Abstract
Introduction
Despite the oncologic safety of laparoscopic surgery in colon cancer management, laparoscopy is not regarded as a standard treatment for T4 colon cancer. The aim of this study was to investigate the short-term and long-term oncologic outcomes of laparoscopic surgery in patients with locally advanced colon cancer.
Material and method
From March 2003 to June 2013, a total of 109 consecutive patients with proven pathologic T4 colon cancer were enrolled. These patients were divided into the laparoscopy group (LG, n = 52) and the open group (OG, n = 57). Perioperative and long-term oncologic outcomes were compared between the two groups.
Results
In the LG, open conversion occurred in four patients (7.6%). Combined resection was less commonly performed in the LG (13.5%) than in the OG (36.8%, P = 0.005). Operation time was similar between the two groups. In the LG, blood loss (129 mL vs. 437 mL, P < 0.001) and overall complication rate (13.5 vs. 36.8%, P = 0.005) were lower and length of hospital stay was shorter (median 7 vs. 17 days, P < 0.001) than in the OG. The 5-year overall survival rate was 60.7% for the LG and 61.9% for the OG (P = 0.817). Local recurrence-free survival did not differ between the groups (88.9% in LG vs. 88.1% in OG, P = 0.725).
Conclusion
Considering the benefits of early recovery and similar oncologic outcomes, laparoscopic surgery in T4 colon cancer could be a viable option in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is a common disease in Western countries. Recent national data for South Korea showed an increased rate of colorectal cancer, making it the third most common tumor [1].
Although a colonoscopy performed as part of routine examination may detect colorectal cancer in an early subclinical stage, locally advanced colorectal cancer, i.e., tumor invading adjacent organs, was detected in approximately 7–13% of all colorectal cancer patients [2–4]. Multivisceral en bloc resection could increase the chance of cure in the case of locally advanced colorectal cancer [5, 6]. However, management of locally advanced colorectal cancer is associated with relatively high morbidity and mortality rates [7].
Laparoscopy is widely used for colorectal cancer treatment. The benefits of early postoperative recovery and similar long-term oncologic outcomes in comparison to open surgery are the main reasons for the adoption of laparoscopic surgery in colon cancer [8, 9]. However, the efficacy of laparoscopy in T4 colon cancer remains unclear; therefore, the consensus meeting of the European Association of Endoscopic Surgery (EAES) did not recommend laparoscopic surgery in colon cancer with suspected invasion of the abdominal wall or adjacent structures [10]. To date, several studies have compared laparoscopy and open surgery in the context of short-term and long-term oncologic outcomes for T4 colorectal cancers [11–16]. However, these studies showed diverse characteristics of different inclusion criteria (colon cancer only vs. colon and rectal cancer), insufficient patient numbers, mixed hand-assisted surgery and laparoscopic surgery as minimally invasive surgeries, and relatively short follow-up periods. Although high probabilities of open conversion and possible suboptimal oncologic outcomes are the main reasons for hesitation to perform laparoscopic surgery for T4 colon cancer, the true impact of laparoscopy remains unclear.
Therefore, the aim of this study was to investigate the impact of laparoscopic surgery in patients with pathologically proven T4 colon cancer.
Materials and methods
Eligibility
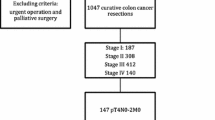
The records of all patients who underwent surgical resection for colorectal cancer in Gangnam Severance Hospital, Yonsei University College of Medicine, have been entered into a prospectively maintained database. From March 2003 to June 2013, 1025 patients underwent surgical resection for colon cancer at our hospital. Pathologic T4 colon cancer was diagnosed in 163 patients. Among them, 47 patients who were stage IV at the initial evaluation, 1 patient who was diagnosed as a hereditary non-polyposis colorectal cancer, and 6 patients who underwent a robotic surgery, were excluded from this study (Fig. 1). Patients who met the inclusion criteria were divided into the laparoscopy group (LG) and the open group (OG) based on the initial surgical approach. Converted patients were included in the LG based on the intention-to-treat analysis. Hand-assisted surgery has never been performed at our institution. This study was approved by the Institutional Review Board of our hospital.
