Abstract
The utilization of FFR remains low. Our study evaluated the per-vessel prognostic value of computational pressure-flow dynamics-derived FFR (caFFR) among patients with stable coronary artery disease. A total of 3329 vessels from 1308 patients were included and analysed. They were stratified into ischaemic (caFFR ≤ 0.8) and non-ischaemic (caFFR > 0.8) cohorts, and the associations between PCI and outcomes were evaluated. The third cohort comprised all included vessels, and the associations between treatment adherent-to-caFFR (PCI in vessels with caFFR ≤ 0.8 and no PCI in vessels with caFFR > 0.8) and outcomes were evaluated. The primary outcome was VOCE, defined as a composite of vessel-related cardiovascular mortality, non-fatal myocardial infarction, and repeat revascularization. PCI was associated with a lower 3-year risk of VOCE in the ischaemic cohort (HR, 0.44; 95% CI, 0.26–0.74; P = 0.002) but not in the non-ischaemic cohort. The risk of VOCE was lower in the adherent-to-caFFR group (n = 2649) (HR, 0.69; 95% CI, 0.48–0.98; P = 0.039).
Graphical Abstract
A novel index that uses coronary angiography images to estimate FFR may have substantial clinical value in guiding management among patients with stable coronary artery disease.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The evaluation of coronary artery disease (CAD) for any inducible myocardial ischaemia can be performed by fractional flow reserve (FFR) during coronary angiography. The Fractional Flow Reserve vs Angiography for Multivessel Evaluation 2 (FAME 2) trial revealed that percutaneous coronary intervention (PCI) in ischaemic lesions, defined by FFR ≤ 0.8, improved clinical outcomes compared with medical therapy alone [1]. On the contrary, PCI has not been recommended for non-ischaemic FFR lesions (defined by FFR > 0.8) because it has not been shown to reduce adverse outcomes and may even be harmful [2, 3]. As a result, FFR has been incorporated in class IA international recommendations to guide decision-making during PCI [4]. A previous study further confirmed that the adoption of an FFR-guided revascularization strategy improved survival compared with an angiography-only revascularization strategy [5].
Of note, FFR has several limitations including the need to pass a pressure wire and hyperaemia induction. The advent of angiography-derived FFR, which circumvents these limitations, has therefore received considerable attention with several models correlating favourably with FFR [6,7,8]. A novel computational pressure-flow dynamics (CPFD)–derived FFR (caFFR) utilizes invasive aortic pressure coupled to computational fluid dynamics (CFD) at the time of angiography and has demonstrated a high degree of accuracy, sensitivity, and specificity compared with wire-based FFR [9, 10]. Recent results from the FAVOR III China trial showed improved 1-year clinical outcomes in CAD patients who underwent PCI guided by quantitative flow ratio (QFR), which utilizes CFD, compared with those who underwent PCI guided by angiography, suggesting a significant role for various angiography-derived FFR models [11]. Like all randomized trials, concerns exist when extrapolating results to routine clinical practice due to strict protocols and selective patient enrolment. The aim of our study was to determine whether the ischaemic status of a coronary vessel, derived by caFFR, could offer long-term prognostic information (up to 3 years) to help strategize revascularization decisions in patients with stable CAD in a routine clinical setting.
