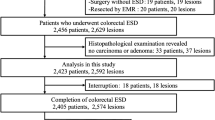
Abstract
Background
The possible risk of colonic perforation during endoscopic submucosal dissection (ESD) for colorectal tumors is a barrier to wide application. This retrospective study was performed to evaluate the risk and the predictive factors for perforation during ESD procedure.
Methods
Between October 2006 and November 2010, a total of 499 consecutive patients (mean age 60.0 ± 11.3 years) who underwent ESD for large-sized (≥20 mm), nonpedunculated colorectal tumor were analyzed. First, incidence rate and clinical course of perforation were evaluated. Second, patient-related variables (age, sex, history of aspirin or antiplatelet agents, and comorbidity), endoscopic variables (tumor size, location, and type), procedure-related variables (experience of procedures, procedure time, and materials of submucosal injection), and pathologic diagnosis were analyzed.
Results
The mean size of the lesions was 28.9 mm. The overall en bloc resection rate was 95.0%. Perforation occurred in 37 out of 499 patients (7.4%). Thirty-four patients could be successfully treated conservatively. The type (laterally spreading tumor) and the location (right-sided colon) of the tumors, less experience of the procedure (<100 cases) in each endoscopist, and submucosal injection without hyaluronic acid were associated with higher frequency of perforation (all P < 0.05). On multivariate analysis, laterally spreading type of tumor [odds ratio (OR) 4.10, 95% confidence interval (CI) 1.17–14.34] and submucosal injection with hyaluronic acid (OR 0.31, 95% CI 0.13–0.72) were independent predictive factors.
Conclusions
Perforation rate was 7.4%, and most cases could be successfully managed nonsurgically. In case of laterally spreading type of tumor, more caution is needed during submucosal dissection and long-lasting submucosal cushion is important for preventing perforation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, endoscopic submucosal dissection (ESD) has emerged as a curative treatment for early gastrointestinal tumors due to its capacity to resect tumors regardless of size. However, when compared with gastric ESD, ESD of colorectal tumors faces more barriers for wide application. Perforation is one of the most serious concerns of colorectal ESD, because peritonitis caused by colonic perforation and secondary contamination by colonic bacteria and feces can be more severe than peritonitis caused by gastric perforation. Moreover, the risk of perforation is higher in colorectal ESD because of the thin colonic wall and tortuous structure of the colon. Based on data from previous studies, the perforation rate for colorectal ESD is 1.4–10.0% [1–5].
Some reports suggest that larger-sized lesions, fibrosis, colonic location, and less experience performing ESD could be risk factors for perforation during ESD of colorectal tumors [4, 6, 7]. When such factors are combined, the risks of incomplete resection and perforation are thought to increase substantially [8]. Also, the rate of colonic perforation is reported to decrease with increased experience of the endoscopist [5, 6, 9].
There have been few studies using multivariate analyses of clinical parameters including patient- and procedure-related variables and pathologic parameters in relationship to perforation. This study was performed to evaluate the risk and predictive factors of perforation during ESD procedures of colorectal tumors.
Patients and methods
Patients and preparations
Between October 2006 and November 2010, a total of 499 consecutive patients who underwent ESD for large-sized (≥20 mm) colorectal tumors were analyzed. We excluded colorectal tumors with stalk or endoscopic findings such as hardness, ulceration, friability, and spontaneous bleeding, which are suggestive of massive submucosal invasion. The ESD procedures were performed by three colonoscopists (E.-J.L., E.G.Y., and J.B.L.) who have individually performed more than 1,000 diagnostic colonoscopies annually and are highly experienced in the therapeutic procedures. Before and during this study period, they visited medical centers in Japan and introduced the latest technical methods for ESD procedure. We arbitrarily defined “experienced” as having successfully completed at least 100 ESD procedures. This benchmark was chosen after pinpointing the cutoff value where the colonoscopist’s experience began showing a drastic reduction in the occurrence of perforation.
Adequate cleansing of the whole colorectum was conducted before performing endoscopic treatment, and the patients were restricted to a low-fiber diet the day before treatment. The patients ingested 90 ml oral sodium phosphate solution (Fleet Phospho-soda®; C.B. Fleet Company, Lynchburg, USA) or 4 l polyethylene glycol solution (Colyte Powder®; Taejoon Pharm, Seoul, Korea) before each procedure to achieve good bowel preparation. We chose each solution according to the patient’s age and their hepatic and renal functions.