Surgery and perioperative outcomes
All patients in the LG and the OG underwent a standardized technique of bowel resection. The operation included segmental resection of the primary tumor, radical lymphadenectomy, and combined resection of invaded adjacent organs, if necessary. Perioperative outcomes included operation time (min), blood loss (mL), conversion to an open procedure, morbidity, mortality, time to resuming soft diet, length of hospital stay, number of retrieved lymph nodes, and pathologic TNM staging. Complications were suspected based on the patient’s symptoms or signs. If we tried to confirm our clinical suspicion, laboratory and/or radiologic test were added. Surgical site infection was composed of superficial incisional, deep incisional and organ or space infection defined by Centers for Disease Control and Prevention. Ileus/obstruction was defined as any condition involving abdominal pain or distension, which could be verified by a simple X-ray. Voiding difficulty was defined as urinary retention or urinary incontinence requiring reinsertion of a foley catheter or adding urological medications. Conversion was defined as any case that could not be completed with the intended laparoscopic surgical approach and required more abdominal incision than expected for specimen removal. All tumor staging was re-evaluated and T4a and T4b patients were classified according to the definition of 7th TNM staging [17].
Definition of recurrence
The patterns of local recurrence (LR) confirmed by clinical, radiologic, or pathologic evidence were categorized into four groups (perianastomotic, mesentery/nodal, retroperitoneum, or peritoneum) on the basis of previous studies [18, 19]. Systemic recurrence (SR) was defined as all recurrences other than LRs. Combined recurrence was defined as simultaneous occurrence of LR and SR. When we estimated overall local recurrence rate, combined recurrence was not included in the category of LR. An imaging-guided biopsy was not routinely performed for histologic confirmation.
Postoperative adjuvant chemotherapy and follow-up
FOLFOX (folinic acid, 5-fluorouracil [5FU], and oxaliplatin) has been the most commonly used adjuvant chemotherapy regimen for patients with stage III colon cancer. Patients with stage II colon cancer in this study were potential candidates for adjuvant chemotherapy because all enrolled patients were considered high risk (T4 tumors). Some patients received 5FU-based chemotherapy because use of oxaliplatin was limited by coverage of national insurance reimbursement during the earlier periods of this study. In addition, some patients refused additional chemotherapy for various reasons.
All of the patients who underwent surgery visited our hospital every 3 months for 3 years. Follow-up visits were then reduced to every 6 months until 5 years and annually thereafter. Serum carcinoembryonic antigen (CEA) level was measured at each follow-up visit. Abdominopelvic computed tomography (CT) scans were performed with an average interval of 6 months. A colonoscopy, chest CT, or 18-FDG PET scan was performed as indicated according to the surgeon’s direction. Patient follow-up lasted until the cutoff date (March 2016) or death of the patient. The median follow-up period was 45 months (range, 2–155 months) in OG and 41 months (range, 1–108 months) in LG.
Statistical analysis
All statistical analyses were performed using IBM SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Differences in clinicopathologic features between the LG and the OG were analyzed using the chi-square test or Fisher’s exact test for categorical variables and with Student’s t test or Mann–Whitney U test for continuous variables. Overall survival (OS) was defined as the time from the date of operation to the date of death or last follow-up. Disease-free survival (DFS) was defined as the time from the date of operation to the date of tumor recurrence or last follow-up. Local recurrence-free survival (LRFS) was defined as the time from the date of operation to the date of local recurrence or last follow-up. The Kaplan–Meier method was used for survival analysis. The log-rank test was used to compare survival outcome between the two groups. A P value <0.05 was considered to indicate significance.
Results
Of the 109 patients who met the inclusion criteria and were finally enrolled, 52 patients were allocated to the laparoscopy group (LG) and 57 patients were categorized in the open group (OG). Four patients who were converted from laparoscopic surgery to open surgery were included in the LG on the basis of “intention-to-treat.”
