Abstract
Background
Colorectal endoscopic submucosal dissection (C-ESD) is a promising but challenging procedure. We aimed to evaluate the factors associated with technical difficulties (failure of en bloc resection and procedure time, ≥2 h) and adverse events (perforation and bleeding) of C-ESD.
Methods
We conducted a retrospective exploratory factor analysis of a prospectively collected cohort in 15 institutions. Eight-hundred sixteen colorectal neoplasms larger than 20 mm from patients who underwent C-ESD were included. We assessed the outcomes of C-ESD and risk factors for technical difficulties and adverse events.
Results
Of the 816 lesions, 767 (94 %) were resected en bloc, with a median procedure time of 78 min. Perforation occurred in 2.1 % and bleeding in 2.2 %. Independent factors associated with failure of en bloc resection were low-volume center (<30 neoplasms), snare use, and poor lifting after submucosal injection. Factors significantly associated with long procedure time (≥2 h) were large tumor size (≥4 cm), low-volume center, less-experienced endoscopist, CO2 insufflation, and use of two or more endoknives. Poor lifting was the only factor significantly associated with perforation, whereas rectal lesion and lack of a thin-type endoscope were factors significantly associated with bleeding. Poor lifting after submucosal injection occurred more frequently for nongranular-type laterally spreading tumors (LST) and for protruding and recurrent lesions than for granular-type LST (LST-G).
Conclusions
Poor lifting after submucosal injection was the risk factor most frequently associated with technical difficulties and adverse events on C-ESD. Less experienced endoscopists should start by performing C-ESDs on LST-G lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endoscopic resection is a noninvasive, standard treatment for patients with superficial colorectal neoplasms (adenoma/early cancer) without risk of lymph node metastasis [1–3]. Small colorectal neoplasms can be removed easily with conventional polypectomy or endoscopic mucosal resection (EMR). However, conventional EMR may result in piecemeal resection (i.e., tumor resection in multiple fragments) of large-sized tumors [4–6]. Limitations of piecemeal resection include incomplete histological assessment of the specimen and a greater risk of tumor recurrence [7]. Indeed en bloc resection (i.e., resecting the entire tumor in one piece) is preferred for precise histological assessment of the resected specimen and to ensure elimination of any residual tumor [4].
Endoscopic submucosal dissection (ESD), of superficial gastrointestinal neoplasms results in high en bloc resection rates, regardless of tumor size, location, or fibrosis in the submucosa (SM) [1]. However, colorectal ESD is associated with technical difficulties resulting in poor outcomes, such as failure of en bloc resection and long procedure time [8]. Additionally, the adverse events of colorectal ESD (e.g., perforation and bleeding) may be quite severe [9]. These technical difficulties and adverse events may be associated with lesion characteristics, type of endoscopic device, and operator experience. Limitations in attempting to perform colorectal ESD may be due to a lack of information on these technical difficulties and adverse events. Assessing factors associated with such technical difficulties and adverse events may help in formulating training programs for colorectal ESD and treatment strategies for large colorectal tumors. Although several large case-series have assessed the feasibility and efficacy of colorectal ESD, these were retrospective analyses in well-experienced single centers [10, 11]. Outcomes of colorectal ESD were also assessed in a prospective multicenter study, but those centers were all advanced institutions [12]. Therefore, the outcomes of colorectal ESD performed at institutions with various levels of experience have not yet been evaluated.
Considering that the rates of adverse events and tumor recurrence following EMR and ESD had never been directly compared, we performed a prospective cohort study comparing EMR and ESD for large (≥20 mm) colorectal neoplasms [13, 14]. In the prospectively collected cohort, there was a large number of ESD procedures (816 ESDs vs. 1,029 EMRs). Therefore, we retrospectively explored the factors associated with technical difficulty and adverse events on colorectal ESD in the cohort.
Patients and methods
This retrospective analysis involved the patients undergoing colorectal ESD in the prospectively selected patients undergoing colorectal endoscopic resection at 18 tertiary institutions with various levels of experience. The study was performed by the Japanese Society for Cancer of the Colon and Rectum to compare recurrence rates after EMR and ESD for colorectal neoplasms ≥20 mm [13, 14]. The study protocol was approved by the institutional review board of each center and registered in the University Hospital Medical Information Network Clinical Trials Registry as number UMIN 000001642. This manuscript followed the STROBE guidelines [15]. All the authors had reviewed and approved the final manuscript.
