Abstract
Objective
To systematically evaluate the immune function in patients with colorectal cancer after laparoscopic surgery (LS) and conventional open surgery (OS).
Methods
PUBMED, EMBASE, and the Cochrane library were searched and randomized controlled trials (RCTs) comparing the immunological difference between LS and OS were included. Two authors extracted data and assessed trial quality.
Results
Eleven studies including 695 patients were analysed. Immune-competent cells demonstrated no significant differences between LS and OS in six trials. Eight trials assessed various perioperative plasma cytokine concentrations with no significant differences in interleukin-6 (IL-6) and C-reactive protein (CRP) levels between LS and OS. However, meta-analysis showed higher T suppressor lymphocytes (CD8+) counts on postoperative days (POD) 1–3 and lower plasma levels of CRP on POD 0–1 in LS group compared with OS group.
Conclusion
Although LS groups displayed higher T suppressor lymphocyte (CD8+) counts on postoperative days (POD) 1–3 and lower plasma levels of CRP on POD 0–1, there is no sufficient evidence to support superior preservation of global immune function with LS compared to OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed malignant disease and the fourth most common cause of cancer-related death worldwide [1, 2]. In 2008, an estimated 148,810 new CRC cases would occur, and during the same year, there were around 49,960 CRC deaths in the United States [3]. The number of new cases of CRC worldwide has been increasing rapidly since 1975 [4]. CRC remains a major public health threat, and economic burden due to this disease is enormous. World expenditures for CRC have been estimated in the range of $US14–22 billion per year (year 2000 values) [5].
Curative treatment of CRC relies principally upon surgical resection, supported by adjuvant chemotherapy, radiotherapy, and/or immunotherapy. Open abdominal surgery (OS) remains the predominant approach internationally; however, midline wound and peritoneal damage are associated with significant postoperative pain and longer hospital stay. Laparoscopic colorectal surgery involves inserting laparoscopic instruments through several ports in the abdominal wall to accomplish the same oncologic resection goals. The tumor is usually removed through an abdominal incision, the length of which depends on the size of the tumor. Hand-assisted laparoscopic surgery (LS) utilizes a somewhat larger incision and the surgeon’s hand to guide the dissection in the abdomen. LS is generally considered less invasive and has been consistently associated with a more rapid recovery, with earlier return of bowel function, reduction in pain, shorter hospital stay and better cosmesis [6, 7]. Since the first laparoscopic cholecystectomy was performed by Mouret in 1987, LS has created an explosion of interest [8]. The first laparoscopic colonic resection was performed by Jacobs in 1991 [9].
Surgery, whether conventional or laparoscopic, is a controlled trauma. Postoperative metabolic, inflammatory, and immunologic changes are relative to the degree of surgical trauma. Elimination or reduction of these changes has been shown to decrease the incidence of postoperative complications and to improve survival [10–14]. Surgery induces a generalized state of immunosuppression [10]. The extent and duration of postoperative immune suppression depend on the magnitude and type of the intraoperative injury [15]. Postoperative immune suppression may have considerable consequences; it has been shown to be related to infectious complications, the development of tumor metastases and abdominal implantation in animal studies [10, 16]. These postoperative immunologic changes seem to be particularly important in oncologic patients, because postoperative immunosupression may be responsible not only for postoperative infections but also for tumor spread and metastases.
In comparisons of laparoscopic and conventional surgery, significantly better preservation of the postoperative immune function was shown with laparoscopic cholecystectomy and Nissen fundoplication than with the conventional approach [17, 18]. The postoperative immunologic differences between laparoscopic and conventional CRC operation has not gained universal agreement, and remains controversial in clinical trials [19–23]. We, therefore, performed a systematic review and meta-analysis to evaluate whether there are immunologic advantages of laparoscopic compared to conventional CRC surgery.
