Abstract
Background
There is growing evidence for beneficial prognostic and economic effects of FFR-guided treatment of stable coronary artery disease. We sought to evaluate the real-world use of FFR measurements in patients undergoing elective coronary angiography.
Methods and results
We analyzed the data of the prospective ALKK coronary angiography and PCI registry including data of 38 hospitals from January 2010 to December 2013. A total of 100,977 patients undergoing coronary angiography were included. In 3240 patients (3.2 %) intracoronary pressure measurement was performed. There was a wide range of use of FFR measurement in the different analyzed ALKK hospitals from 0.1 to 8.8 % in elective patients with suspected or known coronary artery disease (median 2.7 %, quartiles 0.9 and 5.3 %), with a successive increase of use over time during the study period. Overall, it was performed in 3.2 % of coronary angiographies. Use in patients with three-vessel disease (2.5 %) and recommendation for bypass surgery (1.6 %) was less frequent. In procedures without PCI, dose area product was higher in the FFR group (2641 cGy × cm2 vs. 2368 cGy × cm2, p < 0.001), while it was lower in procedures with ad hoc PCI (4676 cGy × cm2 vs. 5143 cGy × cm2, p < 0.001). The performing center turned out to be the strongest predictor.
Conclusions
The use of FFR measurement was very heterogeneous between different hospitals and in general relatively low, in particular in patients with multivessel disease or recommendation for bypass surgery, but there was a positive trend during the study period. Technically, FFR measurement was not associated with an increased periprocedural complication rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, functional assessment of coronary stenoses using intracoronary pressure guidewires has become an important diagnostic tool in the catheterization laboratory. Fractional flow reserve (FFR) has demonstrated the inaccuracy of angiography for evaluating the functional significance of coronary lesions with intermediate stenosis [1], and FFR-guidance was associated with reclassification of revascularization strategy in about half of the patients [2]. Furthermore, FFR-guided percutaneous coronary intervention (PCI) has been shown to reduce the need for PCI and to improve clinical outcome and procedural cost-effectiveness [3–6].
Since 2010, based on these data, the guidelines on myocardial revascularization of the European Society of Cardiology recommend measurement of FFR when noninvasive stress imaging is contraindicated, non-diagnostic, or unavailable [7]. In particular, FFR-guidance of PCI is recommended for detection of ischaemia-related lesions when objective evidence of vessel-related ischaemia is not available [7]. Furthermore, in case of multiple angiographically significant non-culprit stenoses or lesions whose severity is difficult to assess, liberal use of FFR measurement is recommended also to decide on the appropriate treatment strategy [7, 8].
The aim of our study was an evaluation of the real-world use of intracoronary pressure measurements in unselected patients with stable coronary artery disease (CAD), analyzing data from the prospective multicenter ALKK coronary angiography and PCI registry.
Methods
The ALKK coronary angiography and PCI registry
The ALKK (Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte) coronary angiography and PCI registry is a prospective registry that was initiated in 1992 to monitor quality control [9]. It contains all consecutive procedures of the participating hospitals on an intention-to-treat basis [9–12]. Since 2004, enrolment and some essential information in this registry are based on an obligatory quality control program that has been introduced by German authorities. Data were obtained using the standardized questionnaires in 50 participating hospitals, including information about the medical history (prior coronary interventions, diabetes, renal insufficiency, etc.), indication for the procedure, adjunctive antithrombotic therapy, the procedure itself (target vessel, use of intracoronary pressure measurement, interventional success rate, etc.), and complications until hospital discharge. The registry has been approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz, Mainz, Germany. All data were analyzed centrally at the Karl Ludwig Neuhaus Datenzentrum, Ludwigshafen, Germany. The study complies with the Declaration of Helsinki, and consent for anonymous analysis of their data was obtained from all patients.
Patient selection
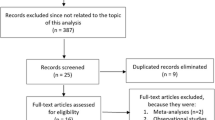
From 2010 to 2013, a total of 256,356 patients underwent coronary angiography in the 50 ALKK hospitals. We excluded hospitals with performance of no FFR (n = 6) or less than five (n = 6) FFR procedures during the study period. Therefore, 38 hospitals were included into this analysis. The analysis population consisted of elective admissions for coronary angiography with a diagnosis of significant or insignificant coronary artery disease. Patients with acute coronary syndrome or cardiogenic shock on admission were excluded from the analysis. Further exclusion criteria comprised angiographic exclusion of CAD, primary cardiomyopathy, significant valvular heart disease, aortic aneurysm with indication for surgery, and procedures with PCI of chronic total occlusions (Fig. 1).