Methods
Study Population and Design
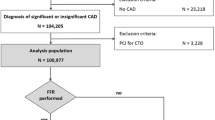
Patients aged ≥ 18 years with a clinical diagnosis of CAD who underwent coronary angiography at Queen Mary Hospital, Hong Kong between January 1, 2014, and December 31, 2016, were retrospectively included for caFFR analysis. Exclusion criteria included: acute coronary syndrome (ACS) or TIMI flow grade below 3 in at least 1 vessel, congenital heart disease, prior coronary artery bypass grafting (CABG) or CABG as preferred treatment, severe valvular heart disease, angiographically significant left main (LM) disease (> 70% diameter stenosis) or prior LM-PCI, and no lesions with ≥ 30% diameter stenosis in any vessel (Fig. 1). Angiographic data of patients who fulfilled the inclusion and exclusion criteria were retrieved and caFFR analysis was performed. Vessel-level exclusion criteria included the presence of chronic total occlusion, right coronary artery (RCA) aorto-ostial lesion ≤ 3 mm from the aorta, left anterior descending artery (LAD), and left circumflex artery (LCx) ostial lesions, true bifurcation lesions (medina 1,0,1; 0,1,1 or 1,1,1) and non-measurable caFFR (Fig. 1) [12]. Baseline demographics and prescribed medication were retrieved from the inter-hospital electronic system. The scoring system of quantitative coronary angiography in the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) study was calculated by three independent operators (CKLL, LYL, KYL) to characterize the severity of CAD. This study was approved by the ethics committee of the West Cluster Hospital Authority of Hong Kong (UW 19–575).
Study design. Study flowchart with details of the cohorts. Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; caFFR, computational pressure-flow dynamics-derived fractional flow reserve; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LM, left main coronary artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction
Measurement of caFFR
The principles and procedures of caFFR analysis have been described previously [9]. Technical requirements for caFFR analysis include [1] contrast opacification of the entire vessel using standard manual force, without table movement during injection of contrast; and 2) ≥ 2 coronary angiograms with projections separated by ≥ 30°.Coronary angiograms were recorded at a standard frame number count of 15 frames per second. Along with mean aortic pressure, a simulated three-dimensional mesh reconstruction of the coronary artery of interest was generated and the analysis was completed using CPFD methods. In our study, caFFR was generated for the main branch of all three major coronary arteries in the patients undergoing caFFR analysis using FLASH software (Fig. 1). The 3D mesh reconstruction was done from the inlet of the vessel until the most distal point (at least 10 mm downstream of the most distal stenosis). In case the distal segment of the vessel had more than 2 daughter branches (e.g. the posterolateral branch and posterior descending artery in RCA), the branch with a larger diameter was considered part of the main vessel. The mean aortic pressure data required for caFFR analysis was computed based on arterial tracing records of coronary angiography sessions. Supplemental Fig. 1 illustrates an example of caFFR analysis. All caFFR analyses were performed by an independent investigator (YF), who was blinded to patient data and outcomes.
Despite a paucity of data regarding the optimal cut-off value of caFFR to predict adverse events, caFFR has been proven to be closely correlated with FFR [9]. In our study, caFFR was considered a surrogate of FFR and a threshold of 0.80 was adopted as the cut-off value for caFFR measurements. Subsequently, two separate cohorts of vessels were created for analysis: vessels with caFFR ≤ 0.80 formed the ischaemic cohort (cohort 1), and vessels with caFFR > 0.80 the non-ischaemic cohort (cohort 2). In cohort 1, vessels were assigned to ischaemic PCI + or ischaemic PCI- group based on whether PCI was performed on any lesion. Similarly, in cohort 2, vessels were assigned to non-ischaemic PCI + or non-ischaemic PCI- group accordingly.
To further assess the association of treatment adherence to the caFFR threshold with clinical outcomes, cohort 3 was formed to include all vessels from cohorts 1 and 2. The adherent-to-caFFR group (hereinafter called the adherent group) included the ischaemic PCI + and non-ischaemic PCI- groups. The term ‘adherent-to-caFFR’ refers to the treatment of the vessel being adherent to its caFFR value, that is vessels with caFFR ≤ 0.8 being treated with PCI and those with caFFR > 0.8 not. Correspondingly, the group non-adherent-to-caFFR (hereinafter called the non-adherent group) included the ischaemic PCI- group and the non-ischaemic PCI + group (Fig. 1).