Methods
Inclusion criteria
Only randomized controlled trials (RCTs) were included. All selected articles compared immune function changes in patients with CRC after LS and OS. Trials were excluded if they involved patients with benign diseases or included patients receiving adjuvant radiotherapy, chemotherapy or immunotherapy. The primary outcome measures were either assessments of immune-competent cell counts/function or plasma Inflammatory cytokine concentration, immunoglobulins, and postoperative infectious complications.
Search strategy
Two authors independently developed an electronic database search strategy to identify studies that met the eligibility criteria. Published and unpublished RCTs were identified by searching the PUBMED (1966 to March 2009), EMBASE (1950 to March 2009), the Cochrane Library (CENTRAL/Cochrane Central Register of Controlled Trials, Issue 1, 2009). The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. Searches were carried out using medical subject headings (MeSH) and free text words in combination with the search strategy for RCTs described by Cochrane Highly Sensitive Search Strategy for identifying randomized trials (2008 revision). The following search was adapted for each database: laparoscopy[MeSH], colorectal neoplasms[MeSH], immunology[MeSH], immunity[MeSH], monocyte[MeSH], lymphocyte[MeSH], cytokine[MeSH], immunoglobulin[MeSH], immunoprotein[MeSH], (neoplasm* OR cancer OR carcinoma OR tumor OR tumour OR adenocarcinoma) And (colorectal OR colon OR colonic OR rectal OR rectum OR sigmoid OR large intestine). Our searches were carried out without language restrictions.
Data extraction
Two authors independently scrutinized all articles and decided which trials were to be included. A data extraction form was developed and used to extract and record information on results of included studies. The following variables from each trial were extracted: author and year, participant selection criteria, patient baseline characteristics, generation of the allocation sequence, allocation concealment, blinding, number of randomized patients, number of patients excluded after randomization and reasons for this, dealing with drop outs, data analysis based on the “intention-to-treat” principle, immunological parameters and values. Results will be compared between reviewers. Any disagreement about data extraction was resolved by discussion among the authors.
Assessment of methodological quality
Two reviewers independently assessed the methodological quality of each trial in terms of generation of allocation sequence, allocation concealment, blinding, incomplete outcome data addressed, free of selecting reports, free of other bias. For each trial, we classified each quality component as “yes”, “no”, “unclear”; an answer of “Yes” indicates a low risk of bias, “No” indicates a high risk of bias, and “unclear” indicates an uncertain risk of bias, according to the Cochrane Handbook 5.0. In case of differences in opinion, a third reviewer was contacted.
Statistical analyses
When data were available for a pooled estimate of the impact of intervention, it was intended that meta-analyses would be conducted for direct comparisons. When data were not available for pooling, we performed a descriptive analysis. The software REVMAN 5.0, provided by Cochrane Collaboration, was used for statistical analysis. We calculated a weighted mean difference (WMD) or standard mean difference (SMD) between mean values for continuous variables with a 95% confidence interval (CI), and overall weighted estimate of the relative risk (RR) for dichotomous variables. We examined intervention effects by a fixed–effect model with the two-sided significance set at P < 0.05. We explored the presence of statistical heterogeneity by χ 2 test with significance set at P < 0.10 and measured the quantities of heterogeneity by I 2. For the continuous variable means with preoperative and postoperative level values, we calculated the changes. The mean changes were calculated as final values minus baseline values. The standard deviations (SD) of the mean changes (SDChange) were calculated according to the following formula: \( {\text{S}}{{\text{D}}_{\text{Change}}} = \sqrt {{{\text{SDpr}}{{\text{e}}^2} + {\text{SDpos}}{{\text{t}}^2} - 2p {\text{SDpre}} * {\text{SDpost}}}} \), where p is assumed to be moderately high with a correlation coefficient of 0.5 between pre- and post-intervention values. We performed subgroup analyses with postoperative day 0–1 (POD0-1), postoperative days 1–3 (POD1-3), and postoperative days 3–8 (POD3-8) according to the majority of studies that assessed their immunological outcomes within these periods. When two time points were investigated within one period, the later one was taken for the analysis.