Patient selection. Flow chart of patient selection according to inclusion and exclusion criteria and treatment recommendations. CAD coronary artery disease, FFR fractional flow reserve, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, CMP primary or hypertensive cardiomyopathy, CTO chronic total occlusion. Surgical indication = significant valvular heart disease or aortic aneurysm
The analyzed study population was stratified in the subgroups of patients receiving diagnostic coronary angiography exclusively, patients undergoing PCI, and patients with recommendation for bypass surgery. The subgroups of patients with three-vessel disease and patients with non-diagnostic stress test were considered as well.
Statistical methods
The patient population is described by absolute numbers and relative frequencies. Categorical data are presented as percentages, and distributions of metrical variables are characterized by median with quartiles, that of age by mean and standard deviation. The prevalence of binary characteristics was compared between patient groups by Pearson Chi-squared test, rates of in-hospital events by Fisher’s exact test. Distributions of metrical or ordinal variables were compared by Mann–Whitney–Wilcoxon test. The descriptive statistics were calculated from the available cases.
Determinants for the use of intracoronary pressure measurements were analyzed using multivariable logistic regression. Year of inclusion, center, and gender were included as factors, age as a linear term. In addition, clinically reasonable variables that showed a significant association in the univariate comparison were tested: prior PCI, prior CABG, diabetes, renal insufficiency, hypertension, current smoking, CCS classification of angina pectoris, and use and results of stress tests. For missing values in the explanatory variables of regression models, means were imputed. Predictors that exhibited statistical significance in the Wald test were included in the final model. Adjusted odds ratios (OR) with 95 %-confidence intervals were calculated, and the values of the log-likelihood ratio statistic (log-LR) are shown as a measure of the information added by each factor when comparing the full model to that without the respective factor.
A significance level of 0.05 was assumed and all p values are the results of two-tailed tests. The statistical computations were performed using SAS, version 9.3 (Cary, NC, USA).
Results
According to the inclusion and exclusion criteria, we identified 100,977 patients of the German ALKK coronary angiography and PCI registry as appropriate for this analysis. Of these, 3240 patients (3.2 %) underwent intracoronary pressure measurement with FFR calculation. There was a wide range in the use of intracoronary pressure measurement between the different analyzed ALKK hospitals with a minimum of 0.1 %, a maximum of 8.8 %, and a median of 2.7 % (quartiles 0.9 and 5.3 %) (Fig. 2). During the study period, there was a continuous increase in the use of FFR from 2.3 to 4.1 % (p < 0.001) (Fig. 3).
Annual use of FFR. Percentage use of FFR in elective patients with suspected or known coronary artery disease for the years 2010–2013, stratified in the subgroups of patients with recommendation for medical therapy (CONS), coronary intervention (PCI), or bypass surgery (CABG). FFR fractional flow reserve, ALKK Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte
Study population
Clinical characteristics of included patients are summarized in Table 1. Patients undergoing intracoronary pressure measurement were younger, more often male, suffered significantly less often from arterial hypertension, diabetes, peripheral artery disease, and renal insufficiency. Patients with acute heart failure underwent significantly less frequently intracoronary pressure measurement (2.4 vs. 4.0 %, p < 0.001).
Patients undergoing FFR measurement less suffered from typical angina symptoms (Table 1). This finding was consistent in the subgroups of patients with three-vessel disease and patients receiving conservative treatment, PCI, or CABG, also. The rate of stress test performed before angiography was somewhat lower in the FFR group (55.1 vs. 59.4 %, p < 0.001), but the rates of pathologic results of stress testing were not significantly different (47.5 vs. 47.4 %, p = 0.90) (Table 1; Fig. 4).
