively validated and is based on consensus and frequently used in clinical trials. In up to 44% of all patients with acute ischemic stroke, “atrial runs” are found in the acute phase [30, 37] and the prognostic relevance has not been conclusively clarified. According to the authors, the current data suggest that the duration of clinically detected AF episodes has rather a minor relevance for the risk of stroke [38, 39]. A meta-analysis of prospective studies on the (primary) prevention of ischemic stroke postulated a slightly higher risk of stroke in patients with persistent or permanent AF compared to patients with paroxysmal AF [40]. A retrospective analysis of the ASSERT study in pacemaker patients also suggests that the risk of stroke only increases when atrial tachycardia persists for at least 24 h [41]. A correlation of AF type with the risk of stroke has not been proven for the secondary prevention of stroke. It should not influence therapeutic decisions on anticoagulation. In particular, since in a relevant proportion of patients the clinical classification of AF type cannot be done correctly, because continuous monitoring data are not readily available in all patients.
Quality standards of ECG monitoring
So far, few studies on the systematic evaluation of ECG monitoring on the stroke unit are available [20, 21]. A survey on the detection of AF from 2014 involving 171 operators of a certified German stroke unit participated, illustrates several possibilities for optimizing ECG diagnostics in daily clinical practice [42]. Therefore, the authors consider it important to have standards of a high-quality “rhythm visit” that also includes involvement of cardiologists. It should be ensured that recorded ECG data should be analyzed in a standardized manner at least once daily by trained and experienced staff [21]. In this context automated analysis is helpful, however, requires a “manual” validation of the ECG findings and a medical diagnosis [20, 21, 43]. Besides detecting AF and atrial flutter, potentially life-threatening arrhythmias have to be considered. Stroke unit derived ECG data should be recorded for off-line analysis. A standardized diagnostic scheme can improve rhythm monitoring [21].
Duration of ECG monitoring after ischemic stroke
Due to limited resources and economic constraints, it is not possible (for stroke units) to immediately expand the diagnostic standard of cardiac arrhythmia diagnostics at scale. From the authors’ point of view and the Stroke Unit Commission of the German Stroke Society this requires appropriate structural adjustments in the respective specialty departments. There is consensus among the authors that a baseline ECG monitoring after ischemic stroke should be performed, ideally for at least 72 h to increase the likelihood of detecting AF, which was also recommended by the current ESC guidelines [4]. It seems to be irrelevant whether this monitoring is done entirely by telemetry on the stroke unit or by Holter ECG, as long as a high-quality, standardized evaluation of the ECG is ensured [21]. No distinction should be made between patients with (confirmed) ischemic stroke and those with TIA if relevant differential diagnoses of TIA are unlikely. In case of a shorter inpatient length of stay an outpatient continuation of ECG monitoring is recommended for TIA patients to reach 72 h in total. In addition, ECG monitoring should be performed even if a non-cardio-embolic cause of ischemic stroke is suspected. A diagnosis of AF is also relevant for the secondary prevention of these patients, which has to be multifactorial [44]. Even in the presence of absolute contraindications for permanent oral anticoagulation ECG monitoring in the acute phase of ischemic stroke is recommended. Besides the occurrence of other relevant cardiac arrhythmias, interventional occlusion of the left atrial appendage may be considered in certain patients upon detection of AF [4]. The costs, the decreasing compliance in non-invasive monitoring procedures and the probability of AF detection have to be weighed for ECG monitoring beyond 72 h. Currently, a continuous ECG monitoring of 30 days, as recommended by the US guidelines for stroke patients with cryptogenic stroke [28], is not regularly feasible in clinical practice. A number of clinical, ECG and echocardiographic parameters have been described (Table 2) that increase the probability to detect a first episode of AF after ischemic stroke. The current evidence is presented in the following.
Relevance of clinical parameters to predict AF
The strongest predictor of AF is age [45, 46]. Therefore, patient age should be considered for the cumulative duration of ECG monitoring (see Table 2). At present, the use of published risk scores including classical cardiovascular risk factors to tailor ECG monitoring after ischemic stroke or TIA does not seem to be efficient.