There was no difference in gender, age, body mass index (BMI), American Society of Anesthesiologists (ASA) grade, tumor location, prior abdominal surgery history, and rate of emergency operation between the two groups. Operation types were as follows: right hemicolectomy/extended right hemicolectomy (RHC) (38.5%), left hemicolectomy (LHC) (7.7%), anterior resection/low anterior resection (AR/LAR) (50%), total/subtotal colectomy (1.9%), and Hartmann procedure (1.9%) in the LG; RHC (35.1%), LHC (14%), AR/LAR (35.1%), total/subtotal colectomy (12.3%), and Hartmann procedure (3.5%) in the OG. Palliative surgery was performed in one patient (1.9%) in the LG and two patients (3.4%) in the OG (Table 1).
Combined resection was less commonly performed in the LG (13.5%) than in the OG (36.8%) (P = 0.005). Bladder and small bowel were the most commonly resected organs. Open conversion occurred in four patients (7.7%). The reasons for conversion were anatomical uncertainty due to severe adhesions (n = 2), bladder invasion (n = 1), and small bowel invasion (n = 1). There was no difference in operation time between the two groups (249.1 ± 71.5 min in LG vs. 252.9 ± 101.8 min in OG, P = 0.821). When the combined resection group and no combined resection group were analyzed separately, the operation time was not different between the LG and OG.
Blood loss was significantly lower in the LG than in the OG (129.2 ± 196.7 mL in LG vs. 437.1 ± 494.1 mL in OG, P < 0.001). Overall complication rate was significantly lower in the LG than the OG (13.5% in LG vs. 36.8% in OG, P = 0.005). Among the complications, ileus/obstruction was significantly less frequent in the LG than in the OG (3.8% in LG vs. 15.8% in OG, P = 0.039). There was one in hospital mortality within 30 days after operation in the LG due to postoperative severe pneumonia. The median time to resuming a soft diet was 3 days (range, 2–21 days) in the LG and 10 days (range, 3–27 days; P < 0.001) in the OG. The median length of hospital stay was 7 days (range, 4–29 days) in the LG and 17 days (range, 8–38 days; P < 0.001) in the OG (Table 2).
The ratio of stage II and stage III was similar between the two groups. The rate of pT4b was significantly lower in the LG than the OG (13.5% in LG vs. 31.6% in OG, P = 0.025). There was no difference in the number of positive and total retrieved lymph nodes between the two groups. The rate of adjuvant chemotherapy did not differ between the two groups (80.8% in LG vs. 75.4% in OG, P = 0.502). There was no difference in crude events of recurrence between the two groups (P = 0.978) (Table 3).
Patterns of local recurrence were three retroperitoneum type and two peritoneum type in the LG and two mesenteric/nodal type, two retroperitoneum type, and three peritoneum type in the OG (P = 0.747). There was no perianastomotic type local recurrence in either group (Table 4).
There was no statistically significant difference in 5-year overall survival (60.7% in LG vs. 61.9% in OG, P = 0.817), 5-year disease-free survival (53.6% in LG vs. 62.6% in OG, P = 0.980), and 5-year local recurrence-free survival (88.9% in LG vs. 88.1% in OG, P = 0.725) between the LG and the OG (Fig. 2). Patient who experienced postoperative mortality was included in all survival analyses.
Discussion
This study demonstrated that laparoscopy for pathologic confirmed T4 colon cancer showed earlier recovery and similar long-term oncologic outcomes in comparison to open surgery. Thus, laparoscopic surgery for T4 colon cancer could be a viable option in selected patients.
