Abstract
Background
This study aimed to evaluate the influence of conversion on perioperative and short- and long-term oncologic outcomes in laparoscopic resection for rectal cancer and to compare these with those for an open control group.
Methods
The data of 276 consecutive patients who underwent surgery for rectal cancer between 2006 and 2010 at a single institution were prospectively collected. Of the 276 patients, 114 underwent primarily open surgery, and 162 underwent laparoscopic surgery (on an intention-to-treat basis). Of the 162 laparoscopic patients, 38 (23.5 %) underwent conversion to open surgery. The three groups of patients were compared: the conversion surgery group, the open surgery group, and the completed laparoscopy surgery group.
Results
The converted patients had more wound infections (18.4 vs 4.8 %, p = 0.009), but the wound infection rate in the primarily open group also was significantly higher than in the laparoscopic resection group (p = 0.007). No further differences in perioperative morbidity, including anastomotic leakage, were found. The perioperative 30-day mortality rate was comparable between all the groups (0.6 vs 2.6 vs 2.6 %, nonsignificant difference). The oncologic parameters such as number of harvested lymph nodes and rate of R0 resection were equal in all the groups. The completed laparoscopy group had a shorter hospital stay [12 vs 16 days in the primarily open group (p = 0.02) vs 15 days in the converted group (p = 0.03)]. The rates for survival, local recurrence (4.5 vs 3 vs 3 %), and metachronous metastasis (10.1 vs 9.3 vs 9 %) did not differ significantly between the three groups after a period of 3 years.
Conclusion
Conversion to open surgery in laparoscopic rectal resection has no negative effect on perioperative or long-term oncologic outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic operations for malignant diseases must have proven equivalence to established conventional procedures in terms of perioperative morbidity, practiced oncologic surgical standards (free margins, adequate number of harvested nodes), and long-term oncologic outcome (survival, local recurrence rates). Several randomized prospective trials [6, 23–25] and three recent metaanalyses [19–21] have suggested that laparoscopic resection for rectal cancer is safe and oncologically adequate. Compared with open resections, the laparoscopic approach was advantageous in terms of blood loss, hospital length of stay, return to normal bowel function, and cosmesis [1–3].
However, there is concern that patients with operations begun laparoscopically and then converted not only have a worse outcome in terms of their early perioperative course than patients who undergo conventional surgery, but also have a poorer long-term oncologic outcome [4, 8, 10, 23]. For colorectal cancer, the data are conflicting. One study demonstrated a negative effect of conversion on disease-free survival of colonic cancer patients [5]. For rectal cancer, this has not been shown to date, but the data are limited [6, 9].
Laparoscopic total mesorectal excision (TME) is a challenging operation with a slow learning curve. As a result, conversion rates reaching 38 % are reported in the literature [7–14], showing that this group of patients is clinically relevant. If the results after converted laparoscopic rectal resection are indeed significantly worse than after conventional surgery, the focus must be centered on reducing the conversion rate, either by more adequate patient selection or by improvement of the surgical technique.
Because only a few trials with a limited number of patients have addressed this issue, the current study aimed to evaluate the short- and long-term outcomes for patients requiring conversion compared with those for patients who had completed laparoscopic resection and control patients who had primarily open resections.
Patients and methods
This study was based on a prospective database of patients with colorectal cancer. Three groups were compared: open surgery patients (OS), completed laparoscopic surgery patients (LS), and converted laparoscopic surgery patients (CON). To show that the basic patient and tumor characteristics were comparable, all patients with laparoscopically begun surgery (on an intention-to-treat basis) also were analyzed and compared with the open surgery group.
Consecutive patients with isolated rectal cancer up to 16 cm from the anal verge (measured by rectoscopy) who underwent curative resection between January 2006 and December 2010 at the Surgical Department of the University Hospital Mannheim were included in the study.
To allow valid comparisons, the inclusion criteria specified a tumor classification of cT1–T3Nx, resection with curative intent, and no distant metastases (Union internationale contre le cancer 1-3). To rule out a potential bias, the study excluded T4 carcinomas because these tumors usually are managed with an open technique. Only patients who met the inclusion criteria for a laparoscopic resection were included in the study.
Laparoscopic rectal resection was introduced to our department in 2006. From this year on, the rate of laparoscopic resections increased to the current rate exceeding 90 %. The choice of approach (laparoscopic vs conventional) was determined by one of the two experienced laparoscopic surgeons managing all the rectal cancer cases.