Use and results of noninvasive stress imaging. Use and results of noninvasive stress imaging in elective patients with suspected or known coronary artery disease, stratified for the performance of FFR measurement and the subsequent treatment recommendation. FFR fractional flow reserve, CONS conservative recommendation, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, ALKK Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte
Procedural characteristics
Procedural characteristics of included patients are summarized in Tables 2 and 3. In total, coronary artery disease was classified as functional significant more frequently in the FFR group (79.1 vs. 70.4 %, p < 0.001), but the extent of multivessel CAD was lower in the FFR group (1VD 34.8 vs. 27.5 %, p < 0.001, 2VD 34.5 vs. 28.9 %, p < 0.001, 3VD 30.7 vs. 43.6 %, p < 0.001, left main disease 6.4 vs. 10.3 %, p < 0.001). Analyzing the entire study population, FFR measurement led to higher rates of recommendation for conservative treatment (50.6 vs. 47.7 %, p < 0.05) and lower rates of recommendation for CABG (4.5 vs. 9.4 %, p < 0.001) while there were no differences in the rate of PCI (41.5 vs. 40.2 %, p = 0.16).
In procedures without PCI, dose area product (2641 cGy × cm2 vs. 2368 cGy × cm2, p < 0.001) and the amount of contrast media (110 ml vs. 90 ml, p < 0.001) were higher in the FFR group. In procedures with ad hoc PCI, dose area product was lower in the FFR group (4676 cGy × cm2 vs. 5143 cGy × cm2, p < 0.001), but the amount of contrast media was also higher (180 ml vs. 170 ml, p < 0.001). This phenomenon was evident in both single-vessel and multivessel PCI (Table 2).
Subgroup of patients with three-vessel disease
In the subgroup of 29,727 patients with three-vessel disease, use of FFR was lower when compared with the entire study population (2.5 vs. 3.2 %). While there were no differences for the recommendation of a conservative treatment approach between the FFR and no FFR group (30.0 vs. 29.6 %, p = 0.54), performance of FFR measurement was associated more frequently with PCI (59.4 vs. 49.8, p < 0.001), and less frequently with CABG (10.5 vs. 20.6 %, p < 0.001) in this subgroup.
CABG was recommended for 9063 patients (9.0 %) after coronary angiography. Of these, 144 patients (1.6 %) underwent FFR measurement. The latter had higher rates of two-vessel (30.0 vs. 20.0 %, p < 0.01) and lower rates of three-vessel disease (58.5 vs. 70.5 %, p < 0.01).
Subgroup of patients undergoing PCI
The increase in the use of FFR was most evident for patients with recommended or ad hoc performed PCI: in the first 2 years, FFR was associated more frequently with a recommendation of medical treatment instead of PCI (2.8 vs. 1.9 % in 2010, 3.1 vs. 2.8 % in 2011), while it was vice versa in the last 2 years (3.6 vs. 4.0 % in 2012, 4.0 vs. 4.4 %) (Fig. 3).
In the subgroup of 40,160 patients undergoing ad hoc PCI, FFR measurement was used in 1334 patients (3.3 %). In the FFR group, PCI was performed more frequently in the left anterior descending artery (LAD) when compared with the no FFR group, and less frequently in the right coronary artery (RCA) and the circumflex artery (CX) (Table 3). PCI was performed in coronary bypasses of 1688 patients, of whom a total of 12 underwent FFR measurement (0.7 %). In 28,185 patients (67.7 %), PCI was performed despite non-diagnostic noninvasive stress testing, and 6161 of these patients (21.9 %) had no angina symptoms either. In the latter two subgroups, FFR measurement was used in 853 patients (3.0 %) and 206 patients (3.3 %).
The mean number of treated lesions was 1.3 ± 0.6 in both the FFR and No FFR group. The group of patients with FFR measurement had a lower rate of complex coronary lesions (AHA type >B1) (49.2 vs. 53.3 %, OR 0.85), a higher rate of TIMI 3 flow before (86.3 vs. 73.2 %) and after intervention (97.3 vs. 95.9 %, p < 0.05), and they underwent less additional angiographies during the same hospital stay (2.7 vs. 4.0 %, p < 0.05). There were no differences in periprocedural complications, but the rate of major adverse cardiac and cerebrovascular events (MACCE) was lower in patients undergoing FFR measurement (0.1 vs. 0.6 %, p < 0.05). There were no differences in bleeding or puncture site complications or in the rate of acute renal failure (Table 4).