The benefits of laparoscopy surgery, such as earlier recovery and similar long-term oncologic outcomes, have been studied in various multicenter randomized trials [8, 9]. Other proven advantages of a reduction in long-term adhesion formations [20, 21] and a reduction in the time to initiation of postoperative adjuvant chemotherapy [22, 23] may be additional reasons for application of laparoscopy in colorectal surgery. However, laparoscopic surgery was not recommended in cases of colon cancer with suspected invasion of adjacent organs [10]. Laparoscopic resection of T4 colon cancer is regarded a technical demanding procedure and its efficacy remains controversial. The main reason for reluctance to perform laparoscopy surgery in this setting is the fear of worse oncologic outcomes due to incomplete resection [24]. R0 resection is known to be one of the most important prognostic factors in the management of locally advanced colorectal cancer [25]. In our study, curative R0 resection could be achieved in most of the cases (98% of LG and 96% in OG) with no difference between the two groups. The number of retrieved lymph nodes did not differ between the two groups. In addition to these similar short-term parameters of oncologic safety, the 5-year overall survival was similar between the groups (60.7% in LG and 61.9% in OG). One of the most important parameters for locally advanced colon cancer might be the rate of local recurrence. In this study, local recurrence was divided into four categories: perianastomotic, mesentery/nodal, retroperitoneum, or peritoneum [18]. It was already demonstrated that the rate of salvage R0 re-resection was higher for the perianastomotic type than the other types [18]. In our analysis, there was no perianastomotic type of local recurrence in either group and the types of local recurrence did not differ between the two groups. Local recurrence-free survival showed no difference between the groups. These long-term outcomes demonstrated that laparoscopic surgery could give similar oncologic results to open surgery for T4 colon cancer.
The conversion rate from laparoscopy to open surgery in T4 colorectal cancer ranges from 7.1 to 24.7% [11, 13, 14, 24, 26]. The conversion rate in this study was 7.6%. As Vignali and colleagues already noted, accumulated experience of laparoscopic surgery and exclusion of rectal cancer patients might explain the low conversion rate [11]. In contrast, Kim et al. reported that the success rate of the laparoscopic approach was significantly higher in rectal cancer than in colon cancer (86.3% for rectal cancer vs. 50% for colon cancer, P < 0.001) in their laparoscopic multivisceral resection series [26]. The reasons for the higher success rate of completion with laparoscopy in rectal cancer were suggested to be the magnified view of laparoscopy, selective application of preoperative chemoradiotherapy for rectal cancer, and possible en bloc resection of pelvic organs using the perineal approach [26]. However, selection bias may play an important role in determining the actual conversion rate and it is difficult to be sure that low conversion rates directly reflect the safety of laparoscopic surgery in retrospective studies. It has been debated whether conversion impacts the postoperative outcomes in colorectal cancer surgery; some authors reported that conversion adversely impacted the long-term outcomes [27, 28] whereas others did not observe this [29, 30]. However, there are limited data on the impact of conversion in the management of locally advanced colon surgery performed by laparoscopy. Recently, Kim et al. demonstrated that converted patients showed similar short-term recovery outcomes and long-term oncologic results compared to those undergoing open surgery and the non-converted laparoscopy group, although the case number was relatively low [13]. While the pitfalls of conversion in locally advanced colon cancer should be evaluated in a large cohort, this result suggests that converted laparoscopy surgery might not jeopardize the long-term oncologic outcomes.
The weakness of this study is the non-randomization of cases and the inherent limitations of a retrospective designed study. Our study included patients treated from 2003 to 2013, thus different postoperative management strategies are inevitable. Fast discharge was not strongly forced to the patients in the earlier periods of this study and the earlier discharge becomes more emphasized in recent years in Korea, although enhanced recovery protocol after surgery was not applied for the enrolled patients. The fact that open surgeries were more commonly selected in the earlier periods could be a possible reason for longer hospital stay in open surgery group, which might act as a bias. The surgeon’s preference for laparoscopy, patients’ condition, and preoperative local tumor staging might influence selection of laparoscopy or open surgery. However, it is impossible to determine the exact reasons for deciding on either one of the modalities. It is likely that an open approach was chosen in cases where the tumor was suspected to be more advanced. As combined resections were more common, a more extensive surgical resection was anticipated in open surgery group. This may explain in part the significantly higher rate of morbidity and longer hospital stay in open surgery group. One patient in this study underwent a right hemicolectomy and pylorus-preserving pancreaticoduodenectomy (PPPD) simultaneously by an open approach in 2008. At that time, this kind of combined resection was considered too technically demanding for a laparoscopic approach. However, as a result of recent developments of laparoscopic instruments as well as the surgeon’s accumulation of laparoscopic skills, laparoscopic PPPD is now technically feasible with similar oncologic outcomes to open surgery [31, 32]. Likewise, the indication for applying laparoscopic surgery could also be expanded to include potential T4b colon cancers in preoperative diagnosis. Preoperative tumor staging cannot definitely determine which patients are really pathologic T4 stage. The positive predictive value of computed tomography for T4 colon cancer in non-metastatic colon cancers ranges from 19.4 to 73.5% [33, 34]. This relatively low predictive rate might be a substantial hurdle to application of our conclusions. In contrast, several cases of clinically suspected T4 colon cancers were proved to be pathologically T2 or T3. This result might inversely advocate that clinical application of laparoscopic surgery in suspected T4 cancer could be possible although further studies into the limitations of preoperative staging are warranted. In addition, our study had possibilities of underpowered analysis due to relatively small number of cases both in laparoscopy and open surgery groups.