A history of using aspirin or antiplatelet agents was recorded, and all applicable patients were asked to stop their medication at least 7 days prior to the ESD procedure. Comorbidity included hypertension, diabetes, cardiovascular disease, or dyslipidemia, and multiple comorbidity was defined as having more than one of the former diseases.
ESD procedure
The ESD procedures were carried out using a single-channel HD colonoscope (Olympus CF-H260AI; Olympus Optical Co., Tokyo, Japan) and a high-frequency generator with an automatically controlled system (VIO300). A transparent attachment (D-201-13404 or D-201-14304; Olympus Optical Co., Ltd., Tokyo, Japan) was fixed to the tip of the endoscope to provide a constant endoscopic view and to apply tension to the connective tissue for submucosal dissection. A CO2 insufflation system was applied to reduce any patient discomfort, particularly in the right colon by promoting the absorption of leaked air from perforation.
At first, we used a mixed solution of normal saline, epinephrine, and indigo carmine dye (Carmine; Korea United Pharma, Inc., Seoul, Korea). Next, glycerol (cerol injection; Choongwae Pharma Co., Seoul, Korea) and hyaluronic acid (1:3 sodium hyaluronate injection; Huons, Seoul, Korea) were introduced instead of saline to acquire higher and longer lifting of the submucosal layer (Fig. 1).
After the tumor was lifted from the muscle layer, a mucosal incision was made with the tip of a flex knife (Olympus KD-630L). We adjusted the tip of the flex knife to a length of only 1–2 mm and gently pressed onto the mucosa to produce a cutting effect, using the endocut I mode. The distal third of the mucosa was incised first, and submucosal trimming was performed for the introduction of the cap between the tumor and the muscle layer. After trimming, dissection of the submucosal layer with a flex knife using the forced coagulation mode was completed. A hook knife (Olympus KD-620LR) was used for cases with difficulties in further dissection and high risk of muscular injury. The patient’s position was adjusted in order to allow gravity to reposition the dissected tumor downwards. To control bleeding, hemostatic forceps, such as a Coagrasper (Olympus FD-410LR), were used in soft coagulation mode (output 80 W). An additional mucosal incision to the proximal part of the colon was then performed, and further submucosal dissection was carried out. To prevent leakage of the submucosal solution, circumferential mucosal incisions were staggered with submucosal dissection until the tumor was completely resected. After complete resection of the tumor, visible vessels in the exposed layer were treated with a Coagrasper in soft coagulation mode. All procedures were carried out in the inpatient setting, and the length of the hospital stay was 2–3 days unless immediate complications occurred.
Perforation was diagnosed when mesenteric fat or the intraabdominal space was directly observed during the procedure (frank perforation). Although visible colonic wall defects were not present during ESD, any free air could be detected through routine postprocedure radiologic examinations such as plain chest X-rays, simple abdominal X-rays, or computed tomographies (microperforation).
Histopathologic evaluation
All specimens were evaluated after being cut into 2–2.5-mm slices and examined microscopically for histological type, classified in accordance with the Vienna classification [10]. Laterally spreading tumors (LST) were defined as lesions with a low vertical axis extending laterally along the interior luminal wall. En bloc resection was defined as a resection of one single piece of tumor, regardless of pathologic-free margins. The tumor locations were divided as follows: (1) cecum or ascending colon, (2) transverse colon, (3) descending colon, (4) sigmoid colon, and (5) rectum. The tumor locations were then grouped into right colon (cecum, ascending and transverse colon), left colon (descending and sigmoid colon), and rectum for logistic regression analyses.
Statistical analysis
Patient-related variables (age, sex, history of aspirin or antiplatelet agents, and comorbidity), endoscopic variables (tumor size, location, and macroscopic type), procedure-related variables (ESD experience, procedure time, and materials of submucosal injection), and pathologic diagnosis were analyzed as potential risk factors for colon perforation. All of the continuous variables are presented as mean and standard deviation. In univariate analysis, a χ2 test was used for comparisons between categorical variables, and an independent t-test was used for comparisons between continuous variables. All calculations were conducted using the SPSS statistical software package (SPSS version 15.0; Chicago, IL, USA). In multivariate analysis, a logistic regression model was used. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated for predicting the relative risk of perforation. P-values less than 0.05 were considered statistically significant.