Determinants for the use of FFR
Regression analysis revealed center, year, age, sex, diabetes, prior PCI, prior CABG, renal insufficiency, acute heart failure, and angina as significant determinants for the use of FFR. The performing center turned out to be the strongest predictor. Furthermore, prior PCI, and year of angiography were positive predictors, while age, diabetes, renal insufficiency, prior CABG, acute heart failure, and angina CCS II or III were negative predictors for the use of FFR (Table 5).
Discussion
The aim of this study was an evaluation of the real-world use of intracoronary pressure measurements, analyzing data of the ALKK coronary angiography and PCI registry. Since 2010, the European guidelines on myocardial revascularization recommend the use of FFR when noninvasive stress imaging is contraindicated, non-diagnostic, or unavailable, as well as in case of multiple angiographically significant non-culprit stenoses whose severity is difficult to assess, to decide on the appropriate treatment strategy [7]. Furthermore, FFR-guided PCI is recommended for detection of ischaemia-related lesion(s) when objective evidence of vessel-related ischaemia is not available [7]. These recommendations base mainly upon the results of two studies: the DEFER and the FAME trial. In the DEFER trial, deferral of intervention in intermediate coronary stenoses based on a FFR cut-point ≥0.75 lead to an excellent clinical outcome after 5 years [4]. The subsequent FAME trial demonstrated inaccuracy of angiography for evaluating the functional significance of coronary lesions with a 50–90 % diameter stenosis [1], and FFR-guided revascularization based on a cut-point ≤0.8 improved outcome due to a significant reduction of myocardial infarction [13].
Real-world use of intracoronary pressure measurements
Interestingly, a main indicator for the use of intracoronary pressure measurement was the policy of the performing hospital in our study. The rate of FFR measurement in the single ALKK hospitals was very heterogeneous with a range of 0.1–8.8 %, and 12 hospitals not performing FFR measurement at all (excluded from analysis). Patient characteristics did not account for the observed differences between the hospitals. This finding is in line with previously published data regarding implementation of other new technologies in the catheterization laboratory [14, 15], reflecting subjectivity of decision making process despite an identical scientific database.
During the total study period, the overall rate of FFR measurement was 3.2 % with a successive increase over the years. In respect to the existing evidence for both medical and economic beneficial effects of FFR-guided intervention [3–6], this is a quite low rate. The reasons for the rare use of FFR in our study population remain speculative. However, a reduced workflow in the catheterization laboratory due to the additional time required for measurements, increased procedural costs in the absence of a cost-covering reimbursement in Germany until the year 2013, adenosine side effects including patient discomfort, and general skepticism of this technology might be reasonable explanations. It might be assumable that the use of FFR has increased following implementation of a nearly cost-covering reimbursement in the year 2014.
While FFR led more frequently to the recommendation of a medical treatment in the first 2 years of the study when compared with PCI, this situation was vice versa in the last 2 years. This effect might reflect a change in patient selection due to a growing evidence and acceptance of FFR-guidance of PCI during the study period [16]. On the other hand, the cutoff value for significance changed from 0.75 to 0.8 after publication of the FAME trial [6]. A temporally delayed implementation of this new cutoff into daily practice might be another possible reason.
Impact of angina symptoms and stress test results
It is unsurprising that patients in the FFR group suffered less from angina symptoms as typical and severe angina symptoms in patients with intermediate coronary stenosis will provoke an interventional treatment. Nevertheless, evidence of inducible myocardial ischemia should be a fundamental prerequisite for revascularization in stable coronary artery disease [7]. It is important to realize that in stable coronary artery disease, asymptomatic patients with a significant mass of ischaemic myocardium have prognostic benefit from revascularization, while symptomatic patients with no or little evidence of ischaemia have not [17, 18]. Therefore, FFR-guided PCI is recommended for detection of ischaemia-related lesions when objective evidence of vessel-related ischaemia is not available [1, 4, 6]. As expected, the rate of stress imaging was lower in patients undergoing FFR measurement in our study. However, there was no significant difference in the rate of non-diagnostic stress imaging, and PCI was performed in a relevant number of patients without angina symptoms and non-diagnostic stress test without performance of FFR measurement. On the other hand, a relevant number of patients underwent FFR measurement despite pathologic results of noninvasive stress imaging. The latter finding underlines the important advantages in identification of distinct culprit lesions for myocardial ischemia using FFR.