Endpoints and Follow-Up
The primary endpoint was the vessel-oriented composite endpoint (VOCE) at 3 years, defined as a composite of vessel-related cardiovascular mortality, vessel-related non-fatal myocardial infarction (MI), and any repeat revascularization [13,14,15]. Secondary endpoints included individual components of the primary endpoint. All deaths were considered cardiovascular mortality unless a non-cardiovascular cause was indisputable. In cases of single index vessel stenosis, cardiovascular mortality was designated as index vessel related. In cases of stenoses in multiple index vessels, cardiovascular mortality was assigned to each stenotic index vessel [15]. In cases where only side branch(es) had stenoses but not in any main vessels and an outcome event occurred, the caFFR was re-measured in the side branch(es) by reconstruction from its ostium to its most distal part, and during data analysis, it would replace the main vessel where it originated from. Endpoint events would be considered related to the relevant side branch(es). The fourth universal definition of MI was used as the definition of MI, based on a significant increase in cardiac troponin, symptoms, new electrocardiographic changes, and imaging findings of myocardium viability loss [16]. Electrocardiograms and coronary angiograms performed during the episode of MI were reviewed to designate the event as vessel-related or non-vessel-related. Any repeat revascularization was defined as subsequent PCI or CABG to the vessel that occurred after index admission. All clinical outcomes were ascertained from the inter-hospital centralized electronic medical records that contain detailed clinical data of patients, including clinical notes, procedural records, and radiological and laboratory investigation results. These clinical data were used for adjudication of endpoints and the follow-up rate was complete in our cohort of patients.
Statistical Analysis
The primary purpose of data analysis was to evaluate the 3-year probability of VOCE in the three cohorts. Categorical variables were expressed as proportions and compared using the chi-square test or Fisher exact test. Continuous variables were expressed as mean ± standard deviation.
Survival curves were constructed using Kaplan–Meier estimates and differences between groups were tested using the log-rank test [17]. Cox proportional hazards models were used to evaluate the association of PCI + vs. PCI- group (cohort 1 and cohort 2) and adherence-to-caFFR (cohort 3) with the risk of VOCE [18]. The following parameters were entered into the model for univariate analysis: age, gender, Charlson comorbidity index (CCI), hypertension, diabetes mellitus, hyperlipidaemia, ever-smoker, prior PCI, SYNTAX score, presence of multivessel disease and PCI (cohort 1 and cohort 2) or adherence-to-caFFR (cohort 3). Parameters with P < 0.1 upon univariate analysis were entered into multivariable Cox models. To account for a possible significant interaction between vessels, generalized estimating equations (GEE) were used to further evaluate the predictive value of clinical parameters and PCI (ischaemic and non-ischaemic cohorts) or adherence-to-caFFR (adherent-to-caFFR cohort) for VOCE, with adjustment for within-patient vessel non-independence [19, 20]. The proportionality assumption was verified by a non-significant interaction between exposure variables and time. A robust variance estimator was used to estimate the standard error for all regression models [21]. Effect estimates from Cox proportional hazards models were reported as hazard ratios (HRs) with the associated 95% confidence interval (CIs). A two-sided P value < 0.05 indicated statistical significance. All statistical analyses were performed with R (v.4.0.4) and SPSS (v.26.0).
Patient and Public Involvement
Patients and the public were not involved in the design, conduct, outcomes, or dissemination of this study.
Results
Baseline Characteristics
A total of 1367 patients were eligible for caFFR analysis. Among all vessels analysed, 772 (18.8%) were excluded based on vessel-level exclusion criteria. Ultimately, 3329 vessels from 1308 patients were included for data analysis (Fig. 1). There was no side branch included among the 3329 vessels. The median follow-up duration was 1693 days (interquartile range: 1583 to 1897 days). Baseline characteristics at both the patient-level and the vessel-level are listed in Table 1 and Supplemental Table 1. Vessel-level baseline characteristics within each cohort are listed in Table 2, and noteworthily, vessels treated without adherence to caFFR were more likely left anterior descending arteries and have lesions with ≥ 70% diameter stenosis. Table 3 shows the stent characteristics of the vessels with PCI done. Almost half of the vessels were the LAD, and drug-eluting stents were used in most cases (92.1%). The endpoint event rates at 3 years are shown in Table 4. The detailed distribution of caFFR measurements is shown in Supplemental Table 2.