Ethics
The study protocol was approved by the Institutional Review Board at Daehang Hospital, and informed written consent from all patients for each specific colonoscopic treatment and all scheduled follow-up examinations were received by clinicians and repeated by specially trained nurses.
Results
Characteristics of the patients and the tumors
The mean age of the patients was 60.0 years (male:female = 293:206). A total of 91 patients were taking aspirin or antiplatelet agents for cardio- or cerebrovascular disease. In all, 78 patients had multiple comorbidities. The mean size of the lesions was 28.9 mm. The most common location of the tumors was the cecum or ascending colon (n = 161), followed by the sigmoid colon. Morphologically, LSTs (358/499) were more common than non-LSTs (0–I). Pathologically, mucosal cancer was observed in 30.5% of cases (152/499), and submucosal cancer was observed in 11.9% of cases (59/499). Hospital stay was longer for patients with perforation-based complications compared with patients without perforations (5.6 vs. 3.5 days) (Table 1).
Outcome of ESD (Table 1)
The overall en bloc resection rate was 95.0% (474/499). The procedure time was shorter for patients without perforation (60.3 vs. 75.1 min). Perforation occurred in 37 out of 499 patients (7.4%). Forty-one patients with perforations were successfully treated nonsurgically with a combination of endoscopic clipping (Fig. 2), fasting, and broad-spectrum antibiotics; 3 patients among these 37 did not complain of any subjective symptoms after perforation, and for these patients, perforations were detected only by routine radiologic examination (Fig. 3). Only three patients underwent emergency surgery due to frank perforation during ESD. In these exceptional cases, the perforated colonic areas were too large to be directly repaired with endoscopic clipping (Fig. 4).
Successful clipping. A During ESD on the ascending colon, the muscle layer was mistaken as submucosal fibrosis. B The muscle layer was cut with a flex knife, and the cut surface of the muscle layer was detected. C, D After applying four endoscopic clips, the patient recovered without severe complications
Risk factors of perforation
Univariate analysis
The type (laterally spreading tumor) and the location (right colon) of the tumor, less experience with the ESD procedure (<100 cases), and submucosal injection with either saline or glycerol (without hyaluronic acid) were associated with higher frequency of perforation (P = 0.005, P = 0.008, P = 0.03, and P = 0.001, respectively). Age, sex, size of tumor, pathology, comorbidity, and use of aspirin or antiplatelet agents were not associated with higher frequency of perforation (Table 2).
Multivariate analysis
On multivariate logistic regression analysis, laterally spreading type of tumor (OR 4.10, 95% CI 1.17–14.34) and submucosal injection with hyaluronic acid (OR 0.31, 95% CI 0.13–0.72) were significant factors for predicting colonic perforation during ESD procedure. Procedure experience, tumor type, and tumor location were not independently related to perforation (Table 3).
Discussion
To date, en bloc resection of large and nonpedunculated colorectal tumors has been a challenge to therapeutic colonoscopists because precise pathological evaluation is difficult to achieve for piecemeal resection. For treating early gastric cancer, several clinical studies have been conducted to demonstrate the superiority of ESD over piecemeal endoscopic mucosal resection [4, 5, 11, 12]. However, colorectal ESD is not widely used around the world, and limited data regarding colorectal ESD have been collected. The reason is that ESD is a newer technique, which is not only difficult and time consuming to master but also carries an additional risk of perforation due to anatomical features of the colon (i.e., thinner wall and more tortuous structure when compared with the stomach). In addition, the paradoxical movement of the endoscope during ESD, due to the winding nature of the colorectum, may cause knife coagulation on the muscularis propria and perforation [13].
Similar to previous reports [1–5] from Japan, the rate of perforation was 7.4% in this study, and most cases of perforations could be successfully treated without operation, as reported in other previous studies [1, 14]. Although the rate of delayed perforation is reported to be 0.3–0.7% [4, 8, 15], there were no cases of delayed perforation in our study. Peritonitis secondary to colonic perforation following ESD can be more disastrous, particularly in delayed perforation, because such perforations are typically large in size and require emergency surgery.