Study participants
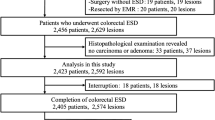
Consecutive patients >20 years old with superficial colorectal neoplasms ≥20 mm in diameter undergoing endoscopic resection between October 2007 and December 2010 were eligible for inclusion in the original cohort trial. Lesions predicted to be noninvasive neoplasms and carcinomas with minute (<1,000 μm) SM invasion, thought to have no risk of lymph node metastasis, were removed by endoscopic resection. The subjects in that trial who underwent ESD were included in this retrospective exploratory factor analysis (Fig. 1). The choice between EMR and ESD was made by each participating colonoscopist, based on the proposed guidelines of the Colorectal ESD Standardization Implementation Working Group [16, 17]. Lesions with contraindications to endoscopic resection, as determined by the colonoscopist, including lesions involving the orifice of the appendix, those encompassing the entire circumference of the colonic wall, those showing massive invasion of the ileum, and lesions inaccessible by colonoscopy, were excluded and treated by surgical colectomy. Written informed consent was obtained from each patient.
Procedures
All procedures were performed by colonoscopists who had been physicians for at least 5 years and were either board-certified by the Japanese Gastroenterological Endoscopy Society (JGES) or had knowledge and endoscopic techniques equal to that of board-certificated colonoscopists. Therefore, no trainees were involved in any of these cases. Endoscopic devices (endoknives), endoscopes, endoscopic systems, and medications were not regulated by the study protocol, and all procedures were performed according to each institution’s standard procedure. Patients were considered admitted to hospital when they underwent ESD. Although the fasting and hospitalization periods and examination after colonic ESD were determined according to each institution’s protocol, in Japan the usual fasting period is 2 days, including the day on which ESD is performed, and the hospitalization period is 7 days with blood tests performed the day after ESD. The histopathology of each resected specimen was assessed at each institution, following the Japanese classification of colorectal carcinoma [2]. Lesions histopathologically diagnosed as low/high-grade adenoma, intramucosal carcinoma, or carcinoma with minute SM invasion (<1,000 μm), without high pathologic risk features (lymph-vascular involvement and/or poorly differentiated adenocarcinoma), were regarded as curable because they had no risk of lymph node metastasis. By contrast, lesions histopathologically diagnosed as carcinoma with deep SM invasion (≥1,000 μm) or with high-risk pathologic features were regarded as incurable, and these patients were referred for additional surgery, including lymph node dissection.
Data collection and measured outcomes
Detailed data sheets on each participating patient were completed by the investigators and faxed to the independent data center. Information about endoscopic resection (e.g., endoscopic devices, endoscopes, and medications) was collected after the procedure. Data included patient characteristics (age and sex), diagnostic modality prior to endoscopic resection (with or without magnifying endoscopy), tumor characteristics (location, estimated size, type, and history of biopsy), institution, the experience of each colonoscopist (<11 or ≥11 years), fluid injected to form a SM cushion (sodium hyaluronate or others), type of power source used for electrical cutting and its setting, type of electrosurgical endoknife, type of insufflation gas (CO2 or air), lifting condition after SM injection (good or poor), completeness of the endoscopic resection (en bloc, piecemeal, or unresected), diagnostic modality for assessment of residual tumor after endoscopic resection (with or without magnifying endoscopy), procedure time (from the beginning of SM injection until lesion removal), adverse events (perforation, bleeding, and others), treatments administered for adverse events and their outcomes, histopathological diagnosis of the resected specimen (histological type, lymph-vascular involvement, and tumor involvement on the lateral and proximal margins) according to the Japanese classification of colorectal carcinoma [2], and additional therapy for incurable lesions.
Outcomes indicating technical difficulties included failure of en bloc resection and procedure time and adverse events included perforation and bleeding. The factors associated with each were also evaluated.