Results
Description of studies
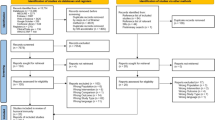
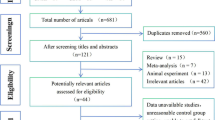
We identified 186 references through electronic searches. We excluded 170 irrelevant references, nonrandomized clinical studies, or observational studies. The remaining 16RCTs compared LS and OS on immune function in CRC patients. Four references included patients with benign disease and one reference only with abstract were excluded. Eleven references involving 695 patients were included in our analysis (see Fig. 1). Four studies were included in meta-analysis of immunological parameters, and seven trials were included in meta-analysis of postoperative infectious complications. All included studies were published as full articles. All studies included patients with CRC. All trials assessed several immunological parameters to describe the immunology change. The characteristics of the studies included in the present review are summarized in Table 1.
Risk of bias in included studies
Most of the studies had the same methodological problems: inadequate allocation sequence generation, inadequacy of allocation concealment. Three [24, 26, 28] of 11 trials described not only adequate randomization method but also adequate allocation concealment. Four studies [22, 25, 27, 29] only demonstrated that the allocation sequence was adequately generated, and the other four trials [19–21, 30] simply mentioned “randomized”. Because of the nature of the trials, all authors did not report blinding assessment. And the outcomes (objective immunological laboratory measurements) and the outcomes measurement were not likely to be influenced by lack of blinding, so there is low risk of blinding bias. All studies included in this analysis contain adequate descriptions of inclusion and exclusion criteria. Reasons for excluding patients after randomization are given in all the studies. In six studies [20, 21, 24, 28–30], no patients were excluded after randomization; there is a low risk of incomplete outcome data bias. In the study of Tang et al. [26], 62 of 223 patients were missed because of missing preoperative or postoperative measurement outcome. Although missing outcome data balanced in numbers and with similar reasons for missing across groups, we regarded it may have an unclear risk of incomplete outcome data bias due to the proportion of missing outcomes was 28% (62/223). The missing outcomes may be enough to have an impact on observed effect size. In Delgado et al.’s study [25], 30 of 69 patients were excluded because of technical problems in obtaining samples or converting to open surgery in laparoscopic group, and 13 of 71 patients were excluded because of technical problems obtaining samples in the open group. The missing data were large and not balanced between groups; we believe this creates a high risk of incomplete outcome data bias. In the other three studies [19, 22, 27], the missing data were few, and these studies were considered at low risk of incomplete outcome data bias. In all of trials, the outcomes were reported in detail. We considered all studies with a low risk of selective reporting bias. There was insufficient information to assess whether an important risk of bias exists in a number of trials; we argued all trials had unclear risk of other potential sources of bias. Four studies [21, 24, 26, 28] performed an intention-to-treat analysis. Patients who were intraoperatively converted to open resection were excluded from further analysis in three studies [19, 22, 25]. Only two studies [21, 26] calculated the sample size. The methodological quality of the included trials is summarized in Table 2.
Effects of interventions
Total lymphocytes counts
The circulating total lymphocyte counts were reported in three studies [28–30], and all were observed decrease after surgery. When compared with OS group, lymphocyte counts returned to normal sooner in LS group, but no significant statistical difference was demonstrated, except in the study of Zhao et al. [30], where the LS group was significantly higher.