Relevance of brain imaging to predict AF
Multilocular lesion patterns affecting various cerebral vascular territories suggest a cardio-embolic origin or an embolic source in the aortic artery [47, 48]. Thus, prolonged ECG monitoring may be considered in these stroke patients (Table 2). However, the detection of a first episode of AF did not correlate with brain lesion pattern in the CRYSTAL-AF study (Table 1) [49]. Of note, about 10% of all patients with a “lacunar” stroke have AF [13, 14]. Thus, standardized (baseline) ECG monitoring is also indicated in patients with “lacunar” stroke, although the likelihood of detecting AF for the first time is lower than in patients with non-lacunar infarction [49]. Furthermore, some brain lesions (e.g., in the right insular region) have shown to induce autonomous imbalance and may thus mediate the occurrence of AF [8, 9]. The question whether AF detected after stroke is the cause or consequence of the stroke may not be conclusively answered in individual cases.
Relevance of cardiac imaging to predict AF
Structural and functional changes of the heart and neighboring structures that indicate an increased risk of AF can be detected by echocardiography. Valvular abnormalities, in particular rheumatic mitral valve stenosis or severe mitral and tricuspid valve insufficiency are predictive for AF. Transesophageal echocardiography provides a higher resolution for the imaging of atrial structures (e.g., atrial appendage) and atheroma load of the thoracic aorta [50] compared to transthoracic echocardiography. Spontaneous echo contrast and solid thrombi in the atrium can be predictors of AF. Whether the morphology of the atrial appendage correlates with thrombogenicity is currently under investigation [51]. The possible role of left atrial deformation characteristics measured by tissue Doppler as left atrial strain [52, 53] after ischemic stroke needs confirmation. The size of the left atrium measured by transthoracic echocardiography can be considered as a predictor of paroxysmal AF [54], and therefore, can be considered for the decision on a prolonged ECG monitoring after ischemic stroke (Table 2). However, a normal left atrium does not exclude the prevalence of AF. In addition, the combination of left atrial volume index and atrial function appear to have a predictive value for the detection of AF [55, 56]. Nevertheless, validation is necessary in larger stroke cohorts. Currently, the importance of magnetic resonance imaging or computed tomography is examined in dedicated studies [57]. Both methods have increasingly better spatial and temporal resolution for the functional characterization of the heart.
Relevance of biomarkers to predict AF
Based on pathophysiological concepts of AF development, several biomarkers have been in the focus of interest: biomarkers of atrial dilatation and impaired cardiac function (natriuretic peptides), myocyte damage (troponins), markers of inflammation (interleukin-6, C-reactive protein), pro-coagulatory pathways (D-dimer), vascular damage (interleukin-6, C-reactive protein, glomerular filtration rate, cystatin C, markers of endothelial function), or impaired hemodynamics (natriuretic peptides, glomerular filtration rate, cystatin C) [9, 58, 59]. For most of the listed biomarkers, convincing data from large prospective studies are missing. The acute phase reactant C-reactive protein and the natriuretic peptides N-terminal pro B-type natriuretic peptide (Nt-proBNP) and B-type natriuretic peptide (BNP) are most promising for the detection of AF [60, 61] and should be considered for a prolonged ECG monitoring after ischemic stroke (Table 2). For natriuretic peptides, validation data in stroke cohorts are available [62,63,64]. Despite an increase in natriuretic peptides in the acute phase of a stroke, it can be assumed that BNP values of > 100 pg/mL and Nt-proBNP values > 400 pg/mL have a predictive value for the occurrence of AF in stroke patients [62]. A retrospective analysis of the WARSS study for stroke patients without known AF showed a benefit of oral anticoagulation by warfarin compared to secondary prevention by acetylsalicylic acid in case of elevated Nt-proBNP [65].
Relevance of ECG parameters to predict AF
Increased excessive supraventricular ectopic activity (ESVEA) over 24 h or the detection of supraventricular tachycardia (atrial run) lasting less than 30 s may indicate an increased risk of AF and should be considered for the cumulative duration of ECG monitoring (Table 2). In the Copenhagen Holter Study [66] patients with ESVEA had a significantly higher risk of stroke and clinically manifest AF. Stroke patients with ESVEA also show AF more frequently later on [67]. However, there is a lack of reliable standard values for the acute phase of stroke. A retrospective analysis of the EMBRACE study (Table 1) also showed that the detection of frequent atrial extra beats in stroke patients was associated with an increased probability of AF detection [68].