Definitions
ESD was defined as endoscopic dissection of a colorectal tumor using an electrosurgical endoknife, consisting of circumferential mucosal cutting and SM dissection or circumferential SM incision prior to EMR (CSI-EMR) [18], as it was difficult to distinguish whether CSI-EMR was initially planned prior to the procedure or was used to rescue a procedure which was difficult to complete. As ESD is intended for en bloc resection, a failed procedure was defined as failure of en bloc resection (i.e., piecemeal resection or incomplete procedure). Tumors were classified as being located on the colon (cecum, ascending, transverse colon, descending or sigmoid colon) or the rectum. Endoknives were classified into three categories (needle knife, IT knife, and scissors types), as well as with or without water-jet function. Tumors were classified into five categories, based on the Paris classification and models of tumor growth during the development of colorectal neoplasia [3, 19]. The five types were: (1) granular-type laterally spreading tumor (LST-G), (2) nongranular-type laterally spreading tumor (LST-NG), (3) protruding tumor, (4) recurrent tumor after endoscopic resection, and (5) unclassified. Lifting conditions after SM injection were assessed as good or poor [20]. Histopathological diagnoses were based on the Japanese classification and were re-classified according to the Vienna classification [21]. Low-grade adenomas according to the Japanese classification were equivalent to noninvasive low-grade neoplasias according to the Vienna classification, whereas high-grade adenomas and intramucosal carcinomas according to the Japanese classification were equivalent to noninvasive high-grade neoplasias according to the Vienna classification. Based on the median number of ESDs performed at each institution during the study period (30 cases/3 years; i.e., 10 cases/year), institutions were classified as low (<30 lesions) and high (≥30 lesions) volume centers. Colonoscopists were classified as those who were less (<11 years) and more (≥11 years) experienced, because it takes at least 5 years to be a board-certified member of JGES and it is thought that it takes more 5 years to experience enough ESD cases. Procedure time >2 h was defined as long, because 30 % of the ESDs needs procedure time >2 h, and it can be said they are relatively difficult cases than average. Lesion size was classified as <40 and ≥40 mm. A bleeding episode was defined as bleeding resulting in (1) apparent hematochezia or melena after the procedure, (2) a ≥2-g/dL decrease in hemoglobin concentration, or (3) a blood transfusion (the decision for transfusion was left each institution’s criterion and 7.0 g/dL in hemoglobin concentration is generally accepted as a criterion for transfusion). Perforation was defined as a full-thickness defect of the colonic wall with visible peritoneal fat or the presence of extra-gastrointestinal air on X-ray or abdominal computed tomography. Although observation period for delayed adverse events was not defined, the patients were generally followed up for at least one year because the follow-up period of the original cohort study was one year. Therefore, we could collect the information about late adverse events for two to four weeks [22].
Sample size estimation and statistical analysis
This study was a retrospective exploratory factor analysis of a prospective cohort study. The cohort involved 1,845 colorectal neoplasms ≥20 mm in diameter. The lesions from patients who underwent ESD were included to this exploratory analysis. Multiple lesions in the same patient were counted as independent lesions.
All data were collected and analyzed at an independent data center. Continuous, parametric variables are reported as means (standard deviation (SD)) and nonparametric data as medians (interquartile range (IQR) or range). Categorical variables were reported as incidence or rates (%) and compared using the χ 2 test or Fisher’s exact test, as appropriate. Univariate and multivariate logistic regression analyses were performed to examine the factors associated with technical difficulty (failure of en bloc resection and procedure time, >2 h), whereas the number of adverse events (perforation and bleeding) was too small for multivariate analysis and only univariate analysis was done to examine the factors associated with adverse events. Variables with p values for association ≤0.2 on univariate analysis were considered potential risk factors in multivariate logistic regression analysis. All statistical analyses were performed using JMP version 10 (SAS Institute Inc, Cary, NC). All analysis were exploratory and P values were two-tailed, with p < 0.05 defined as statistically significant.
Results
Study design and baseline patient characteristics
The participants’ flow is shown in Fig. 1. Between October 2007 and December 2010, 1,845 colorectal neoplasms ≥20 mm in diameter were enrolled in the prospective cohort study. Of these, 816 lesions underwent colorectal ESD and were included in this analysis, and the remainder underwent conventional EMR.
Baseline patient characteristics are shown in Table 1. ESD procedures were performed at 15 of the 18 participating institutions, with a median of 30 lesions (IQR, 11–94 lesions) treated per center. The median lesion size was 35 mm (IQR, 28–47 mm). Almost two thirds of the lesions (64 %) were located in the colon. LST-G was the most frequent type (56 %), with 55 % of the lesions biopsied prior to ESD. Approximately 90 % of the lesions were removed by colorectal ESD at a high-volume center, with 65 % of these procedures performed by more experienced colonoscopists. One fourth of the lesions (25 %) showed poor lifting after SM injection.