There have been a few clinical studies focusing on the risk factors predicting perforation during ESD for colorectal tumors. Some reports suggested that large-sized lesions, fibrosis, colonic location (due to a thinner wall than in the rectum), and less experience performing ESDs might be risk factors for perforation during ESD [4, 6, 7, 16]. According to another report on perforations, there were no statistical differences regarding the location of the tumor, i.e., in the colon or in the rectum [17]. Isomoto et al. [8] reported that right-side colonic location and fibrosis were the significant contributors to incomplete resection and that perforation was associated with large tumor size (>30 mm) and presence of fibrosis. They also reported that, when the contributive factors for each were combined, the risks of incomplete resection and perforation increased substantially. Matsumoto et al. reported that, in cases of lesions with severe fibrosis, the rate of complete en bloc resection was low and the perforation rate was high even when ESD was performed by an experienced operator. However, clarification of the presence and extent of fibrosis before actual colorectal ESD was obviously impossible [18]. In fact, in our study, we could not find any contributor predicting presence of fibrosis. We did not include presence of fibrosis in this analysis since this study was designed to clarify a clinical predictive factor before performing ESD procedure. However, fibrosis encountered during ESD procedure can be an important factor for complete submucosal dissection without complication, hence further study to predict presence of fibrosis as well as risk of colonic perforation is necessary.
Tanaka et al. [5, 9] have previously reported that an increase in operator experience is associated with a reduction in the rate of perforation during ESD. In a recent novel multicenter study in Japan, Saito et al. [7] reported that less experience performing ESDs (fewer than 50 cases) was an independent risk factor for complications. In our study, the perforation rate was lower in cases where each operator’s skill seemed to be mature and experienced (≥100 cases) than immature and less experienced (<100 cases) (10.1% vs. 5.0%); however, this experience was not an independent risk factor on multivariate analysis. This insignificance associated with experience may be explained through various considerations. First, it was observed that, the more experienced the operators were, the larger the tumors were indicated. The mean size of tumors was 27.3 ± 8.7 cm in less-experienced (<100) cases and 30.4 ± 13.4 cm in experienced (≥100) cases (P < 0.01). Second, despite this insignificance on multivariate analysis, subgroup analysis demonstrated that experience was not a significant risk factor in small-sized tumors (<30 mm) but, on the other hand, a significant risk factor in large-sized tumors (≥30 mm). Third, techniques and devices for performing ESD developed over time, and they were introduced regardless of the number of cases. Therefore, we should acknowledge that experience performing ESDs can be a significant factor but a variety of factors exist that influence the perforation risk of the ESD procedure beyond the absolute numbers of cases. The indication of ESD according to the colonoscopist’s skill, appropriate application of ESD, and the choice of suitable devices in each case are important in preventing perforations.
Hyaluronic acid is a macromolecular polysaccharide composed of d-glucuronate and N-acetyl-glucosamine, which is physicochemically very water retentive and viscoelastic. Yamamoto et al. [19] reported that, when hyaluronic acid was injected into the submucosal layer during endoscopic mucosal resection (EMR), mucosal lesions could be adequately lifted for sufficient duration to allow safe, reliable, and complete resection without sodium hyaluronate diffusion or absorption by the submucosal layer. Some experimental studies have described the effectiveness of sodium hyaluronate for EMR [20, 21]. Fujishiro et al. [22] reported that a mixed solution of high-molecular-weight hyaluronic acid, glycerin, and sugar for treatment of gastrointestinal (GI) tumors gave far better results in comparison with the treatments used in previous studies. They recommended the mixed solution of hyaluronic acid for ESD of GI tumors, especially for difficult ESD cases, because hyaluronic acid creates a long-lasting and sufficiently thick submucosal fluid cushion. This thick submucosal fluid cushion sufficiently lifts up the lesion from the muscle layer and prevents perforation. However, to our best knowledge, no clinical studies have proven that long-lasting submucosal cushioning such as that provided by hyaluronic acid injection could lessen the risk of perforation during colorectal ESD significantly. Our study showed that submucosal injection with hyaluronic acid is an independent factor to prevent perforation during colorectal ESD. The significant decrease in the rate of perforation associated with use of hyaluronic acid may be the result of the following technique: After mucosal incision, we blindly dissected submucosal tissue, making a gap between the specimen and muscle layer to introduce a transparent hood. During this trimming step, a submucosal injection of hyaluronic acid was used to maintain sufficient thickening of the submucosal tissue to prevent perforation. Finally, a transparent hood is used to open up the incised mucosa as a substitute for countertraction. With this method, not only the lateral margin but also the vertical margin of the resection can also be precisely determined, as submucosal dissection is carried out under direct visualization of the submucosal tissue [23].