Consensus recommendation on stratification for prolonged ECG monitoring
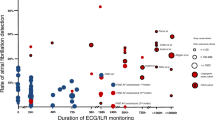
Following baseline ECG monitoring, ideally of 72 h, the authors suggest that AF risk estimation should be considered using the parameters listed in Table 2 to pragmatically specify the duration of prolonged ECG monitoring (also see Fig. 1). The decision on prolonged ECG monitoring should be based on a written standard procedure as required by the current certification criteria of the German Stroke Unit Commission [29].
Staged diagnostic scheme for the duration of (post-) inpatient ECG monitoring after acute ischemic stroke as suggested by the authors [36]. Dashed lines indicate that it may be considered, asterisk indicates if feasible
Ambulatory ECG monitoring after ischemic stroke
Guidelines recommend ECG monitoring after the acute phase of stroke [27, 28]. This appears to be feasible in practice but is often performed only to a limited extent [7, 31, 33]. A focused and standardized communication between the neurology acute clinic and the outpatient clinicians seems important for the practical implementation of a continued ECG monitoring. Systematic analyses are lacking. It is not conclusively clarified which device and technique could ensure a cost-efficient ambulatory ECG monitoring after ischemic stroke or TIA. Apart from the ECG, pulse palpitation is an established method of rhythm monitoring which can easily be taught to patients and their caregivers. The procedure achieves relatively high sensitivity and specificity for the detection of AF [69].
Which stroke patients should receive continuous ECG monitoring?
A comparatively small monocentric study showed that the consideration of the cardiovascular risk profile as well as specific ECG and echocardiographic parameters could increase the post-discharge detection rate of AF in stroke patients by an implanted event recorder [23]. Therefore, stroke patients with specific risk factors for AF should receive prolonged ECG monitoring. Non-invasive ECG monitoring should be based on practicability and should ideally last for at least 7 days. The compliance for longer non-invasive monitoring decreases significantly [32]. Therefore, the majority of the authors suggest the implantation of event recorders in stroke patients without AF identified during prolonged non-invasive ECG monitoring, but high probability of AF (Table 2).
Reimbursement of extended ECG monitoring
Currently, the reimbursement of post discharge ECG monitoring constitutes a relevant socio-economic problem. To date, health insurance companies have not reimbursed the costs for non-invasive ECG monitoring adequately. Extended monitoring appears to be a cost-effective measure, although information varies. In the US a quality-adjusted life year using a 30-day ECG monitor costs 2,000 USD in the US [70], while using a 7-day long-term ECG in Germany costs approximately 3,900 EUR [71], and £ 13,296 when using an implanted event recorder in the Crystal AF Study (Table 1) [72]. In Germany, the implantation of event recorders in stroke patients has been financed so far by a corresponding increase of the DRG during the hospital stay. However, an ambulatory implantation is not always reimbursed, neither is the interpretation of the ECG data recorded over years.
Summary
As part of the diagnostic work-up after an ischemic stroke, a timely ECG diagnosis to detect previously undiagnosed AF is essential. Due to an extended and high-quality ECG monitoring and patient selection based on clinical, laboratory, echocardiographic and electrographic parameters the likelihood of non-permanent AF detection and thus the efficiency of ECG monitoring can be improved.