In conclusion, it is possible to apply laparoscopic surgery selectively in the treatment of pathologic T4 colon cancer patients with favorable recovery outcomes and without decreasing the long-term oncologic safety. Thus, laparoscopy should not be regarded as an absolute contraindication in the management of locally advanced colon cancer. Although only a true randomized controlled trial (RCT) can eliminate any potential selection bias, our study provides meaningful retrospective data that may be useful when launching an RCT.
References
Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH (2016) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat 48(2):436–450
Gebhardt C, Meyer W, Ruckriegel S, Meier U (1999) Multivisceral resection of advanced colorectal carcinoma. Langenbeck's Arch Surg 384(2):194–199
Croner RS, Merkel S, Papadopoulos T, Schellerer V, Hohenberger W, Goehl J (2009) Multivisceral resection for colon carcinoma. Dis Colon rectum 52(8):1381–1386
Lehnert T, Methner M, Pollok A, Schaible A, Hinz U, Herfarth C (2002) Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 235(2):217–225
Kapoor S, Das B, Pal S, Sahni P, Chattopadhyay TK (2006) En bloc resection of right-sided colonic adenocarcinoma with adjacent organ invasion. Int J Color Dis 21(3):265–268
Lee WS, Lee WY, Chun HK, Choi SH (2009) En bloc resection for right colon cancer directly invading duodenum or pancreatic head. Yonsei Med J 50(6):803–806
Nakafusa Y, Tanaka T, Tanaka M, Kitajima Y, Sato S, Miyazaki K (2004) Comparison of multivisceral resection and standard operation for locally advanced colorectal cancer: analysis of prognostic factors for short-term and long-term outcome. Dis Colon rectum 47(12):2055–2063
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365(9472):1718–1726
Buunen M, Veldkamp R, Hop WC et al (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10(1):44–52
Veldkamp R, Gholghesaei M, Bonjer HJ et al (2004) Laparoscopic resection of colon cancer: consensus of the European Association of Endoscopic Surgery (EAES). Surg Endosc 18(8):1163–1185
Vignali A, Ghirardelli L, Di Palo S, Orsenigo E, Staudacher C (2013) Laparoscopic treatment of advanced colonic cancer: a case-matched control with open surgery. Color Dis 15(8):944–948
Shukla PJ, Trencheva K, Merchant C, Maggiori L, Michelassi F, Sonoda T, Lee SW, Milsom JW (2015) Laparoscopic resection of T4 colon cancers: is it feasible? Dis Colon rectum 58(1):25–31
Kim IY, Kim BR, Kim YW (2016) The short-term and oncologic outcomes of laparoscopic versus open surgery for T4 colon cancer. Surg Endosc 30(4):1508–1518
Bretagnol F, Dedieu A, Zappa M, Guedj N, Ferron M, Panis Y (2011) T4 colorectal cancer: is laparoscopic resection contraindicated? Color Dis 13(2):138–143
Ng DC, Co CS, Cheung HY, Chung CC, Li MK (2011) The outcome of laparoscopic colorectal resection in T4 cancer. Color Dis 13(10):e349–e352
Huh JW, Kim HR (2012) The feasibility of laparoscopic resection compared to open surgery in clinically suspected T4 colorectal cancer. J Laparoendosc Adv Surg Tech A 22(5):463–467
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474
Bowne WB, Lee B, Wong WD, Ben-Porat L, Shia J, Cohen AM, Enker WE, Guillem JG, Paty PB, Weiser MR (2005) Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon rectum 48(5):897–909
Park JH, Kim MJ, Park SC, Kim MJ, Hong CW, Sohn DK, Han KS, Oh JH (2015) Difference in time to locoregional recurrence between patients with right-sided and left-sided colon cancers. Dis Colon rectum 58(9):831–837