Intracoronary pressure measurements in multivessel disease
The rate of FFR measurement was even lower in the subgroup of patients with three-vessel disease (2.5 %), and in particular very low in patients with a recommendation for CABG with FFR measurement in only 1.6 % of patients. This is most worrying, because it was known from the FAME trial that only 14 % of patients with angiographic three-vessel disease had functional three-vessel disease, while 43 % had two-vessel disease, 34 % suffered from single-vessel disease, and 9 % had no functionally relevant coronary artery disease at all [1]. Furthermore, it was demonstrated in patients with multivessel disease that PCI of hemodynamically nonsignificant stenoses as identified by an FFR >0.75 can be safely deferred [19], and PCI in those patients with one or two hemodynamically significant lesions yielded a similar favorable outcome as CABG in those patients with three or more culprit lesions despite a similar angiographic extent of disease [8]. Based on these data, the European guidelines recommended in case of multiple angiographically significant stenoses whose severity is difficult to assess, a liberal use of FFR measurement to decide on the treatment strategy [7]. Accordingly, use of FFR led to reclassification of the revascularization decision determined by angiography alone in about half of patients in the R3F study [2].
Interestingly, coronary artery disease was more frequently classified as functional significant using FFR (79.1 vs. 70.4 %, p < 0.001), while the extent of multivessel CAD was lower. This demonstrates that visual misinterpretation of angiography happens to both directions: over-and underestimation. When compared with angiography-guided interventions, PCI was performed more frequently in the LAD and less frequently in the RCA and CX in the FFR group. This finding may represent an operator dependent bias, which might be explained by the prognostic impact of LAD stenosis [20]. On the other hand, this finding might reflect differences in the results of FFR measurements in different coronary territories. Indeed, we recently published data showing systematic higher FFR values in posterior vessels (RCA and CX) when compared with anterior vessels (LAD). This would explain higher PCI rates in the LAD, and we hypothesize an influence of hydrostatic pressure on intracoronary pressure measurements as reason [21, 22]. Moreover, there is a complex interactive relationship of coronary flow and pressure [23], therefore differences in flow could be causal also. Right and left coronary systems have usually drastically different subtended myocardial mass [24], and therefore absolute maximal flow through each of the systems might be vastly different. According to Poiseuille’s and Bernoulli’s law, the magnitude of trans-stenotic pressure drop will increase with increasing coronary flow, and flow is the highest in the proximal LAD [25]. This aspect might explain discrepancies between the left and right coronary system also. Further research is necessary to investigate this phenomenon.
Procedure related aspects of intracoronary pressure measurements
Even though FFR measurement is an additional procedure with necessity of a venous femoral sheath in many cases [26], MACCE rate was slightly lower in the FFR group and there were no other differences of in-hospital complications between both groups, in particular there was no higher rate of bleeding or puncture site complications. For patients undergoing FFR measurement, the amount of contrast media was slightly higher (about 10 ml) in all subgroups, but there were no differences in the already low rate of acute renal failure. In patients without performance of PCI, the mean dose area product was higher in the FFR group. These findings are unsurprising since FFR measurement is an additive diagnostic procedure with necessity of guidewire positioning. Surprisingly, in patients undergoing ad hoc PCI, the mean dose area product was significantly lower in the FFR group. The reason for this phenomenon remains unclear. It might be speculated that the use of FFR measurement reduces the number of pre- and postinterventional angiograms. Even though it is difficult to quantify the absolute risk caused by radiation exposure, according to the linear no-threshold model this finding would implicate a relative increase of risk of long-term biological damage due to radiation of 11.3 % for the group of patients without PCI and a relative decrease of 9.1 % for patients with performance PCI [27, 28]. Furthermore, patients in the FFR group underwent less additional coronary angiographies in the same hospital stay.