References
Heuschmann PU, Busse O, Wagner M, Endres M, Villringer A, Röther J, Kolominsky-Rabas P, Berger K (2010) Schlaganfallhäufigkeit und Versorgung von Schlaganfallpatienten in Deutschland. Aktuelle Neurologie 37(07):333–340
Gesundheit-Todesursachen in Deutschland 2014. Fachserie 12 Reihe 4 Wiesbaden (2016) Statistisches Bundesamt. http://www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Todesursachen/Todesursachen2120400147004.pdf
Schnabel R, Johannsen S, Wild P, Blankenberg S (2015) Prevalence and risk factors of atrial fibrillation in Germany: data from the Gutenberg Health Study. Herz 40(1):8–15
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K (2016) 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962. https://doi.org/10.1093/eurheartj/ehw210
Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A (2005) Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Stroke 36(6):1115–1119
Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener H-C, Bernstein RA, Rymer M, Ziegler PD, Liu S, Passman RS (2016) Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients. Circ Arrhythm Electrophysiol 9 (1):e003333
Lip GY, Hunter TD, Quiroz ME, Ziegler PD, Turakhia MP (2017) Atrial fibrillation diagnosis timing, ambulatory ECG monitoring utilization, and risk of recurrent stroke. Circ Cardiovasc Qual Outcomes 10 (1):e002864
Sposato LA, Riccio PM, Hachinski V (2014) Poststroke atrial fibrillation: cause or consequence? Critical review of current views. Neurology 82(13):1180–1186
Scheitz JF, Erdur H, Haeusler KG, Audebert HJ, Roser M, Laufs U, Endres M, Nolte CH (2015) Insular cortex lesions, cardiac troponin, and detection of previously unknown atrial fibrillation in acute ischemic stroke. Stroke 46(5):1196–1201
Fauchier L, Clementy N, Bisson A, Stamboul K, Ivanes F, Angoulvant D, Babuty D, Lip GY (2017) Prognosis in patients with atrial fibrillation and a presumed “temporary cause” in a community-based cohort study. Clin Res Cardiol 106(3):202–210
Higgins P, Dawson J, MacFarlane PW, McArthur K, Langhorne P, Lees KR (2014) Predictive value of newly detected atrial fibrillation paroxysms in patients with acute ischemic stroke, for atrial fibrillation after 90 days. Stroke 45(7):2134–2136
Herm J, Konieczny M, Jungehulsing GJ, Endres M, Villringer A, Malzahn U, Heuschmann PU, Haeusler KG (2013) Should transesophageal echocardiography be performed in acute stroke patients with atrial fibrillation? J Clin Neurosci 20(4):554–559
Kamel H, Okin PM, Elkind MS, Iadecola C (2016) Atrial fibrillation and mechanisms of stroke. Stroke 47(3):895–900
Demeestere J, Fieuws S, Lansberg MG, Lemmens R (2016) Detection of atrial fibrillation among patients with stroke due to large or small vessel disease: a meta-analysis. J Am Heart Assoc 5(9):e004151
Hart RG, Pearce LA, Aguilar MI (2007) Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146(12):857–867
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM (2014) Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383(9921):955–962. https://doi.org/10.1016/S0140-6736(13)62343-0
Ottosen TP, Svendsen ML, Hansen ML, Brandes A, Andersen G, Husted SE, Johnsen SP (2014) Preadmission oral anticoagulant therapy and clinical outcome in patients hospitalised with acute stroke and atrial fibrillation. Dan Med J 61(9):A4904
Hellwig S, Grittner U, Audebert H, Endres M, Haeusler KG (2018) Non-vitamin K-dependent oral anticoagulants have a positive impact on ischaemic stroke severity in patients with atrial fibrillation. Europace 20(4):569–574
Sposato LA, Cipriano LE, Saposnik G, Vargas ER, Riccio PM, Hachinski V (2015) Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 14(4):377–387
Rizos T, Güntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R, Reinhardt R, Hepp T, Kirchhof P, Aleynichenko E (2012) Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 43(10):2689–2694
Kallmünzer B, Breuer L, Hering C, Raaz-Schrauder D, Kollmar R, Huttner HB, Schwab S, Köhrmann M (2012) A structured reading algorithm improves telemetric detection of atrial fibrillation after acute ischemic stroke. Stroke 43(4):994–999
Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, Smith CJ (2014) Detection of atrial fibrillation after ischemic stroke or transient ischemic attack. Stroke 45(2):520–526