The preoperative investigations principally included colonoscopy, rectoscopy with biopsies, rectal endosonography, magnetic resonance imaging (MRI) of the pelvic region, abdominal sonography, chest X-ray, and tumor markers [carcinoembryonic antigen (CEA)]. The pre-therapeutic tumor stage was assessed through rectal endosonography and MRI using the tumor node metastasis (TNM) classification.
According to national guidelines, clinical stages cT3 and cN+ generally were treated by neoadjuvant chemoradiation. The therapeutic concept for every patient was discussed and chosen in an interdisciplinary tumor conference.
Surgery was scheduled 6–8 weeks (median 48 days) after the end of radiotherapy. A bowel preparation generally was performed for patients with a tumor located in the lower two-thirds of the rectum for these patients nearly always underwent TME with protective ileostomy.
The operating procedures were anterior rectum resection and partial mesorectal excision (PME) (for cancer in the upper third of the rectum), deep anterior resection and TME (for cancer in the mid or lower rectum), or abdominoperineal resection (for sphincter infiltration or sphincter insufficiency).
All the operations were performed by one of two surgeons with a large experience performing laparoscopic colorectal surgery. Open and laparoscopic surgeries were standardized as described in the following discussion.
For the laparoscopic operation, we use four or five trocars. For open resection, a lower median laparotomy is used.
The dissection starts with identification of the inferior mesenteric artery and vein. The artery is clipped 1 cm distal to its origin, and the vein is clipped just below the pancreas. Afterward, the left colonic flexure is always mobilized up to the medial colic vessels. Dissection of the rectum is performed in the “holy plane,” with visualization and preservation of the hypogastric nerves, as previously described with TME for deep tumors (≤12 cm from the anal verge) and with PME for high tumors.
After mobilization, the rectum is dissected with a linear stapler either 5 cm distal to the tumor in PME or at the anorectal junction in TME. The specimen is removed via minilaparotomy in the left lower abdomen (4–5 cm) or via a small Pfannenstiel incision using a wound protector. After stapling, the anastomosis (usually side-to-end) generally is tested by anal insufflation of air. The operation ends with placement of drainage to the anastomosis. An ileostomy is added for patients with TME.
Conversion to open surgery was defined as the use of an incision (median laparotomy or Pfannenstil′s incision) larger than the minilaparotomy normally used for retrieval of the bowel (i.e., an incision through which surgery is performed in the abdomen). Hence, a laparoscopically assisted resection with laparoscopic ligature of the mesenteric artery and mobilization of the splenic flexure followed by open TME via a Pfannenstiel incision was defined as a converted operation if the operation was planned as a completely laparoscopic operation. An operation planned to be laparoscopically assisted from the beginning was not defined as a converted operation. Patients with diagnostic laparoscopy only and then open surgery were included in the open surgery group.
The postoperative course followed a standardized clinical pathway (fast-track regimen including a peridural catheter, fluids on day 0, full nutrition on day 1). Between days 7 and 10, an endoscopy of the anastomosis was performed.
The short-term outcome was measured through perioperative morbidity and mortality. Morbidity was classified according to the Clavien classification introduced in 2004 [15]. Grades 1 and 2 morbidity include complications managed by conservative treatment (pharmaceutical drugs, blood transfusions, parenteral nutrition). Grade 3 morbidity involves complications treated surgically or interventionally. Grade 4 morbidity includes those that lead to organ dysfunction requiring intensive care. Grade 5 morbidity equals death of the patient. Anastomotic leakage was defined as any leakage seen on endoscopy or computed tomography (CT) or during reoperation.
Data were entered into a prospective database. Follow-up assessment was performed according to the national guidelines for the treatment of colorectal cancer [16]. After surgery, the patients were followed up every 6 months.
The data were collected prospectively and entered into a database by a single person (study nurse). The Kruskal–Wallis test and the Mann–Whitney U test were used for qualitative data. Univariate analysis of variance (ANOVA, one-way) with Bonferroni correction was performed for normally distributed quantitative data. Normal distribution was verified by the Kolmogorov–Smirnov test. For quantitative data, median (range) was calculated. The Kaplan–Meier and log-rank-tests were used for evaluation of recurrence and survival rates. Statistical significance was determined by a p value lower than 0.05. Statistical analyses were performed with SPSS 15.0, Windows version (SPSS Inc., Chicago, IL, USA).