T-lymphocytes (CD3+) and subsets
The T-lymphocytes and subset counts were reported in six studies [19, 20, 26, 28–30]. Leung et al. [28] showed a decrease from the preoperative counts in the total T cells (CD3+), helper T cells (CD4+), CD8+, NK-like T cells (CD3+, CD16+, and CD56+), and activated T-cell (CD3+ and HLA-Dr+) in all patients. However, there was no significant difference in the postoperative levels between LS and OS group. Subset analysis on POD8 showed a significantly reduced return of CD8+, NK-like T cells (CD3+, CD16+, and CD56+), and activated T-cell (CD3+ and HLA-Dr+) in the OS group. Similarly, Wu et al. [29] described reductions in CD4+, CD8+ cells out to POD4 in both groups. The ratio CD4+/CD8+ on POD1 also dropped; however, there was no significant difference between groups. Hewitt et al. [19] described a decrease in the percentage of lymphocytes (especially NK cells) and an increased CD4+/CD8+ ratio in all patients without significant difference between LS and OS groups. Zhao et al. [30] showed similar findings for CD4+, CD8+ and the CD4+/CD8+ ratio related to colectomy with no impact of technique. Conversely, Ordemann et al. [20] reported no postoperative changes in the numbers of lymphocyte CD4+, CD8+, and ratio CD4+/CD8+ with either the laparoscopic or the conventional open approach. Tang et al. [26] showed no significant difference in the ratio CD4+/CD8+ and percentage of lymphocytes between two groups on POD3. The pooled data on POD3 from the studies Wu et al. [29] and Zhao et al. [30] showed no significant change of postoperative circulating CD4+ counts (WMD 0.05, Fix 95% CI −0.03 to 0.13 × 106/ml, P = 0.20) between LS and OS groups. The data did suggest less of a reduction of postoperative circulating CD8+ counts (WMD 0.08, Fix 95% CI 0.04 to 0.13 × 106/ml, P = 0.0004) in the LS group compared to the OS group. However, there was no significant difference in the postoperative change of the CD4+/CD8+ ratio (WMD −0.10, Fix 95% CI −0.25 to 0.05, p = 0.20) between two groups (Fig. 2).
NK cell
Four studies [19, 26–28] reported the NK cell counts and function. Leung et al. [28] described suppression in NK cell function and drop in NK cell count postoperatively comparing to preoperative levels in both groups, but a comparison of the laparoscopic and open groups postoperatively showed no significant difference. Hasegawa et al. [27] reported that NK cell activity decreased slightly after surgery, and showed no significant difference when comparing LS with OS group. Tang et al. [26] reported the NK cell phagocytosis function was similar between the laparoscopic and open groups. Hewitt et al. [19] showed that NK cells decreased after surgery with no difference by technique.
B-cell
Only two studies [28, 29] reported the number and percentage of B cell; all showed no significant changes at all time periods.
Levels of HLA-DR expression
Three studies [19, 20, 29] reported the levels of HLA-DR expression on monocytes. All trials observed reduced expression after surgery in both groups, but levels of HLA-DR expression returned to normal sooner in the laparoscopic group compared to open group. Ordemann et al. [20] showed a significant increase on POD8 in LS group compared to OS group. The other two studies [19, 29] showed no significant difference between two groups.
IL-6
Eight studies [19–22, 24, 25, 27, 29] reported the IL-6 serum levels. All trials showed an increased level after surgery and a maximum peak at postoperative 2–4 h in all surgical groups. On POD0-1, when compared to OS groups, five studies [20, 21, 24, 25, 29] demonstrated a significant lower increase, and two studies [19, 27] described no significant differences, while Stage et al. [22] observed significantly higher increases in the laparoscopic group. All the eight studies did not observe significant differences between the two groups on POD1-8. The pooled data by Delgado et al. [25] and Wu et al. [29] showed a trend towards less of an increase in the serum level of IL-6, but there were no significant difference on POD0-1 (WMD −37.07, Fix 95% CI −77.66 to 3.52 pg/ml, P = 0.07) and POD1-3 (WMD −8.05, Fix 95% CI −17.24 to 1.14 pg/ml, P = 0.09) (Fig. 3)
CRP
Seven studies [21, 22, 24, 25, 27, 29, 30] reported the CRP serum levels. Each trial showed an increased level after surgery and a maximum peak on POD1-3 in every surgical group. When compared with open groups, two studies [21, 27] observed a significantly lower increase, while Stage et al. [22] demonstrated a significantly higher increase in laparoscopic groups on POD0-1. Four studies [21, 24, 25, 27] observed significantly lower increases, while Stage et al. [22] observed a significantly higher increase in the laparoscopic group on POD1-3. Schwenk et al. [21] observed a significant lower increase in laparoscopic group on POD3-8. Two studies [29, 30] observed no significant difference between groups after surgery on POD0-8. The pooled data by Delgado et al. [25] and Zhao et al. [30] showed a significantly a lower increase on POD0-1 (WMD −1.26, Fix 95% CI −2.49 to −0.03 mg/l, P = 0.05,) in the LS group compared to the OS group, and no significant difference on POD1-3 (WMD −0.16, Fix 95% CI −0.65 to 0.32 mg/l, P = 0.51) between groups (Fig. 4).