Poli S, Diedler J, Härtig F, Götz N, Bauer A, Sachse T, Müller K, Müller I, Stimpfle F, Duckheim M (2016) Insertable cardiac monitors after cryptogenic stroke—a risk factor based approach to enhance the detection rate for paroxysmal atrial fibrillation. Eur J Neurol 23(2):375–381
Laufs U, Hoppe U, Rosenkranz S, Kirchhof P, Böhm M, Diener H, Endres M, Grond M, Hacke W, Meinertz T (2010) Cardiac workup after cerebral ischemia. Consensus paper of the Working Group on Heart and Brain of the German Cardiac Society and German Stroke Society. Der Nervenarzt 81(4):444
Neurologie DGf (2012) Leitlinien für Diagnostik und Therapie in der Neurologie-Akuttherapie des ischämischen Schlaganfalls. http://www.dgn.org/leitlinien/2310-ll-22-2012-akuttherapie-des-ischaemischen-schlaganfalls
Deutsche Schlaganfall-Gesellschaft (DSG) DGfND S3-Leitlinie: Sekundärprophylaxe ischämischer Schlaganfall und transitorische ischämische Attacke-Teil 1. Version 1.0. 31.01.2015
Committee ESOEECEW (2008) Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dise (Basel Switzerland) 25(5):457–507. https://doi.org/10.1159/000131083
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 45(7):2160–2236
Nabavi D, Ossenbrink M, Schinkel M, Koennecke H, Hamann G, Busse O (2015) Revised certification criteria for regional and national stroke units in Germany. Der Nervenarzt 86(8):978–988
Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR (2013) Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke. Stroke 44(9):2525–2531
Sanna T, Diener H-C, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F (2014) Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 370(26):2478–2486
Gladstone DJ, Spring M, Dorian P, Sanna T, Diener H, Passman R, Abboud H, Berroir S, Labreuche J, Orjuela K (2014) Cryptogenic stroke and atrial fibrillation. New Engl J Med 370:2467–2477
Wachter R, Gröschel K, Gelbrich G, Hamann GF, Kermer P, Liman J, Seegers J, Wasser K, Schulte A, Jürries F (2017) Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AF RANDOMISED): an open-label randomised controlled trial. Lancet Neurol 16(4):282–290
Haeusler KG, Kirchhof P, Heuschmann PU, Laufs U, Busse O, Kunze C, Thomalla G, Nabavi DG, Röther J, Veltkamp R (2016) Impact of standardized MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke (MonDAFIS): rationale and design of a prospective randomized multicenter study. Am Heart J 172:19–25
Dussault C, Toeg H, Nathan M, Wang ZJ, Roux J-F, Secemsky E (2015) Electrocardiographic monitoring for detecting atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation Arrhythm Electrophysiol 8(2):263–269
Häusler KG, Gröschel K, Köhrmann M, Schnabel R, Anker SD, Brachmann J, Böhm M, Diener HC, Doehner W, Endres M, Gerloff C, Huttner HB, Kaps M, Kirchhof P, Nabavi DG, Nolte CH, Pfeilschifter W, Pieske B, Poli S, Schäbitz WR, Thomalla G, Veltkamp R, Steiner T, Laufs U, Röther J, Wachter R (2018) Positionspapier zur Detektion von Vorhofflimmern nach ischämischem Schlaganfall. Akt Neurol 45:93–106
Stahrenberg R, Weber-Krüger M, Seegers J, Edelmann F, Lahno R, Haase B, Mende M, Wohlfahrt J, Kermer P, Vollmann D (2010) Enhanced detection of paroxysmal atrial fibrillation by early and prolonged continuous holter monitoring in patients with cerebral ischemia presenting in sinus rhythm. Stroke 41(12):2884–2888
Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE (2013) Device-detected atrial fibrillation and risk for stroke: an analysis of> 10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 35(8):508–516
Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE (2015) Atrial fibrillation burden and short-term risk of stroke: a case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol 8(5):1040–1047
Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, McGavigan AD (2016) The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 37(20):1591–1602
Van Gelder IC, Healey JS, Crijns HJ, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau C-P, Morillo CA, Hobbelt AH (2017) Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 38(17):1339–1344
Rizos T, Quilitzsch A, Busse O, Haeusler KG, Endres M, Heuschmann P, Veltkamp R (2015) Diagnostic work-up for detection of paroxysmal atrial fibrillation after acute ischemic stroke. Stroke 46(6):1693–1695