Burns EM, Currie A, Bottle A, Aylin P, Darzi A, Faiz O (2013) Minimal-access colorectal surgery is associated with fewer adhesion-related admissions than open surgery. Br J Surg 100(1):152–159
Dowson HM, Bong JJ, Lovell DP, Worthington TR, Karanjia ND, Rockall TA (2008) Reduced adhesion formation following laparoscopic versus open colorectal surgery. Br J Surg 95(7):909–914
Strouch MJ, Zhou G, Fleshman JW, Birnbaum EH, Hunt SR, Mutch MG (2013) Time to initiation of postoperative chemotherapy: an outcome measure for patients undergoing laparoscopic resection for rectal cancer. Dis Colon rectum 56(8):945–951
Poylin V, Curran T, Lee E, Nagle D (2014) Laparoscopic colectomy decreases the time to administration of chemotherapy compared with open colectomy. Ann Surg Oncol 21(11):3587–3591
Elnahas A, Sunil S, Jackson TD, Okrainec A, Quereshy FA (2016) Laparoscopic versus open surgery for T4 colon cancer: evaluation of margin status. Surg Endosc 30(4):1491–1496
Smith JD, Nash GM, Weiser MR, Temple LK, Guillem JG, Paty PB (2012) Multivisceral resections for rectal cancer. Br J Surg 99(8):1137–1143
Kim KY, Hwang DW, Park YK, Lee HS (2012) A single surgeon’s experience with 54 consecutive cases of multivisceral resection for locally advanced primary colorectal cancer: can the laparoscopic approach be performed safely? Surg Endosc 26(2):493–500
Moloo H, Mamazza J, Poulin EC, Burpee SE, Bendavid Y, Klein L, Gregoire R, Schlachta CM (2004) Laparoscopic resections for colorectal cancer: does conversion survival? Surg Endosc 18(5):732–735
Chan AC, Poon JT, Fan JK, Lo SH, Law WL (2008) Impact of conversion on the long-term outcome in laparoscopic resection of colorectal cancer. Surg Endosc 22(12):2625–2630
Franko J, Fassler SA, Rezvani M, O’Connell BG, Harper SG, Nejman JH, Zebley DM (2008) Conversion of laparoscopic colon resection does not affect survival in colon cancer. Surg Endosc 22(12):2631–2634
Rickert A, Herrle F, Doyon F, Post S, Kienle P (2013) Influence of conversion on the perioperative and oncologic outcomes of laparoscopic resection for rectal cancer compared with primarily open resection. Surg Endosc 27(12):4675–4683
Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ (2015) Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann Surg 262(1):146–155
Senthilnathan P, Srivatsan Gurumurthy S, Gul SI, Sabnis S, Natesan AV, Palanisamy NV, Praveen Raj P, Subbiah R, Ramakrishnan P, Palanivelu C (2015) Long-term results of laparoscopic pancreaticoduodenectomy for pancreatic and periampullary cancer-experience of 130 cases from a tertiary-care center in South India. J Laparoendosc Adv Surg Tech A 25(4):295–300
Smith NJ, Bees N, Barbachano Y, Norman AR, Swift RI, Brown G (2007) Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer 96(7):1030–1036
Huh JW, Jeong YY, Kim HR, Kim YJ (2012) Prognostic value of preoperative radiological staging assessed by computed tomography in patients with nonmetastatic colon cancer. Ann Oncol 23(5):1198–1206
Acknowledgments
The authors are grateful to Dong-Su Jang, (Medical Illustrator, Medical Research Supporting Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, J., Baik, S.H., Lee, K.Y. et al. Outcomes of laparoscopic surgery in pathologic T4 colon cancers compared to those of open surgery. Int J Colorectal Dis 32, 531–538 (2017). https://doi.org/10.1007/s00384-016-2720-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2720-5