Results
The study included 276 consecutive patients who underwent curative rectal resection for rectal cancer between January 2006 and December 2010 in the surgical department of the University Clinic Mannheim, University of Heidelberg. Of the 276 patients, 114 had open surgery and 162 had laparoscopic surgery. In the latter group, 38 patients (23.5 %) required conversion to an open procedure. Hence, 124 patients had a completely laparoscopic resection.
For statistical calculation and comparison, three groups were defined: the completely laparoscopic surgery group (LS), the open surgery group (OS), and the conversion group (CON). Additionally, an intention-to treat analysis was performed for all patients whose surgery began laparoscopically. Patients with distant metastasis or suspected invasion of organs were excluded from the study. The characteristics of the patients are shown in Table 1.
The group intended for laparoscopic treatment and the OS group showed similar patient and tumor characteristics in terms of age, body mass index (BMI), sex, and American Society of Anesthesiology (ASA) score. Tumor stage, tumor location, surgical procedure, and neoadjuvant therapy were well balanced between the groups. However, the patients in the converted group were older (age 69 years) than those in the laparoscopic group (age 63 years) (p = 0.03). The conversion and open groups showed no significant differences with regard to their basic characteristics.
Conversion was necessary for 23.5 % of the patients treated laparoscopically. In 27 of these cases, a laparoscopically facilitated operation with addition of a Pfannenstiel incision then was performed (16.7 % of all cases begun laparoscopically). A median laparotomy was necessary in 11 cases (6.8 % of all the cases begun laparoscopically). The reasons for conversion are shown in Table 2. Intraoperative complications (i.e., bleeding) were rarely causes for conversion.
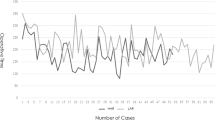
Between 2006 and 2010, the laparoscopy rate increased from 0 to 93 %. The conversion rate decreased from 37 % at the beginning of the study period to 13 % at the end (p = 0.02) (Figs. 1, 2).
The postoperative mortality and morbidity rates were similar in all the groups (Table 3). The overall mortality rate was low (1.4 %). Two patients died of septic multiorgan failure after anastomotic leakage. The one patient died after pulmonary embolism, and the other patient died after severe bleeding from a gastric ulcer. Fewer wound infections were observed in the laparoscopic surgery group than in the open surgery group or the converted surgery group [4.8 % vs 14.9 (p = 0.007) vs 18.4 % (p = 0.009)].
Anastomotic leakage appeared to be higher in the conversion group than in the other groups, but the difference did not reach statistical significance. Half of the leakages in all the groups were asymptomatic, detected during routine endoscopy only on days 7–10.
The rate of reoperations due to complications did not differ among the groups. The surgery time was shortest in the open surgery group (p < 0.001). The converted patients did not differ in procedure time from the patients whose surgery was completely laparoscopic.
The patients who underwent a completely laparoscopic operation had a significantly shorter hospital stay than the patients in the open and converted groups [12 vs 16 (p = 0.02) vs 15 (p = 0.03) days]. No 30-day mortalities occurred in the laparoscopic group, compared with a 2.6 % 30-day mortality rate in the open and conversion groups. The rate of R0 resections and the number of harvested lymph nodes did not differ between the three groups (Table 4).
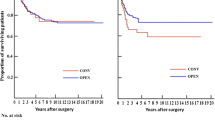
The median follow-up period was 34 months (range 1–70 months). The 3-year overall survival rate for all the patients did not differ significantly between the laparoscopic, open, and converted surgery groups (Fig. 3). This was unchanged when the patients were stratified according to tumor stage. The local recurrence rate during the observation period was 3 % in the laparoscopic group, 4.5 % in the open group and 3 % in the converted group (p = 0.84). No port-site metastasis occurred. No differences in occurrence of distant metachronous metastases were found (OS 10.1 %, LS 9.3 %, CON 9 %, p = 0.97) (Table 5).
Discussion
The study enrolled 276 patients with rectal cancer. Of these patients, 124 had completely laparoscopic surgery, and 38 had to be converted. The conversion rate of 23.5 % is very comparable with rates in other published series (7–38 %) [7–11]. The current conversion rate of 13 % in 2010 in our study compared to 37 % in 2008 reflects the learning curve of the surgeons. The relatively low conversion rate of 16 % in 2007 probably can be attributed to patient selection because the overall rate of laparoscopic rectal resections in that year was only 39 %.