Clinical Outcomes
Ischaemic Cohort (Cohort 1)
The ischaemic cohort comprised 926 vessels, with 708 treated with PCI and 218 not treated with PCI. The 3-year rate of VOCE was lower in the PCI + group than in the PCI- group (4.8% vs. 11.5%, P < 0.001) (Table 4, Fig. 2). After multivariable adjustment, PCI was associated with a 56% lower risk of VOCE than no PCI (HR, 0.44; 95% CI, 0.26–0.74; P = 0.002) (Table 5). The GEE analysis further showed that PCI was an independent negative predictor of VOCE, whereas the male gender was an independent predictor of VOCE (Table 6). For secondary endpoints, PCI was associated with a lower rate of vessel-related cardiovascular mortality, vessel-related non-fatal MI, and any repeat revascularization (Supplemental Table 3, Supplemental Figs. 2–4).
Non-Ischaemic Cohort (Cohort 2)
The non-ischaemic cohort comprised 2403 vessels, with 462 receiving PCI and 1941 not receiving PCI. The 3-year rate of VOCE was similar in the PCI + and PCI- groups (3.9% vs. 3.8%, P = 0.891) (Table 4, Supplemental Fig. 5). In univariate analysis, PCI was not associated with a significant change in risk of VOCE (HR, 1.04; 95% CI, 0.62–1.79; P = 0.888) (Table 5). The GEE analysis showed CCI ≥ 3 and ever-smoker status were independent predictors of VOCE (Table 6). There were no associations between PCI and all three secondary endpoints (Table 4, Supplemental Table 4, Supplemental Figs. 6–8).
Adherent Group vs. Non-Adherent Group (Cohort 3)
The adherent-to-caFFR cohort comprised 3329 vessels, with 2649 receiving treatment adherent-to-caFFR and 680 receiving treatment non-adherent-to-caFFR. The 3-year rate of VOCE was lower in the adherent group than in the non-adherent group (4.0% vs. 6.3%, P = 0.010) (Table 4, Fig. 3). After multivariable adjustment, treatment adherent-to-caFFR was associated with a 31% reduction in VOCE compared with the non-adherent group (HR, 0.69; 95% CI, 0.48–0.98; P = 0.039) (Table 5). The GEE analysis further confirmed that treatment adherent-to-caFFR was an independent negative predictor of VOCE, while male gender, Charlson comorbidity index ≥ 3, and ever-smoker status were independent predictors (Table 6). In the analysis of secondary outcomes, the reduction in VOCE was mainly driven by a reduction in vessel-related non-fatal MI, but not vessel-related cardiovascular mortality or any repeat revascularization (Table 4, Supplemental Table 5, Supplemental Figs. 9–11).
Adherent-to-caFFR Cohort: Cumulative Occurrence of VOCE. In the adherent-to-caFFR cohort, a significantly higher cumulative occurrence of VOCE was observed in vessels that were treated without adherence to caFFR treatment threshold. Abbreviations: caFFR, computational pressure-flow dynamics-derived fractional flow reserve; VOCE, vessel-oriented composite endpoint
Discussion
In the present study, we characterized coronary artery ischaemic status by caFFR, an angiography-derived index, in a large population of patients with stable CAD. Our results showed that among ischaemic lesions (caFFR ≤ 0.8) (cohort 1), PCI was associated with a lower incidence of VOCE at 3 years, compared with those without PCI. Conversely, in vessels with non-ischaemic lesions (caFFR > 0.8) (cohort 2), no reduction of VOCE was observed in those that underwent PCI compared with no PCI. Finally, using a caFFR threshold of 0.8, PCI performed only in vessels with caFFR-defined ischaemia had a significantly lower risk of VOCE than those that did not adhere to their ischaemic status (cohort 3). The present study, conducted in a routine clinical setting, demonstrates favourable long-term clinical performance (up to 3 years) for adherence to non-wire-based angiography-derived functional assessment in patients with stable CAD lesions.