Our study has a few limitations. First, the potential protective effect of hyaluronic acid might be biased by a learning curve because it was introduced at a latter phase of the study, hence we carried out multivariate analysis of relatively large numbers of cases to overcome the bias of this retrospective study as far as possible. Second, there were three therapeutic colonoscopists involved. Our study could have been more authoritative if all procedures were performed by a single colonoscopist. However, the introduction of these procedures and the learning curve of the three colonoscopists were similar. Third, we did not define a clear-cut value for experienced cases (100 cases or more), although we attempted to determine a cutoff value for each colonoscopist’s cases statistically. Fourth, procedure time was excluded from this risk analysis because the time for managing the perforation was included in perforated cases. However, longer procedure time can be an important factor which affects the operator’s mental or physical exhaustion.
In conclusion, our perforation rate was 7.4%, and most cases could be successfully managed without operation. In the case of laterally spreading type of tumor, more cautious management is needed during submucosal dissection and long-lasting submucosal cushion such as that provided by hyaluronic acid injection is important for preventing perforation during ESD procedure.
References
Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T (2010) Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 24(2):343–352
Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K (2001) Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 54(1):62–66
Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K (2007) Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 39(5):418–422
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2007) Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 5(6):678–683 quiz 45
Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K (2007) Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 66(1):100–107
Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D (2007) Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc 66(5):966–973
Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D (2010) A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72(6):1217–1225
Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S (2009) Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 41(8):679–683
Tanaka S, Oka S, Chayama K (2008) Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 43(9):641–651
Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H (2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47(2):251–255
Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S, Ishikawa T, Saito D, Fujii T, Kaneko E, Yoshida S (2007) Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol 22(9):1409–1414
Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2006) Endoscopic submucosal dissection for rectal epithelial neoplasia. Endoscopy 38(5):493–497
Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS, Gossner L (2005) Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy 37(2):178–182
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2006) Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy 38(10):1001–1006
Toyanaga T, Man IM, Ivanov D, Sanuki T, Morita Y, Kutsumi H, Inokuchi H, Azuma T (2008) The results and limitations of endoscopic submucosal dissection for colorectal tumors. Acta Chir Iugosl 55(3):17–23
Jeong G, Lee JH, Yu MK, Moon W, Rhee PL, Paik SW, Rhee JC, Kim JJ (2006) Non-surgical management of microperforation induced by EMR of the stomach. Dig Liver Dis 38(8):605–608
Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N, Naito Y, Yanagisawa A, Yoshikawa T (2009) Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy 41(9):758–761
Matsumoto A, Tanaka S, Oba S, Kanao H, Oka S, Yoshihara M, Chayama K (2010) Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol 45(11):1329–1337
Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K (2002) Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc 56(4):507–512
Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, Kakushima N, Kobayashi K, Hashimoto T, Iguchi M, Shimizu Y, Ichinose M, Omata M (2004) Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 36(7):579–583
Fujishiro M, Yahagi N, Kashimura K, Matsuura T, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ichinose M, Omata M (2005) Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc 62(6):933–942
Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2006) Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 63(2):243–249
Kita H, Yamamoto H, Miyata T, Sunada K, Iwamoto M, Yano T, Yoshizawa M, Hanatsuka K, Arashiro M, Omata T, Sugano K (2007) Endoscopic submucosal dissection using sodium hyaluronate, a new technique for en bloc resection of a large superficial tumor in the colon. Inflammopharmacology 15(3):129–131
Disclosures
Authors Eun-Jung Lee, Jae Bum Lee, Yong Sung Choi, Suk Hee Lee, Doo Han Lee, Do Sun Kim, and Eui Gon Youk have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, EJ., Lee, J.B., Choi, Y.S. et al. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc 26, 1587–1594 (2012). https://doi.org/10.1007/s00464-011-2075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-2075-5