Immunoglobulin
Only two studies [26, 30] reported the serum immunoglobulin (G, M, A) levels after surgery. A postoperative decrease was observed, but no significant difference was shown between LS and OS groups, except in the work of Zhao et al. [30], where a higher level of IgM was observed in the LS group. We pooled the data on POD3 from the two studies, and no significant differences were shown in subgroups: IgG (SMD, Fix 95% CI 0.00 −0.26 to 0.27, P = 0.97), IgM (SMD 0.01, Fix 95% CI −0.26 to 0.27, P = 0.95). The IgA data were not pooled due to the heterogeneity I 2 = 97%.
Postoperative infectious complications
Seven studies [20, 21, 24–28] reported postoperative infectious complications (wound infection, urinary tract infection, pulmonary infection, intra-abdominal abscess). The pooled data showed significant less infection risk (RR 0.73 M-H, Fixed 95% CI 0.24 to 2.24, P = 0.008) in the LS group compared to the OS group (Fig. 5).
Discussion
In our current descriptive summary of results and limited meta-analysis, we found insufficient evidence to support the contention that laparoscopic resection for CRC preserved host immune function better than conventional open surgery, although there was a trend towards improvement. Major abdominal surgery clearly results in impairment of immunologic function, especially cell-mediated response, and this impact seems to correlate with the severity of injury [31, 32]. This review described very limited evidence of differences between OS and LS surgery. Leung et al. [28] described a higher T-lymphocyte subsets count on POD8. Zhao et al. [30] displayed a higher total lymphocytes count on POD7. Ordemann et al. [20] reported an elevated expression of HLA-DR on POD4 in LS group. However, clinical relevance of these isolated findings is likely very limited.
The inflammatory response is part of an effective host immune process, but hyperinflammation has been linked to immunosuppression [33]. Production of the proinflammatory cytokines IL-6, TNF-alpha, and CRP are felt to be accurate markers of the overall acute-phase response. Certain plasma cytokine levels are used to monitor the impact of surgical trauma [34]. In the present review, a majority of trials showed significantly lower peak levels for IL-6 and CRP (Table 1) after LS compared to open OS.
In hopes of defining clinical relevance better, we performed subgroup analysis (POD0-1, POD1-3 and POD3-8). These groupings were selected because they broadly fit differences in study design for the available trials, and clinically these time frames may represent differences in surgical response from initial insult to recovery. In subgroup POD0-1, T-lymphocyte and subsets, B-lymphocyte, NK cell counts and function, the levels of HLA-DR expression on monocytes failed to demonstrate a significant difference between LS and OS groups [19, 20, 22, 27–29]. The majority of trials identified a lower peak level for IL-6 [20, 21, 24, 25, 29] and CRP [21, 27] after laparoscopic resection, and some studies did not [19, 27, 29, 30]. The data became more contradictory for the other two subgroups (POD1-3 and POD3-8) (Table 1).
The advantage of a meta-analysis is that it provides a method for aggregating similar studies to determine the best estimate for the treatment effect [35]. Owing to the lack of uniformity of data presentation, only the four studies reporting mean ± SD were used in our meta-analysis of immunological parameters. Our analysis was limited by the fact that not all studies reported the same mediators, so we could only use two studies which greatly limited the overall effect assessment. Thus, we performed descriptive summary and limited meta-analyses. Our meta-analysis identified a higher level of CD8+ count on POD1-3, a lower level of CRP on POD0-1 in LS group, and no significant differences in circulating CD4+ counts, CD4+/CD8+ ratio, levels of IL-6, CRP and Ig (DM) on POD1-3 between the two groups. However, our analysis was underpowered because the full panel of immunologic parameters was only present in two of 11 studies.