Procedures for colorectal ESD
Almost all procedures used CO2 gas and sodium hyaluronate (Table 2). Various types of electrosurgical endoknives were used. Of the colorectal ESDs, 68 % required one electrosurgical endoknife, with the remaining 32 % requiring two or more. Endoknives with water-jet function were used to remove 29 % of the colorectal ESDs, with only 5 % requiring an endoscopic snare. Most of the colonoscopists (86 %) preferred to use a thin endoscope (thin caliber colonoscope or gastroscope). A gastroscope was used in 25 % of the procedures, whereas endoscopes equipped with a water-jet function were utilized in 70 %.
Therapeutic outcomes
Therapeutic outcomes are shown in Table 3. The median procedure time was 78 min (IQR, 50–120 min). Procedure times were longer than 2 h for 30 % of the lesions and longer than 3 h for about 10 %. We found that 57 % of the lesions were noninvasive high-grade neoplasms, 24 % were noninvasive low-grade neoplasms, and 18 % were invasive adenocarcinomas, including 7 % that were unexpectedly deep (≥1,000 μm) invasive SM cancers. These latter tumors were regarded as incurable by endoscopic local resection and were referred for additional surgery. Almost all the tumors (94 %) were resected en bloc, with 6 % requiring piecemeal resection or surgical colectomy.
Perforation occurred in 17 patients (2.1 %). Although most perforations were treated endoscopically using endoclips without surgical intervention, one required emergency surgery. Bleeding occurred in 20 patients (2.2 %), with most (19 patients) being postoperative. One patient with severe uncontrolled intraoperative bleeding required emergency surgery. There were no fatal adverse events.
Factors associated with difficulty and adverse events of colorectal ESD
Tables 4, 5, 6, and 7 show the results of univariate and multivariate analyses of factors associated with technical difficulties (failure of en bloc resection and long procedure time) and adverse events (perforation and bleeding). Univariate analysis showed that protruding type tumor, low-volume center (<30 neoplasms), lack of sodium hyaluronate use, snare use, poor lifting after SM injection, noninvasive high-grade dysplasia and deeply invasive carcinoma (≥1,000SMμm) were possible risk factors associated with failure of en bloc resection. Multivariate analysis showed that low-volume center, snare use, and poor lifting after SM injection were independent risk factors associated with failure of en bloc resection (Table 4). Factors associated with long procedure time (≥2 h) on univariate analysis included large tumor size (≥4 cm), colonic lesion, LST-NG, protruding-type tumor, low-volume center (<30 lesions), less-experienced endoscopist, CO2 use, use of two or more electrosurgical endoknives, snare use, noninvasive high-grade neoplasm and deeply invasive carcinoma (≥1,000 μm). On multivariate analysis, large tumor size, low-volume center, less-experienced endoscopist, CO2 use, and use of two or more electrosurgical endoknives were independent risk factors for long procedure time (Table 5).Univariate analysis showed that poor lifting after SM injection was the only risk factor associated with perforation (Table 6). Factors associated with bleeding on univariate analysis included rectal lesions and lack of thin-type endoscope (Table 7).
Poor lifting after SM injection occurred more frequently in LST-NG and in protruding and recurrent lesions than in LST-G, with the incidence of poor lifting after SM injection being extremely high (80 %) for recurrent lesions, although the incidence of poor lifting was not related to history of biopsy (Table 8).
Discussion
We found that colorectal ESD yielded satisfactory outcomes in this prospective cohort treated at several participating institutions with various levels of experience. Acceptable outcomes of colorectal ESD have also been reported in western countries, but improvements are needed because of its technical difficulties [23]. Adverse events such as perforation and bleeding [13] have been reported, as have failure of en bloc resection and long procedure time. We therefore assessed factors independently associated with these technical difficulties and adverse events.