Although recommended as a class IA indication, wire-based FFR is underutilized in clinical practice, with a substantial proportion (> 80%) of patients with intermediate obstructive coronary lesions not undergoing FFR assessment [22]. As a result, coronary angiography-derived FFR has gained considerable attention, as it precludes the need for wire manipulation and hyperaemia stimulus essential in wire-based FFR, and avoids wire-related technical inadequacies, potentially overcoming certain limitations that may have impacted the utilization of FFR [23,24,25].
Importantly, these non-wire-based angiography-derived measurements have been shown to have comparable diagnostic accuracy to conventional FFR [26, 27]. For instance, QFR had a diagnostic accuracy of over 90% compared with wire-based FFR [6], while FFRangio, another coronary angiography-based technology, was validated to have 92% accuracy against wire-based FFR [7]. CPFD-derived caFFR [9], utilized in the present study, employs invasive aortic pressure coupled to computational flow modelling, eliminating the convective and diffusive energy losses associated with the lumped model used by FFRangio and QFR. The diagnostic performance of caFFR is excellent with 96% accuracy compared with wire-based FFR, and its clinical utility is further strengthened by its short operating time with a total operation time below 5 min and computational time below 1 min. Consequently, these advantages point towards the potential widespread adoption of caFFR to guide PCI and utilization of functional assessment in routine clinical settings.
Clinically, the incorporation of angiography-derived FFR measurements into PCI strategies may improve patient outcomes. The recent FAVOR III China trial confirmed that a QFR-guided PCI strategy, compared with a standard angiography-guided PCI strategy, resulted in improved one-year outcomes among patients with CAD [11]. Our data, derived from patients in the routine clinical setting and with extended follow-up, provide additional unique information that supports the adoption of angiography-derived FFR. The current results confirm that PCI was associated with significantly fewer VOCE in vessels with ischaemic lesions, defined by a caFFR ≤ 0.8, while no association between PCI and clinical outcomes was seen in vessels with non-ischaemic lesions, defined by a caFFR > 0.8. Furthermore, we demonstrated that adherence to caFFR-guided PCI was associated with a 31% reduction in VOCE, compared with those that were non-adherent to caFFR. Our findings corroborate those of the IRIS-FFR registry, which demonstrated that major adverse cardiac events (MACE) were similar between deferred and revascularized lesions with FFR ≥ 0.76, but a significant benefit of PCI was observed only in those with FFR < 0.75. The neutral effect of PCI in non-ischaemic lesions was likewise observed in the DEFER study. Conversely, a population-based study demonstrated that PCI in non-ischaemic lesions, compared with no PCI, was associated with a higher MACE [3]. The discrepant observation in their study may have been due to the adoption of a single vessel FFR assessment, unlike our study which included a multivessel assessment. Irrespective of these differences, supported by the current class III recommendation [4], revascularization in non-ischaemic lesions, either by wire-based FFR or angiography-derived FFR, does not provide clinical benefit and should be avoided.