Our review was confined to assessment of circulating peripheral immune cell numbers and the proinflammatory cytokines CRP, IL-6. We did not review other immunological parameters such as delayed-type hypersensitivity (DTH) response, T-lymphocytes Phagocytosis function and Tumor necrosis factor-alpha (TNF-α), due to the insufficient reporting of these parameters. Additionally, the local peritoneal immune responses are not defined as only Wu et al. [29] reported a higher IL-8 level in the peritoneal drain fluid (PDF) after LS was compared to OS. The impact of pneumoperitoneum and insufflation gases on the immune response was also not reviewed, which was not reported in the trials involved in our study.
The data clearly associate the degree of postoperative immunosuppression and the degree of surgical trauma, and by extension the risk of infectious complications. Our meta-analysis, confirmed a significantly lower risk of infection (RR 0.46 M-H, Fixed 95% CI 0.26 to 0.82, P = 0.008) (Fig. 5) with LS. We performed a sensitivity analysis excluding the Delgado et al. (2001) trial, whose weight is largest in the meta-analysis, and still the benefit remained (RR 0.51, 95% CI 0.27–0.96) with low heterogeneity (I 2 = 0%). Using random-effect models to assess overall effect, the result was also not reversed (RR 0.51 M-H, Random 95% CI 0.27 to 0.97, I 2 = 0%). Therefore, we concluded that LS was associated with a significant decrease in postoperative infectious complications.
Our systematic review analyzed 11 trials including 695 patients. This is a low number of patients. The selected trials varied widely in sample size, ranging from 16 to 223. Five of 11 trials included less than 50 subjects, and only one trial [26] included more than 100 subjects. Only two trials [21, 26] reported sample size estimation. We did not attempt to contact all the authors of published trials to clarify results that had not been shown but contributed to the outcomes of literature reviews. Hadhazy et al. [36] stated, “If the information has not been reported, it probably has not been done”. Liddle et al. [37] proposed that if an evaluation criterion was not addressed in an article, it would be safe to assume that the criterion had not been met.
The main areas of methodological quality that were consistently found to be deficient in the trials were the allocation sequence adequately generated, and the reporting of allocation concealment. Allocation concealment and blinding of the assessors have been considered to be the most relevant element in minimizing bias in RCTs [38, 39]. Effect estimates from trials with inadequate or unclear concealment of treatment allocation were on average 25% more beneficial than effect estimates from trials with adequate concealment of allocation [40]. In our review, only three [24, 26, 28] of 11 trials appeared to have adequately concealed treatment allocation, which may produce a high risk of selection bias. Because of the nature of the trials, it was impossible to perform an analysis in which the patients are blinded for the performed procedure. The outcome (objective immunological laboratory measurements) and the outcome measurement were not likely to be influenced by lack of blinding. Hence, not performing blind was not likely to produce performance bias. Participants who were randomized but subsequently found ineligible for inclusion need not always be considered as having missing outcome data (handbook 5.0). In this review, nine [19–22, 24, 27–30] studies had a low risk of incomplete data bias, while one trial [26] had an unclear bias, and the other trial [25] produced a high bias (Table 2). Another quality indicator is to use the “intention-to-treat principle” [41] for analysis in RCTs. In this review, we found that only four [21, 24, 26, 28] trials explicitly reported on an intention-to-treat analysis. In three studies [19, 22, 25], patients who were intraoperatively converted to open resection were excluded from further analysis, which failed to perform an intention-to-treat analysis. If RCTs are to provide unbiased assessments of treatment efficacy, investigators must apply the “intention-to-treat principle”. It is a key issue of external validity [42].