However, the majority of the conversions in our study were to a laparoscopically assisted procedure with a larger Pfannenstiel incision (16.7 %). Only one-third of converted patients needed a conversion to a median laparotomy (overall conversion rate to median laparotomy 6.8 %). Compared with a vertical incision, the Pfannenstiel incision has advantages such as reduced postoperative pain and fewer incisional hernias [17].
In all recently published trials, the term “conversion” either is defined as a midline laparotomy or unfortunately not defined at all [6, 18, 29]. Possibly because the majority of patients were converted to a Pfannenstiel incision and not to a median laparotomy, the differences between the converted and primarily open groups may not have been as clinically evident as expected. Especially when the overall limited number of patients in the group is taken into account, the negative impact of a conversion may indeed have been underestimated in our study.
Laparoscopic rectal resection for rectal carcinoma is assumed to be comparable with open surgery in terms of long-term oncologic outcome and has shown some advantages in early postoperative outcome parameters such as hospital stay and recovery [19–25] (Table 4). But several studies have suggested that patients undergoing laparoscopic rectal resection who require conversion to an open procedure have increased perioperative morbidity [4, 23]. Anastomotic leakage, as one of the major morbidities after rectal cancer resection, has been identified as a risk factor for local recurrence, and hence higher morbidity may lead to a poorer oncologic outcome [26] (Table 6).
One study investigating colonic cancer indeed found a decreased 5-year disease-free survival rate after conversion [5]. But in rectal cancer, no significant differences in oncologic quality indicators (resection margins, number of lymph nodes) or oncologic outcomes between open, laparoscopic, and converted resections have been identified to date in the available studies. Albeit, patient numbers were limited in these trials [6, 29].
The results of the CLASICC trial raised concern because it showed a statistical trend toward more patients displaying infiltrated circumferential resection margins after laparoscopic than after open rectal resection. This finding could not be confirmed in our study because the rate of infiltrated circumferential margins did not differ in the three groups. The number of harvested lymph nodes also was identical in the groups. Thus, the quality of oncologic surgery as measured by the established quality indicators was very comparable in the three groups. This was reflected by the identical long-term oncologic outcome in all the groups, with an equivalent overall survival as well as comparable local and distant recurrence rates.
The long-term follow-up data of the CLASICC trial also demonstrated equal oncologic recurrence and survival rates in all groups, thereby not confirming the voiced concern that the trend in that study toward more patients displaying infiltrated resection margins after laparoscopic than after open surgery indeed translates into a worse oncologic outcome [27].
Our short-term results are well comparable with those of a recently published retrospective study that compared laparoscopic with open rectal resection for rectal cancer. Of 238 patients, 36 had to undergo conversion, and no difference in postoperative mortality or morbidity was found, but no detailed data for the converted group is presented [6]. This is the only hitherto published study showing equal morbidity rates for converted and laparoscopically completed patients undergoing rectal resection for rectal cancer.
A recent large study with more than 8,000 patients that investigated conversion in laparoscopic surgery for colonic cancer reported a similar result, showing no difference in mortality and morbidity rates [5]. But because the two main reasons for conversion are unexpected intraoperative complications and their management and difficult anatomic circumstances (obesity, adhesions, tumor size), which resulted in the converted patient group being a negatively selected one, the perioperative morbidity rate should be higher. These patients cannot take advantage of the potential benefits conferred by purely laparoscopic surgery. The operation is more difficult, and the operation time usually is increased.
The majority of studies in the literature examining the outcome of converted patients after rectal resection have indeed found a higher complication rate [4, 8, 9, 23, 29]. The CLASICC-trial demonstrated higher rates of postoperative mortality and morbidity among converted patients [23], as well as a statistically nonsignificant trend toward lower long-term survival rates among converted rectal cancer patients [28].
A large Japanese study (995 patients, 76 conversions) also found a substantially increased morbidity rate among converted patients [4]. The conversion rate was low (7 %), and the BMI was significantly higher in the converted group, which might in part account for the higher complication rate. A high percentage of carcinomas with favorable tumor characteristics (tumor location in the upper rectum, high rate of low tumor stage T1–T2, low percentage of neoadjuvant therapy) were further limitations of this study, making the comparison with other studies difficult.
A German study in 2008 compared 274 patients with laparoscopic rectal resections for rectal cancer and 26 converted patients [29]. The patient and tumor characteristics were similar to those in our study. An increased overall complication rate after conversion was found, but this was mainly due to a higher wound infection rate in this group. Major complications such as anastomotic leakage did not differ between the converted patients and those not converted.