Uphaus T, Grings A, Gröschel S, Müller A, Weber-Krüger M, Wachter R, Gröschel K (2017) Automatic detection of paroxysmal atrial fibrillation in patients with ischaemic stroke: better than routine diagnostic workup? Eur J Neurol 24(7):990–994
Kirchhof G, Lindner JF, Achenbach S, Berger K, Blankenberg S, Fangerau H, Gimpel H, Gassner UM, Kersten J, Magnus D (2018) Stratified prevention: opportunities and limitations. Report on the 1st interdisciplinary cardiovascular workshop in Augsburg. Clin Res Cardiol 107(3):193–200
Thijs VN, Brachmann J, Morillo CA, Passman RS, Sanna T, Bernstein RA, Diener H-C, Di Lazzaro V, Rymer MM, Hogge L (2016) Predictors for atrial fibrillation detection after cryptogenic stroke results from CRYSTAL AF. Neurology 86(3):261–269
Israel C, Kitsiou A, Kalyani M, Deelawar S, Ejangue LE, Rogalewski A, Hagemeister C, Minnerup J, Schabitz WR (2017) Detection of atrial fibrillation in patients with embolic stroke of undetermined source by prolonged monitoring with implantable loop recorders. Thromb Haemost 117(10):1962–1969. https://doi.org/10.1160/th17-02-0072
Miller DJ, Khan MA, Schultz LR, Simpson JR, Katramados AM, Russman AN, Mitsias PD (2013) Outpatient cardiac telemetry detects a high rate of atrial fibrillation in cryptogenic stroke. J Neurol Sci 324(1–2):57–61. https://doi.org/10.1016/j.jns.2012.10.001
Bhatt A, Majid A, Razak A, Kassab M, Hussain S, Safdar A (2011) Predictors of occult paroxysmal atrial fibrillation in cryptogenic strokes detected by long-term noninvasive cardiac monitoring. Stroke Res Treat 2011:172074
Bernstein RA, Di Lazzaro V, Rymer MM, Passman RS, Brachmann J, Morillo CA, Sanna T, Thijs V, Rogers T, Liu S, Ziegler PD, Diener HC (2015) Infarct topography and detection of atrial fibrillation in cryptogenic stroke: results from CRYSTAL AF. Cerebrovasc Dis 40(1–2):91–96
Nakanishi K, Homma S (2016) Role of echocardiography in patients with stroke. J Cardiol 68(2):91–99
Lupercio F, Ruiz JC, Briceno DF, Romero J, Villablanca PA, Berardi C, Faillace R, Krumerman A, Fisher JD, Ferrick K (2016) Left atrial appendage morphology assessment for risk stratification of embolic stroke in patients with atrial fibrillation: a meta-analysis. Heart Rhythm 13(7):1402–1409
Kim D, Shim CY, Cho IJ, Kim YD, Nam HS, Chang H-J, Hong G-R, Ha J-W, Heo JH, Chung N (2016) Incremental value of left atrial global longitudinal strain for prediction of post stroke atrial fibrillation in patients with acute ischemic stroke. J Cardiovasc Ultrasound 24(1):20–27
Sarvari SI, Haugaa KH, Stokke TM, Ansari HZ, Leren IS, Hegbom F, Smiseth OA, Edvardsen T (2015) Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. Eur Heart J Cardiovasc Imaging 17(6):660–667
Broughton ST, O’Neal WT, Salahuddin T, Soliman EZ (2016) The influence of left atrial enlargement on the relationship between atrial fibrillation and stroke. J Stroke Cerebrovasc Dis 25(6):1396–1402
Stahrenberg R, Edelmann F, Haase B, Lahno R, Seegers J, Weber-Krüger M, Mende M, Wohlfahrt J, Kermer P, Vollmann D (2011) Transthoracic echocardiography to rule out paroxysmal atrial fibrillation as a cause of stroke or transient ischemic attack. Stroke 42(12):3643–3645
Waldenhjort D, Sobocinski Doliwa P, Alam M, Frykman-Kull V, Engdahl J, Rosenqvist M, Persson H (2016) Echocardiographic measures of atrial function may predict atrial fibrillation in stroke patients. Scand Cardiovasc J 50(4):236–242
Haeusler KG, Grittner U, Fiebach JB, Endres M, Krause T, Nolte CH (2015) HEart and BRain interfaces in Acute ischemic Stroke (HEBRAS)–rationale and design of a prospective oberservational cohort study. BMC Neurol 15(1):213
Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Siegbahn A, Yusuf S, Wallentin L (2014) Importance of persistent elevation of cardiac biomarkers in atrial fibrillation: a RE-LY substudy. Heart (British Cardiac Society) 100(15):1193–1200
Camm A, Savelieva I, Potpara T, Hindriks G, Pison L, Blömstrom-Lundqvist C (2016) The changing circumstance of atrial fibrillation-progress towards precision medicine. J Intern Med 279(5):412–427
Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA (2010) Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 121(2):200–207
Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens ACJ, Kronmal RA, Magnani JW (2014) B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace 16(10):1426–1433