We found that poor lifting after SM injection was independently associated with failure of en bloc resection and with increased perforation. Poor lifting after SM injection is thought to be associated with fibrosis in the SM layer. In single center trials, fibrosis was reported related to failure of en bloc resection and perforation [20]; and tumor size and the presence of fibrosis were found to be independent risk factors for perforation [24, 25]. Although a multicenter trial showed that only large tumor size and performance of the procedure at a low-volume institution were risk factors for perforation and postoperative bleeding, that trial did not assess lifting condition or fibrosis [13]. Our finding, that poor lifting was a significant risk factor for failure of en bloc resection and adverse events, was similar to the results of these earlier trials. The causes of fibrosis are not completely known, but we frequently observed the lesions with poor lifting in LST-NG and in protruding and recurrent lesions. These findings suggest that endoscopists in low-volume centers should start by performing colorectal ESDs on LST-G lesions.
We also found that performance of ESD at a low-volume institution was an important risk factor for failure of en bloc resection and long procedure time. Similarly, another study reported that the total number of ESDs performed per institution was inversely associated with the incidence of adverse events [13]. In this study, institutions performing fewer than ten colorectal ESDs per year were regarded as low-volume centers and these institutions should be selective in performing colorectal ESD. Unfortunately, we could not collect the colorectal ESD volume of each colonoscopist and we had to assess the experience of ESD by each institution, not by each colonoscopist. However, since colorectal ESD is a technically challenging and relatively rare procedure, we expect that within each institution such cases are performed by or with the assistance of the most experienced ESD operator whenever possible. Therefore, we believe that the institutional experience with colorectal ESD is an accurate and adequate surrogate marker for the colonoscpists’ ESD experience.
The mechanisms by which rectal location and lack of use of a thin-type endoscope enhance bleeding are unclear. Lesion location in the colon has been reported to be a significant risk factor for delayed bleeding following colonic EMR for large lesions [26]. By contrast, we found that the risk of bleeding was lower for lesions in the colon than the rectum. Differences between the two studies may be due to differences in the resection method (EMR vs. ESD), the race or ethnic background of the patients, and/or lesion characteristics. However, we found that the incidence of bleeding after endoscopic resection for large superficial colorectal tumors was lower (2.2 %) than previously reported (7 %) [26]. This difference may have been due to post-ESD coagulation (PEC), which uses a coagulation forceps to prevent bleeding by visible blood vessels in the resection area [27]. As PEC is not usually performed after conventional EMR, it may explain the reduced bleeding rate after ESD and the different characteristics of bleeding after EMR and ESD. Additionally, thin-type endoscopes are flexible, making it easier for them to access any part of mucosal defects after colorectal ESD. Use of these endoscopes would better detect visible vessels on the mucosal defect after colorectal ESD, resulting in a reduction in bleeding rate due to easier coagulation. Moreover, CO2 and multiple endoknives use were also independent risk factors for longer ESD procedure time in our study, although CO2 was reported to reduce the procedure time of colorectal ESD and endoknives are generally used to make the procedure easier [28]. We suppose these are not causative factors but rather than the opposite, symptoms of an expected complicated clinical situation. That is, the endoscopists may tend to use CO2 or multiple endoknives for especially difficult cases.
Although our prospectively collected large sample size was one of the strengths of this trial, our results may have been limited by selection bias. Our study subjects consisted of consecutive patients who underwent endoscopic resection in a prospective cohort trial, but more than half the subjects screened underwent EMR [16, 17]. Flat-type lesions, rectal lesions, and SM cancers were more frequently removed by ESD than by EMR. Additionally, the mean lesion size was larger in the ESD than in the EMR group (39.4 vs. 26.4 mm). Despite any possible selection bias, however, the en bloc resection rate was greater for ESD than for conventional EMR (94.5 vs. 56.9 %). Moreover, of the 816 lesions removed by ESD, 140 (17.2 %) showed unfavorable results (failure of en bloc resection, perforation, bleeding or operation time longer than 2 h), indicating that colorectal ESD remains difficult even after selection. Although the necessity of en bloc resection for colorectal neoplasm is controversial, especially in western countries, en bloc resection is superior to piecemeal resection in eliminating residual tumor and for accurate histopathological assessment of the resected specimen [4]. We found that 18 % of the enrolled lesions were invasive adenocarcinomas, including 7 % that were unexpectedly deep (≥1,000 μm) invasive SM cancers. Examination of a single resected specimen would be more accurate in assessing lymphovascular involvement and depth of tumor invasion. Moreover, no one knows long-term outcomes after piecemeal resection for large colorectal tumor so far. We believe that endoscopists should therefore attempt to resect these lesions en bloc, with the information about these technical difficulties being valuable for training endoscopists and for selecting patients at less experienced institutions for colorectal ESD. Additionally, we cannot exclude the possibility that as yet unknown associated factors may have been omitted from multivariate analysis, despite our careful selection of variables. Moreover, we could not assess the effect of lesion location on technical difficulty, although colorectal ESD is considered more technically challenging in certain locations (e.g., transverse colon and flextures). Thus, such opinion is not generally established.