In our study, where revascularization strategies were angiography-guided, the frequency of PCI in non-ischemic vessels (defined by caFFR > 0.8) was 19.2%. Observations in other FFR-based studies have demonstrated that physicians tend to opt for PCI when the decision is clinically driven, rather than FFR guided, with the frequencies of PCI in non-ischaemic vessels ranging from 4 to 13% [3, 28, 29]. Perhaps due to a low FFR utilization rate, it is possible that PCI in non-ischaemic lesions (unnecessary coronary stenting) is not uncommon in clinical practice. The FAVOR 3 trial demonstrated that 445 (23.3%) patients had an initial angiography-based revascularization plan that differed from the eventual QFR-guided revascularization strategy [11]. Similarly, 680 (20.4%) vessels in our study that were treated according to angiographic considerations were nonadherent-to-caFFR. This highlights the importance of physiological testing to identify any mismatch between angiographic severity and haemodynamic consequences of coronary artery lesions. Without the need for pressure wire introduction and hyperaemic stimulation, caFFR may also encourage functional assessment of multivessel disease. These compelling advantages of angiographic-based FFR assessment may further improve adherence to evidence-based strategies and improve the clinical outcome of patients with stable CAD.
Clinical Implications
Current guidelines recommend FFR-guided PCI in the management of patients with stable CAD, but its adoption has been exceedingly low due to a myriad of limitations. Non-invasive FFR assessment by computed tomography (FFRCT) has been validated and shows considerable promise for patients with CAD [30]. Nonetheless, it requires patient data transfer offsite to be post-processed, introducing a delay of approximately 24 h. Furthermore, raw data acquired by CT may be of insufficient quality due to artefacts and raises the possibility of high rejection rates. The use of caFFR provides instant ischaemic assessment and usually requires less than 10 min with the advantage of re-evaluation by additional angiography images if the calculation is considered inadequate. Importantly, our study demonstrated that adherence to caFFR-guided PCI (as a surrogate of FFR) reduced the risk of adverse outcomes, without the necessity for wire introduction or hyperaemia induction. Furthermore, offline calculation of caFFR was performed by an investigator blinded to patient clinical characteristics and outcomes. We were also able to comprehensively evaluate the outcomes at 3 years, providing unique long-term prognostic information offered by caFFR. Our findings will be further supported by an ongoing nationwide prospective randomized study to compare the clinical outcome of caFFR vs. wire-based FFR strategy.
Study Limitations
Several limitations should be considered in the present study. First, like most retrospective observational studies, selection bias and confounding factors may have existed, although minimised by our large study population relative to other studies that evaluated coronary physiological indices at a per-vessel level. Future prospective trials will further provide robust clinical data to validate the use of caFFR. Second, the present study included patients with single-vessel and multi-vessel disease compared with a recent study that included only single-vessel FFR assessment and intervention. (3) Nevertheless, we evaluated the use of caFFR at the per-vessel level, which, alongside its ease in multi-vessel assessment, provides generalizable results to patients with multi-vessel disease. Third, the data regarding the reasons to or not to perform PCI, such as the use of other assessment modalities (e.g. intravascular imaging) and chest pain severity, were unknown or inadequate. In our study, 277 (29.9%) vessels with ≥ 70% diameter stenosis in the ischaemic cohort did not receive PCI, whereas 1222 (50.9%) vessels in the non-ischaemic cohort received PCI despite not having ≥ 70% diameter stenosis. This was likely to be driven by multiple factors including the interventionists’ clinical judgement and the patient’s comorbidities, such that the exact decision for revascularization may not be fully illustrated due to the retrospective nature of our study. Fourth, data on periprocedural MI was unavailable from the inter-hospital electronic medical records, leading to possibly lowered event rates and our limited ability to perform multivariable adjustments for study endpoints. Nonetheless, our study focused on evaluating the long-term prognostic value of caFFR and showed significant results at a 3-year follow-up. Fifth, post-PCI caFFR data was not included in this study and there is a lack of quantification of improvement in vessel ischemia. However, the primary aim of this study was to evaluate the prognostic role of treatment adherence to caFFR, which refers to suitable use of PCI based on caFFR, and the benefit of assessing post-PCI residual ischemic burden was not the main focus of this study. Future trials focusing on the clinical value of post-PCI caFFR will be beneficial to further expand the data supporting the clinical use of caFFR in various settings. Sixth, caFFR was treated as a surrogate of FFR and an optimal cutoff value of caFFR per se was not evaluated. However, with a high diagnostic accuracy of caFFR compared with FFR, we believe the ischaemic and non-ischaemic cohorts were correctly identified. Future multicentre prospective trials are warranted and will be in a better position to define the optimal cutoff value of caFFR. Finally, caFFR could not be ascertained in a number of vessels due to several reasons (Supplemental Table 6). Most of them were due to the inability to fulfilling the technical criteria of caFFR analysis, namely inadequate angiographic angulation, unsuitable angiographic projection, and poor angiogram quality. Furthermore, angiographic data of some vessels were missing owing to the incomplete storage of images in the retrospective context of this study. To optimize the use of caFFR in the future, standardized protocols of angiographic image acquisition are needed for maximizing the success rate of caFFR analysis.