Wachter R, Lahno R, Haase B, Weber-Krüger M, Seegers J, Edelmann F, Wohlfahrt J, Gelbrich G, Görlitz A, Kermer P (2012) Natriuretic peptides for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemia—the Find-AF study. PLOS one 7(4):e34351
Llombart V, Antolin-Fontes A, Bustamante A, Giralt D, Rost NS, Furie K, Shibazaki K, Biteker M, Castillo J, Rodríguez-Yáñez M (2015) B-type natriuretic peptides help in cardioembolic stroke diagnosis. Stroke 46(5):1187–1195
Rodríguez-Yáñez M, Arias-Rivas S, Santamaría-Cadavid M, Sobrino T, Castillo J, Blanco M (2013) High pro-BNP levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology 81(5):444–447
Longstreth W, Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, Brey RL, Buchsbaum R, Elkind MS, Tirschwell DL (2013) Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke 44(3):714–719
Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A (2010) Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 121(17):1904–1911
Weber-Krüger M, Gröschel K, Mende M, Seegers J, Lahno R, Haase B, Niehaus C-F, Edelmann F, Hasenfuß G, Wachter R (2013) Excessive supraventricular ectopic activity is indicative of paroxysmal atrial fibrillation in patients with cerebral ischemia. PLoS One 8(6):e67602
Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, Thorpe KE (2015) Atrial premature beats predict atrial fibrillation in cryptogenic stroke. Stroke 46(4):936–941
Kallmünzer B, Bobinger T, Kahl N, Kopp M, Kurka N, Hilz M-J, Marquardt L, Schwab S, Köhrmann M (2014) Peripheral pulse measurement after ischemic stroke a feasibility study. Neurology 83(7):598–603
Yong JHE, Thavorn K, Hoch JS, Mamdani M, Thorpe KE, Dorian P, Sharma M, Laupacis A, Gladstone DJ (2016) Potential cost-effectiveness of ambulatory cardiac rhythm monitoring after cryptogenic stroke. Stroke 47(9):2380–2385
Mayer F, Stahrenberg R, Gröschel K, Mostardt S, Biermann J, Edelmann F, Liman J, Wasem J, Goehler A, Wachter R (2013) Cost-effectiveness of 7-day-Holter monitoring alone or in combination with transthoracic echocardiography in patients with cerebral ischemia. Clin Res Cardiol 102(12):875–884
Diamantopoulos A, Sawyer LM, Lip GY, Witte KK, Reynolds MR, Fauchier L, Thijs V, Brown B, Quiroz Angulo ME, Diener H-C (2016) Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke 11(3):302–312
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A German version of this expert opinion paper of the “Heart and Brain” consortium of the German Cardiac Society and the German Stroke Society has been published previously [36]. KGH received lecture honoraria from Bayer HealthCare, Sanofi-Aventis, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Medtronic; honoraria for consulting from Edwards Lifesciences, Bayer HealthCare, Pfizer, EIP Pharma as well as research Grants from Bayer HealthCare and Sanofi-Aventis. KG received lecture honoraria, honoraria for consulting or travel Grants from Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo and Pfizer. MKö received lecture honoraria and honoraria for consulting from Bayer HealthCare, Pfizer, Bristol-Myers Squibb and Boehringer Ingelheim. SDA received honoraria from Bayer, Boehringer Ingelheim, Novartis, Servier and Vifor Int. and research Grants from Abbott Vascular and Vifor Int. MB received research honoraria and lecture honoraria from Boehringer-Ingelheim, Medtronic, St. Jude, Servier, AstraZeneca and Vifor Pharma. JB received lecture honoraria, honoraria for consulting or travel costs from Bayer HealthCare, Daiichi Sankyo, Boehringer Ingelheim, Novartis, Sanofi-Aventis, Pfizer, Bristol-Myers Squibb, Medtronic, Biotronik. HCD has received honoraria for the participation in clinical studies and collaboration on advisory boards and lectures from: Abbott, Allergan, AstraZeneca, Bayer Vital, Bristol-Meyers-Squibb, Boehringer Ingelheim, BrainsGate, CoAxia, Corimmun, Covidien, Daiichi-Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen Cilag, Lilly, Lundbeck, Medtronic, MSD, MindFrame, Neurobiological Technologies, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Portola, Sanofi-Aventis, Schering, Servier, Solvay, St-Jude, Syngis, Tacrelis, Thrombogenics and Wyeth. Revenues were transferred to the Essen University Clinical Center to finance research bodies for the Headache Center, the Vertigo Center and the Department of Neurology: AstraZeneca, GSK, Boehringer Ingelheim, Novartis, Janssen-Cilag