In conclusion, we found that the outcomes of colorectal ESD in a large cohort of patients at participating institutions with various levels of experience were satisfactory. We found that poor lifting after SM injection was the most frequent risk factor for technical difficulty and adverse events. The lesions with poor lifting were frequently observed in LST-NG and in protruding and recurrent lesions. These findings suggest that less experienced endoscopists should start by performing colorectal ESDs on LST-G lesions.
References
Soetikno RM, Gotoda T, Nakanishi Y et al (2003) Endoscopic mucosal resection. Gastrointest Endosc 57:567–579
Japanese Society for Cancer of the Colon and Rectum (2009) Japanese classification of colorectal carcinoma. Second English Edition. Tokyo: Kanehara & Co Ltd
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58:S3–S43
Iishi H, Tatsuta M, Iseki K et al (2000) Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc 51:697–700
Moss A, Bourke MJ, Williams SJ et al (2011) Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 140:1909–1918
Terasaki M, Tanaka S, Oka S et al (2012) Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol 27:734–740
Hotta K, Fujii T, Saito Y et al (2009) Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis 24:225–230
Saito Y, Kawano H, Takeuchi Y et al (2012) Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc 24(Suppl 1):67–72
Uraoka T, Parra-Blanco A, Yahagi N (2012) Colorectal endoscopic submucosal dissection in Japan and Western countries. Dig Endosc 24:80–83
Fujishiro M, Yahagi N, Kakushima N et al (2007) Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 5:678–683
Lee EJ, Lee JB, Lee SH et al (2013) Endoscopic submucosal dissection for colorectal tumors–1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc 27:31–39
Saito Y, Uraoka T, Yamaguchi Y et al (2010) A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72:1217–1225
Nakajima T, Saito Y, Tanaka S et al (2013) Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 27:3262–3270
Oka S, Tanaka S, Saito Y et al. Multicenter prospective study on local recurrence after endoscopic resection of large colorectal neoplasia conducted by the colorectal endoscopic resection standardization implementation working group of Japanese society for cancer of the colon and rectum. Endoscopy 2012;44:A70 [abstract]
von Elm E, Altman DG, Egger M et al (2007) STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Tanaka S, Oka S, Chayama K (2008) Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 43:641–651
Tanaka S, Terasaki M, Kanao H et al (2012) Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc 24(Suppl 1):73–79
Moss A, Bourke MJ, Tran K et al (2010) Lesion isolation by circumferential submucosal incision prior to endoscopic mucosal resection (CSI-EMR) substantially improves en bloc resection rates for 40-mm colonic lesions. Endoscopy 42:400–404
Kudo S, Lambert R, Allen JI et al (2008) Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 68:S3–S47
Matsumoto A, Tanaka S, Oba S et al (2010) Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol 45:1329–1337
Schlemper RJ, Riddell RH, Kato Y et al (2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47:251–255
Cotton PB, Eisen GM, Aabakken L et al (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 71:446–454
Probst A, Golger D, Anthuber M et al (2012) Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy 44:660–667
Kim ES, Cho KB, Park KS et al (2011) Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy 43:573–578
Lee EJ, Lee JB, Choi YS et al (2012) Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc 26:1587–1594
Metz AJ, Bourke MJ, Moss A et al (2011) Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy 43:506–511
Takizawa K, Oda I, Gotoda T et al (2008) Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 40:179–183
Saito Y, Uraoka T, Matsuda T et al (2007) A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc 65:537–542
Acknowledgments
The authors would like to thank the members of the Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) for valuable discussions. This study was financially supported by a grant-in-aid from JSCCR.
Conflict of interests
None of the authors has any financial relationships to disclose relevant to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeuchi, Y., Iishi, H., Tanaka, S. et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis 29, 1275–1284 (2014). https://doi.org/10.1007/s00384-014-1947-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-014-1947-2