Conclusion
In this retrospective analysis of patients with stable CAD, angiography-derived caFFR provided valuable long-term prognostic information, and in the future may assist decision-making about revascularization in clinical practice. The prognostic information provided by caFFR, which precludes pressure wire introduction and hyperaemia induction, has the potential for widespread clinical application.
Data Availability
Data are available on reasonable request.
Abbreviations
- caFFR:
-
Computational pressure-flow dynamics-derived fractional flow reserve
- CFD:
-
Computational fluid dynamics
- CI:
-
Confidence interval
- CPFD:
-
Computational pressure-flow dynamics
- FFR:
-
Fractional flow reserve
- GEE:
-
Generalized estimating equations
- HR:
-
Hazard ratio
- VOCE:
-
Vessel-oriented composite endpoint
References
De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, et al. Fractional flow reserve–guided pci versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001.
Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van `t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24.
Sud M, Han L, Koh M, Austin PC, Farkouh ME, Ly HQ, et al. Association between adherence to fractional flow reserve treatment thresholds and major adverse cardiac events in patients with coronary artery disease. JAMA. 2020;324(23):2406–14. https://doi.org/10.1001/jama.2020.22708
Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–41.
Li J, Elrashidi MY, Flammer AJ, Lennon RJ, Bell MR, Holmes DR, et al. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375–83.
Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70:3077–87.
Fearon WF, Achenbach S, Engstrom T, Assali A, Shlofmitz R, Jeremias A, et al. Accuracy of fractional flow reserve derived from coronary angiography. Circulation. 2019;139(4):477–84.
Masdjedi K, van Zandvoort LJC, Balbi MM, Gijsen FJH, Ligthart JMR, Rutten MCM, et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve: the FAST study. EuroIntervention. 2020;16:591–9.
Li J, Gong Y, Wang W, Yang Q, Liu B, Lu Y, et al. Accuracy of computational pressure-fluid dynamics applied to coronary angiography to derive fractional flow reserve: FLASH FFR. Cardiovasc Res. 2020;116:1349–56.
Ai H, Zheng N, Li L, Yang G, Li H, Tang G, et al. Agreement of angiography-derived and wire-based fractional flow reserves in percutaneous coronary intervention. Front Cardiovasc Med. 2021;8:654392.
Xu B, Tu S, Song L, Jin Z, Yu B, Fu G, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398(10317):2149–59.
Chen X, Zhang D, Yin D, Li J, Zhao Z, Wang H, et al. Can “true bifurcation lesion” actually be regarded as an independent risk factor of acute side branch occlusion after main vessel stenting?: a retrospective analysis of 1,200 consecutive bifurcation lesions in a single center. Catheter Cardiovasc Interv. 2016;87(Suppl 1):554–63.
Biscaglia S, Tebaldi M, Brugaletta S, Cerrato E, Erriquez A, Passarini G, et al. Prognostic value of QFR measured immediately after successful stent implantation: the international multicenter prospective HAWKEYE study. JACC Cardiovasc Interv. 2019;12(20):2079–88.