Conclusion
Whether LS for CRC produces a lower postoperative systemic immunosuppression and inflammatory response is difficult to confirm with the available data. This is particularly true when assessing the immunological data. There were trends in certain immune measures; however, laparoscopic colectomy is clearly associated with significant reductions in postoperative infections compared to conventional open surgery. It may be that the tools we are using are too imprecise or insensitive to small changes that produce this result. Alternatively, there may other yet-to-be-determined measures that are related to reductions in infection. Either way, we suggest that the literature would benefit from the consistent application of an agreed to set of immunological parameters, uniformity in sample size assessment and randomization, and uniformity of data presentation. Until these data are produced, it will be difficult to rely on biomarkers as surrogate measures for important outcomes such as morbidity, mortality, survival and recurrence.
References
Meyerhardt JA, Mayer RJ (2005) Systemic therapy for colorectal cancer. New Engl J Med 352(5):476–487
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Büchler MW (2005) Seminar: colorectal cancer. Lancet 365:153–165
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA: Cancer J Clin 58(2):71–96
Boyle P, Langman JS (2000) ABC of colorectal cancer: epidemiology. BMJ 321(7264):805–808
Redaelli A, Cranor CW, Okano GJ, Reese PR (2003) Screening, prevention and socioeconomic costs associated with the treatment of colorectal cancer. Pharmacoeconomics 21:1213–1238
Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J (2006) Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg 93(8):921–928
Schwenk W, Haase O, Neudecker J, Muller JM (2005) Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 3:31–45
Frazee RC, Roberts JW, Symmonds RE, Snyder SK, Hendricks JC, Smith RW, Custer MD (1994) A prospective randomized trial comparing open versus laparoscopic appendectomy. Ann Surg 219(6):725–728
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1(3):144–150
Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, Proud G, Taylor RM (1985) The influence of surgical operations on components of the human immune system. Br J Surg 72(10):771–776
Biffl WL, Moore EE, Moore FA, Peterson VM (1996) Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg 224(5):647–664
Weissman C (1990) The metabolic response to stress: an overview and update. Anesthesiology 73(2):308–327
Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC Jr (1990) Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg 77(2)
Wakefield CH, Carey PD, Foulds S, Monson JRT, Guillou PJ (1993) Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg 80(2):205–209
Allendorf JD, Bessler M, Whelan RL, Trokel M, Laird DA, Terry MB, Treat MR (1997) Postoperative immune function varies inversely with the degree of surgical trauma in a murine model. Surg Endosc 11(5):427
Allendorf JDF, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1999) Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc 13(3):233–235
Karayiannakis AJ, Makri GG, Mantzioka A, Karousos D, Karatzas G (1997) Systemic stress response after laparoscopic or open cholecystectomy: a randomized trial. Br J Surg 84(4):467–471
Sietses C, Wiezer MJ, Eijsbouts QAJ, Beelen RHJ, van Leeuwen AM, Von Blomberg BME, Meijer S, Cuesta MA (1999) A prospective randomized study of the systemic immune response after laparoscopic and conventional Nissen fundoplication. Surgery 126(1):5–9
Hewitt PM, Ip SM, Kwowk SPY, Somers SS, Li K, Leung KL, Lau WY, Li AKC (1998) Laparoscopic-assisted vs. open surgery for colorectal cancer: comparative study of immune effects. Dis Colon Rectum 41(7):901–909
Ordemann J, Jacobi CA, Schwenk W, Stosslein R, Muller JM (2001) Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc 15(6):600–608
Schwenk W, Jacobi C, Mansmann U, Bohm B, Muller JM (2000) Inflammatory response after laparoscopic and conventional colorectal resections—results of a prospective randomized trial. Langenbecks Arch Surg 385(1):2–9