Interestingly, patients undergoing PCI with FFR measurements had higher rates of post-interventional TIMI 3 flow. This is explained by the higher rate of TIMI 3 flow before PCI and the avoidance of FFR measurement in more complex coronary lesions, which have a higher risk of slow flow or no-reflow after PCI [29]. Indeed, our data showed a lower rate of AHA type >B1 lesions and a higher rate of TIMI 3 flow before PCI in the FFR group. On the other hand, positive effects of adenosine preconditioning in prevention of no-reflow have been shown for acute myocardial infarction [30, 31] and for PCI in venous bypass grafts [32]. It might be possible that adenosine administration for induction of hyperemia during FFR measurement is a kind of preconditioning with similar positive effects on coronary flow. However, this hypothesis remains speculative to date and needs further investigation. In this context, it will be interesting to see in the future if the instantaneous wave-free ratio (iFR), which is a new adenosine-independent index of coronary stenosis severity [22, 33], will show comparable results.
Limitations
The data presented reflect the use of intracoronary pressure measurement in a real-world setting. Due to the concept of the registry, the study was not randomized and no follow-up data after demission was available. Registry data did neither contain the results of intracoronary pressure measurements nor the measured vessel. Accordingly, we were unable to assess in patients with ad hoc PCI if FFR measurement was performed in the intervened or another vessel. Furthermore, the registry data contained neither data regarding the kind of stress test performed before coronary angiography nor data regarding the way of adenosine administration. In the absence of structured monitoring regarding information on the angiographic procedure, input data errors cannot be excluded.
Conclusion
Our analysis shows a relatively rare use of FFR measurement in daily practice, in particular, in patients with multivessel disease or recommendation for bypass surgery. There was a successive increase during the study period, but FFR measurement was used very heterogeneously across the participating hospitals. FFR measurement was not associated with an increased periprocedural complication rate.
References
Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van’t Veer M, Pijls NH (2010) Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 55:2816–2821
Van Belle E, Rioufol G, Pouillot C, Cuisset T, Bougrini K, Teiger E, Champagne S, Belle L, Barreau D, Hanssen M, Besnard C, Dauphin R, Dallongeville J, El Hahi Y, Sideris G, Bretelle C, Lhoest N, Barnay P, Leborgne L, Dupouy P (2014) Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation 129:173–185
Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, Siebert U (2010) Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 122:2545–2550
Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B (2007) Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 49:2105–2111
Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL (1993) Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 87:1354–1367
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360:213–224
Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D (2010) Guidelines on myocardial revascularization. Eur Heart J 31:2501–2555
Botman KJ, Pijls NH, Bech JW, Aarnoudse W, Peels K, van Straten B, Penn O, Michels HR, Bonnier H, Koolen JJ (2004) Percutaneous coronary intervention or bypass surgery in multivessel disease? A tailored approach based on coronary pressure measurement. Catheter Cardiovasc Interv 63:184–191
Vogt A, Bonzel T, Harmjanz D, von Leitner ER, Pfafferott C, Engel HJ, Niederer W, Schuster PR, Glunz HG, Neuhaus KL (1997) PTCA registry of German community hospitals. Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausarzte (ALKK) Study Group. Eur Heart J 18:1110–1114
Tebbe U, Hochadel M, Bramlage P, Kerber S, Hambrecht R, Grube E, Hauptmann KE, Gottwik M, Elsässer A, Glunz HG, Bonzel T, Carlsson J, Zeymer U, Zahn R, Senges J (2009) In-hospital outcomes after elective and non-elective percutaneous coronary interventions in hospitals with and without on-site cardiac surgery backup. Clin Res Cardiol 98:701–707
Zeymer U, Vogt A, Zahn R, Weber MA, Tebbe U, Gottwik M, Bonzel T, Senges J, Neuhaus KL (2004) Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI); Results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Eur Heart J 25:322–328
Zeymer U, Zahn R, Hochadel M, Bonzel T, Weber M, Gottwik M, Tebbe U, Senges J (2005) Incications and complications of invasive diagnostic procedures and percutaneous coronary interventions in the year 2003. Results of the quality control registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Z Kardiol 94:392–398
Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B (2010) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 56:177–184
Härle T, Zeymer U, Hochadel M, Schmidt K, Zahn R, Darius H, Behrens S, Lauer B, Mudra H, Schächinger V, Elsässer A (2015) Use and impact of thrombectomy in primary percutaneous coronary intervention for acute myocardial infarction with persistent ST-segment elevation: results of the prospective ALKK PCI-registry. Clin Res Cardiol 104:803–811
Härle T, Zeymer U, Schwarz AK, Luers C, Hochadel M, Darius H, Kasper W, Hauptmann KE, Andresen D, Elsässer A (2014) Use of drug-eluting stents in acute myocardial infarction with persistent ST-segment elevation: results of the ALKK PCI-registry. Clin Res Cardiol 103:373–380
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367:991–1001
Davies RF, Goldberg AD, Forman S, Pepine CJ, Knatterud GL, Geller N, Sopko G, Pratt C, Deanfield J, Conti CR (1997) Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation 95:2037–2043
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS (2003) Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 107:2900–2907
Berger A, Botman KJ, MacCarthy PA, Wijns W, Bartunek J, Heyndrickx GR, Pijls NH, De Bruyne B (2005) Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol 46:438–442
Nudi F, Schillaci O, Neri G, Pinto A, Procaccini E, Vetere M, Frati G, Tomai F, Biondi-Zoccai G (2016) Prognostic impact of location and extent of vessel-related ischemia at myocardial perfusion scintigraphy in patients with or at risk for coronary artery disease. J Nucl Cardiol 66:274–284
Härle T, Meyer S, Bojara W, Vahldiek F, Elsässer A (2016) Intracoronary pressure measurement differences between anterior and posterior coronary territories. Herz 1–8. doi:10.1007/s00059-016-4471-z
Härle T, Bojara W, Meyer S, Elsässer A (2015) Comparison of instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR)—first real world experience. Int J Cardiol 199:1–7
van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, Siebes M (2012) Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol 52:786–793
Leone AM, De Caterina AR, Basile E, Gardi A, Laezza D, Mazzari MA, Mongiardo R, Kharbanda R, Cuculi F, Porto I, Niccoli G, Burzotta F, Trani C, Banning AP, Rebuzzi AG, Crea F (2013) Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv 6:29–36
Ofili EO, Kern MJ, St Vrain JA, Donohue TJ, Bach R, al-Joundi B, Aguirre FV, Castello R, Labovitz AJ (1995) Differential characterization of blood flow, velocity, and vascular resistance between proximal and distal normal epicardial human coronary arteries: analysis by intracoronary Doppler spectral flow velocity. Am Heart J 130:37–46
Härle T, Meyer S, Vahldiek F, Elsässer A (2016) Differences between automatically detected and steady-state fractional flow reserve. Clin Res Cardiol 105:127–134
Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A (2009) Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 251:6–12
Seiffert M, Ojeda F, Mullerleile K, Zengin E, Sinning C, Waldeyer C, Lubos E, Schafer U, Sydow K, Blankenberg S, Westermann D (2015) Reducing radiation exposure during invasive coronary angiography and percutaneous coronary interventions implementing a simple four-step protocol. Clin Res Cardiol 104:500–506
Kodama T, Kondo T, Oida A, Fujimoto S, Narula J (2012) Computed tomographic angiography-verified plaque characteristics and slow-flow phenomenon during percutaneous coronary intervention. JACC Cardiovasc Interv 5:636–643
Assali AR, Sdringola S, Ghani M, Denkats AE, Yepes A, Hanna GP, Schroth G, Fujise K, Anderson HV, Smalling RW, Rosales OR (2000) Intracoronary adenosine administered during percutaneous intervention in acute myocardial infarction and reduction in the incidence of “no reflow” phenomenon. Catheter Cardiovasc Interv 51:27–31 (discussion 32)
Marzilli M, Orsini E, Marraccini P, Testa R (2000) Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 101:2154–2159
Kapoor N, Yalamanchili V, Siddiqui T, Raza S, Leesar MA (2014) Cardioprotective effect of high-dose intragraft adenosine infusion on microvascular function and prevention of no-reflow during saphenous vein grafts intervention. Catheter Cardiovasc Interv 83:1045–1054
Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R, Sethi A, Baker CS, Bellamy M, Al-Bustami M, Hackett D, Khan M, Lefroy D, Parker KH, Hughes AD, Francis DP, Di Mario C, Mayet J, Davies JE (2012) Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol 59:1392–1402
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Härle, T., Zeymer, U., Hochadel, M. et al. Real-world use of fractional flow reserve in Germany: results of the prospective ALKK coronary angiography and PCI registry. Clin Res Cardiol 106, 140–150 (2017). https://doi.org/10.1007/s00392-016-1034-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1034-5