and SanofiAventis. The University Department of Neurology has received research funding from the following institutions: DFG, BMBF, EU, NIH, EAST-AFnet, Bertelsmann Stiftung and Heinz-Nixdorf Stiftung. HCD owns no shares or shares of pharmaceutical companies or medical technology companies. HCD was involved in the development of guidelines of the DGN, the DSG, the ESC and EHRA. ME received research Grants from Bayer and Roche as well as fees to the Charité by Amgen, Bayer, BMS, Boehringer-Ingelheim, Ever, GSK, MSD, Novartis, Pfizer and Sanofi. CG received research Grants for stroke studies from the DFG and the EU as well as honoraria for Consulting from Bayer Vital, Boehringer Ingelheim, GlaxoSmithKline, Pfizer, Bristol-Myers Squibb, Sanofi Aventis, Amgen and Prediction BioSciences. HBH received lecturer and consultant honoraria and travel Grants as well as research funding from Bayer HealthCare, Boehringer-Ingelheim, Novartis and Medtronic. CHN received honoraria for lectures and consulting as well as travel Grants from Boehringer Ingelheim, Bayer HealthCare, Sanofi-Aventis, Pfizer, Bristol-Myers Squibb and Gore. MKa received honoraria for lectures and consulting from Bayer, Boehringer Ingelheim, Daiichi Sankyo. DGN received lecture honoraria from Bayer HealthCare, Sanofi-Aventis, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Medtronic; honoraria for lectures and travel Grants from AstraZeneca, Bayer HealthCare, Bristol-Myers Squibb and Pfizer. PK received research support from the European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), DZHK and pharmaceutical companies. A detailed declaration of interest, updated annually, is publicly available on the ESC web site. WP received honoraria for consulting and travel Grants from Sanofi Aventis, research and lecture honoraria from Boehringer Ingelheim, travel Grants from Bayer, lecture honoraria, research Grants and travel Grants from Stryker Neurovascular, research Grants from Novartis Pharma. BP received honoraria for consulting and participation in study committees from Bayer Healthcare, MSD, Novartis, Stealth Peptides, Astra-Zeneca, Menarini and BMS. SP received honoraria for lectures, consulting and travel Grants from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo and Werfen as well as research Grants from Bristol-Myers Squibb, Pfizer, Boehringer-Ingelheim, Daiichi Sankyo und Helena Laboratories. WRS received honoraria for lectures, consulting and travel Grants from Bayer HealthCare, Daiichi Sankyo, Boehringer Ingelheim, Sanofi-Aventis, Pfizer, Bristol-Myers Squibb and Medtronic. TS received honoraria from Bayer HealthCare, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Daiichy Sankyo and consulting honoraria from Bayer, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Daiichy Sankyo and Medtronic. GT received consulting honoraria for participation in adisory boards, for lectures by Acandis, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Daichii Sankyo, GlaxoSmithKline and Stryker as well as research Grants from Bayer Vital. RV received honoraria for lectures and consulting from Bayer, Boehringer Ingelheim, Daiichi Sankyo, BMS, Pfizer, Medtronic, ApoplexMedical Technologies, Biogen, Amgen, Morphosys, Sanofi, AZT Therapeutics, Portola as well as research Grants from Bayer, Boehringer Ingelheim, BMS, Pfizer, Daiicihi Sankyo, Biogen, ApoplexMedical Technologies. UL received honoraria for lectures and consulting from Amgen, Bayer, Berlin-Chemie, Boehringer-Ingelheim, Daiichi-Sankyo, MSD, Sanofi and Servier. JR received honoraria for lectures and/or consulting from Sanofi-Aventis, Pfizer, Lundbeck, Boehringer Ingelheim, BMS, Bayer Vital, Servier as well as the Deutsche Allgemeine Krankenversicherung (DAK) and is PI of studies by Astra Zeneca and Servier. RW received honoraria for consulting and lectures and the care of patients involved in clinical studies from Bayer, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Johnson&Johnson, Medtronic, Novartis, Pfizer, Sanofi and Servier.
Rights and permissions
About this article
Cite this article
Haeusler, K.G., Gröschel, K., Köhrmann, M. et al. Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol 107, 871–880 (2018). https://doi.org/10.1007/s00392-018-1256-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1256-9