Kogame N, Takahashi K, Tomaniak M, Chichareon P, Modolo R, Chang CC, et al. Clinical implication of quantitative flow ratio after percutaneous coronary intervention for 3-vessel disease. JACC Cardiovasc Interv. 2019;12(20):2064–75.
Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ: Cardiovasc Interv. 2017;10(8):e005233.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2018;40(3):237–69.
Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–110.
Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodological). 1972;34(2):187–220.
Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30.
Hanley JA, Negassa A, Edwardes MDD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–75.
Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–49.
Parikh RV, Liu G, Plomondon ME, Sehested TSG, Hlatky MA, Waldo SW, et al. Utilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J Am Coll Cardiol. 2020;75:409–19.
Tu S, Barbato E, Köszegi Z, Yang J, Sun Z, Holm NR, et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv. 2014;7:768–77.
Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani S, Bourantas CV, et al. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire - fractional flow reserve. EuroIntervention. 2014;10:574–83.
Tröbs M, Achenbach S, Röther J, Redel T, Scheuering M, Winneberger D, et al. Comparison of fractional flow reserve based on computational fluid dynamics modeling using coronary angiographic vessel morphology versus invasively measured fractional flow reserve. Am J Cardiol. 2016;117:29–35.
Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv. 2019;9:2024–35.
Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B, Kornowski R, et al. Diagnostic performance of angiography-derived fractional flow reserve: a systematic review and Bayesian meta-analysis. Eur Heart J. 2018;39(35):3314–21.
Ahn J-M, Park D-W, Shin E-S, Koo B-K, Nam C-W, Doh J-H, et al. Fractional flow reserve and cardiac events in coronary artery disease. Circulation. 2017;135(23):2241–51.
Belle EV, Rioufol G, Pouillot C, Cuisset T, Bougrini K, Teiger E, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography. Circulation. 2014;129(2):173–85.
Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–97.
Acknowledgements
We thank the medical staff of the Division of Cardiology, Queen Mary Hospital for their support during this study.
Funding
This work was supported by HKU-SZH Fund for Shenzhen Key Medical Discipline, Grant Number: SZXK2020081, and Sanming Project of Cardiology, the university of Hong Kong Shenzhen hospital, Sanming Grant from the Ministry of Health, Shenzhen, China, Grant Number: SZSM201911020.
Author information
Authors and Affiliations
Contributions
Study concept and design: CKLL, LYL, KYL, KHY. Data analysis and interpretation: CKLL, LYL, KYL, YF, SYY. Manuscript drafting: CKLL, LYL, KYL, KHY. Critical revision of the manuscript and intellectual input: CKLL, GC, MW, RW, MZW, QWR, SYY, YKT, HLL, HFT, BX. Guarantor: KHY.
Corresponding author
Ethics declarations
Human Subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. This study was approved by the ethics committee of the West Cluster Hospital Authority of Hong Kong. The need for informed consent was waived.
Animal Studies
No animal studies were carried out by the authors for this article.
Conflict of Interest
KHY receives research support from Novartis; consultancy fees from service on the Advisory Board/Steering Committee for Abbott Diagnostics, Bayer, Boehringer Ingelheim, Boston Scientific, Medtronic, and Novartis. All other authors report no conflict of interest.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical Relevance
This study, to the best of our knowledge, is the first to demonstrate a favourable clinical performance in patients with stable CAD by adhering to values derived by a non-wire-based per-vessel angiography-derived functional assessment. These findings support the use of caFFR and its potential for widespread application in clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leung, C.KL., Lam, LY., Li, KY. et al. Clinical Value of Computational Angiography-derived Fractional Flow Reserve in Stable Coronary Artery Disease. J. of Cardiovasc. Trans. Res. 16, 1166–1176 (2023). https://doi.org/10.1007/s12265-023-10381-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10381-x