Stage JG, Schulze S, Moller P, Overgaard H, Andersen M, Rebsdorf-Pedersen VB, Nielsen HJ (1997) Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg 84(3):391–396
Santoro E, Carlini M, Carboni F, Feroce A (1999) Colorectal carcinoma: laparoscopic versus traditional open surgery. A clinical trial. Hepatogastroenterology 46(26):900–904
Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, Lau WY (2000) Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg 231(4):506–511
Delgado S, Lacy AM, Filella X, Castells A, Garcia-Valdecasas JC, Pique JM, Momblan D, Visa J (2001) Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum 44(5):638–646
Tang CL, Eu KW, Tai BC, Soh JGS, Machin D, Seow-Choen F (2001) Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg 88(6):801–807
Hasegawa H, Kabeshima Y, Watanabe M, Yamamoto S, Kitajima M (2003) Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg Endosc 17(4):636–640
Leung KL, Tsang KS, Ng MH, Leung KJ, Lai PB, Lee JF, Lau WY (2003) Lymphocyte subsets and natural killer cell cytotoxicity after laparoscopically assisted resection of rectosigmoid carcinoma. Surg Endosc 17(8):1305–1310
Wu FPK, Sietses C, Von Blomberg BME, Van Leeuwen PAM, Meijer S, Cuesta MA (2003) Systemic and peritoneal inflammatory response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum 46(2):147–155
Zhao G, Xiao G, Huang MX, Long HK (2005) Effect of laparoscopic radical operation on systemic immunity in patients with colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 8(5):407–409
Lee SW, Whelan RL (2006) Immunologic and oncologic implications of laparoscopic surgery: what is the latest? Clinics Colon Rectal Surg 19(1):5–12
Cruickshank A, Fraser W, Burns H, Van Damme J, Shenkin A (1990) Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci 79:161–165
Braga M, Vignali A, Zuliani W, Radaelli G, Gianotti L, Martani C, Toussoun G, Di Carlo V (2002) Metabolic and functional results after laparoscopic colorectal surgery: a randomized, controlled trial. Dis Colon Rectum 45(8):1070–1077
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. New Engl J Med 340(6):448–454
Wirtitsch M, Wessner B, Spittler A, Roth E, Volk T, Bachmann L, Hiesmayr M (2007) Effect of different lipid emulsions on the immunological function in humans: a systematic review with meta-analysis. Clin Nutr 26(3):302–313
Hadhazy V, Ezzo J, Berman B (1999) How valuable is effort to contact authors to obtain missing data in systematic reviews. 7th Annual Cochrane Colloquium Abstracts, Rome
Liddle J, Williamson M, Irwig L (1996) Methods for evaluating research guideline evidence. Improving Health Care and Outcomes, NSW Health Department, Sydney, 11
McLeod RS, Wright JG, Solomon MJ, Hu X, Walters BC, Lossing A (1996) Randomized controlled trials in surgery: issues and problems. Surgery 119(5):483–486
Balk EM, Bonis PAL, Moskowitz H, Schmid CH, Ioannidis JPA, Wang C, Lau J (2002) Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA 287(22):2973–2982
Jüni P, Egger M (2002) Allocation concealment in clinical trials. J Am Med Assoc 288(19):2407–2408
Faller H (2004) Intention-to-treat. Rehabilitation (Stuttg) 43(1):52–55
Montori VM, Guyatt GH (2001) Intention-to-treat principle. Can Med Assoc J 165(10):1339–1341
Acknowledgements
First, we thank the patients who took part in this study and the investigators who designed and conducted the reviewed trials. We also thank Youping Li, Taixiang Wu and Guangjian Liu, of the Chinese Cochrane Centre, Chinese EBM Centre, West China Hospital, Sichuan University, for their expert assistance during the preparation of this review. The authors also thank Anthony J. Senagore (Division of Colon and Rectal Surgery/University of Southern California, Los Angeles, CA, USA) for suggestions and English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C., Liu, J. & Zhang, S. Laparoscopic versus conventional open surgery for immune function in patients with colorectal cancer. Int J Colorectal Dis 26, 1375–1385 (2011). https://doi.org/10.1007/s00384-011-1281-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1281-x