In our study, conversion was not associated with greater mortality or morbidity, except with regard to the rate of wound infections. This rate was significantly higher among the converted patients than among the laparoscopically treated patients. But this probably was not the result of the conversion per se but merely the result of the open approach, which is reflected by the finding that primarily open surgery compared with laparoscopic surgery also was associated with a higher wound infection rate. This result of our study accords well with many other studies comparing laparoscopic and open colorectal surgery, which have consistently shown lower wound infection rates in the minimally invasive group Yamamoto [30, 31].
Due to the nonrandomized character of our study, a selection bias can obviously not be ruled out. To minimize this, patients with advanced or metastatic disease were excluded because these patients were more likely to undergo conventional surgery.
In our study, the converted patients were significantly older than the patients in the laparoscopic group. Older age may lead to a lower threshold for conversion because the surgeon may be more inclined to keep the procedure time short. We could not detect a higher conversion rate for obese patients as shown in other studies investigating minimally invasive surgery [32, 33]. Obviously, a randomized study is impossible in this setting because conversion is not a calculable event. Consequently, potential bias will always be an issue in studies dealing with conversion [4, 6].
Conclusion
In conclusion, this study demonstrated that conversion in minimally invasive rectal cancer resection does not lead to higher rates of morbidity and mortality than for an open control group and does not worsen the oncologic outcome. A selection bias cannot be ruled out completely because the patients in the two groups were not entirely comparable (the patients in the laparoscopic group were younger and had a lower BMI). Nevertheless, these data strongly support our view that neither a laparoscopic approach nor conversion after a laparoscopically begun surgery is detrimental in terms of early postoperative and late oncologic outcomes as long as the conversion is performed early enough.
References
Fernández-Cebrián JM, Gil P, Hernández-Granados P, Fiuza C, Ochando F, Loinaz C, Rueda J, Alasala M, Jiménez-Almonacid P, Vega D, Pardo M, Quintans A (2009) Initial surgical experience in laparoscopic total mesorectal excision for middle and lower third rectal cancer: short-term results. Clin Transl Oncol 11:460–464
Glancy DG, Chaudhray BN, Greenslade GL, Dixon AR (2012) Laparoscopic total mesorectal excision can be performed on a nonselective basis in patients with rectal cancer with excellent medium-term results. Colorectal Dis 14:453–457
Pugliese R, Di Lernia S, Sansonna F, Maggioni D, Ferrari GC, Magistro C, Costanzi A, De Carli S, Artale S, Pugliese F (2009) Laparoscopic resection for rectal adenocarcinoma. Eur J Surg Oncol 35:497–503
Yamamoto S, Fukunaga M, Miyajima N, Okuda J, Konishi F, Watanabe M (2009) Japan Society of Laparoscopic Colorectal Surgery: impact of conversion on surgical outcomes after laparoscopic operation for rectal carcinoma: a retrospective study of 1,073 patients. J Am Coll Surg 208:383–389
Ptok H, Kube R, Schmidt U, Köckerling F, Gastinger I, Lippert H, Colon/Rectum Carcinoma (Primary Tumor) Study Group (2009) Conversion from laparoscopic to open colonic cancer resection: associated factors and their influence on long-term oncological outcome. EJSO 35:1273–1279
Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E (2009) Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg 250:54–61
Bretagnol F, Lelong B, Laurent C, Moutardier V, Rulliert A, Monges G, Delpero JR, Rullier E (2005) The oncological safety of laparoscopic total mesorectal excision with sphincter preservation for rectal carcinoma. Surg Endosc 19:892–896
Lelong B, Bege T, Esterni B, Guiramand J, Turrini O, Moutardier V, Magnin V, Moges G, Pernoud N, Blache JL, Giovannini M, Delpero JR (2006) Short-term outcome after laparoscopic or open restorative mesorectal excision for rectal cancer: a comparative cohort study. Dis Colon Rectum 50:176–183
Strohlein M, Grützner KU, Jauch KW, Heiss MM (2008) Comparison of laparoscopic vs open access surgery in patients with rectal cancer: a prospective analysis. Dis Colon Rectum 51:385–391
Law WL, Lee YM, Choi HK, Seto CL, Ho JW (2006) Laparoscopic and open anterior resection for upper and mid rectal cancer: an evaluation of outcomes. Dis Colon Rectum 49:1108–1115
Morino M, Allax M, Giraudo G, Corno F, Garrone C (2005) Laparoscopic versus open surgery for extraperitoneal rectal cancer. Surg Endosc 19:1460–1467
Gonzalez R, Smith CD, Mason E, Duncan T, Wilson R, Miller J, Ramshaw BJ (2005) Consequences of conversion in laparoscopic colorectal surgery. Dis Colon Rectum 49:197–204
Belizon A, Sardinha CT, Sher ME (2006) Converted laparoscopic colectomy: what are the consequences? Surg Endosc 20:947–951
Marusch F, Gastinger I, Schneider C, Scheidbach H, Konradt J, Bruch HP, Köhler L, Bärlehner E, Köckerling F (2001) Importance of conversion for results obtained with laparoscopic colorectal surgery. Dis Colon Rectum 44:207–214
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg 240:205–213
Schmiegel W, Reinacher-Schick A, Arnold D, Graeven U, Heinemann V, Porschen R, Riemann J, Rödel C, Sauer R, Wieser M, Schmitt W, Schmoll HJ, Seufferlein T, Kopp I, Pox C (2008) Update S3-guideline “colorectal cancer”. Z Gastroenterol 46:799–840
Luijendijk RW, Jeekel J, Storm RK, Schutte PJ, Hop WC, Huikeshoven FJ (1997) The low transverse Pfannenstiel incision and the prevalence of incisional hernia and nerve entrapment. Ann Surg 225:365–369
Gervaz P, Pikarsky A, Utech M, Secic M, Efron J, Belin B, Jain A, Wexner S (2001) Converted laparoscopic colorectal surgery: a meta-analysis. Surg Endosc 15:827–832
Gao F, Cao YF, Chen LS (2006) Meta-analysis of short-term outcomes after laparoscopic resection for rectal cancer. Int J Colorectal Dis 21:652–656
Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG (2006) Laparoscopic versus open surgery for rectal cancer: a metaanalysis. Ann Surg Oncol 13:413–424
Anderson C, Uman G, Pigazzi A (2008) Oncologic outcome of laparoscopic surgery for rectal cancer: a systematic review and meta-analysis of the literature. Eur J Surg Oncol 34:1135–1142
Bonjer HJ, Lacy AM (2009) COLOR II–a randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull 56:89–91
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term end points of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V (2007) Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum 50:464–471
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM (2007) Randomized trial of laparoscopic assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 25:3061–3068
Law WL, Choi HK, Lee YM, Ho JW, Seto CL (2007) Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg 11:8–15
Jayne DG, Thorpe H, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97:1638–1645
Green BL, Marshall HC, Collison F, Quirke P, Gouillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the medical research council CLASSIC-trial of conventional vs. laparoscopically assisted resection in colorectal cancer. Br J Surg 100:75–82
Agha A, Fürst A, Iesalnieks I, Fichtner-Feigl S, Ghali N, Krenz D, Anthuber M, Jauch KW, Piso P, Schlitt HJ (2008) Conversion rate in 300 laparoscopic rectal resections and its influence on morbidity and oncological outcome. Int J Colorectal 23:409–417
Poon JT, Law WL, Wong IW, Ching PT, Wong LM, Fan JK, Lo OS (2009) Impact of laparoscopic colorectal resection on surgical site infection. Ann Surg 249:77–81
Biondo S, Kreisler E, Fraccalvieri D, Basany EE, Codina-Cazador A, Ortiz H (2012) Risk factors for surgical site infection after elective resection for rectal cancer: a multivariate analysis on 2,131 patients. Colorectal Dis 14:e95–e102
Schwandner O, Schiedeck T, Bruch H-P (1999) The role of conversion in laparoscopic colorectal surgery: do predictive factors exist. Surg Endosc 13:151–156
Tekkis P, Senagore A, Delaney C, Fazio V (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 242:83–91
Disclosures
Peter Kienle, Florian Herrle, and Stefan Post received grants from the German research council for clinical trials. Stefan Post received payment for lectures from J&J, Roche, Siemens, and the Merck advisory board for clinical trials. Peter Kienle received payment for lectures from Aesculap and Falk. Florian Herrle received payment for tutorship in workshops from the German Cochrane Center. Fabian Doyon and Alexander Rickert have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alexander Rickert and Florian Herrle have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Rickert, A., Herrle, F., Doyon, F. et al. Influence of conversion on the perioperative and oncologic outcomes of laparoscopic resection for rectal cancer compared with primarily open resection. Surg Endosc 27, 4675–4683 (2013). https://doi.org/10.1007/s00464-013-3108-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3108-z