Abstract
Background: Laparoscopically assisted resection of colorectal carcinoma is technically feasible and minimally invasive. Postoperative immunosuppression also may be reduced. This study compared the lymphocyte subsets and natural killer (NK) cell cytotoxicity in patients after laparoscopically assisted resection with those after open resection of rectosigmoid carcinoma. Methods: In this study, 40 patients with rectosigmoid carcinoma, but no evidence of metastasis, were randomized to receive either laparoscopically assisted or conventional open resection of the tumor. Blood was collected before the operation, then 24 h, 72 h, and 8 days after the operation for studies of lymphocyte subsets and NK cell cytotoxicity. Results: The lymphocyte subsets and NK cell cytotoxicity of both groups showed typical suppression after surgery. The suppression of T cell activation and NK-like T cells was significantly less after laparoscopically assisted resection than in after open resection, whereas the difference in other lymphocyte subsets and NK cell cytotoxicity was not significant. Conclusion: This study showed that some cellular components of the immune system are less suppressed after laparoscopically assisted than after conventional open resection of rectosigmoid carcinoma. This may have implications for tumor recurrence and long-term patient survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With accumulation of experience and data, it has been shown that laparoscopically assisted colorectal surgery is less traumatic and allows earlier postoperative recovery than open surgery [9, 10]. However, its application in cases of malignancy is controversial because of the occurrence of port-site metastasis and the uncertain adequacy of tumor clearance [14], both of which affect the long-term result.
In cases of malignancy, immunosuppression induced by the disease and the surgery confers a growth advantage to micrometastases [15]. Nevertheless, the effect of the laparoscopic approach on the host immunity is uncertain. It is hoped that with reduced surgical trauma, the laparoscopic approach may help to preserve host immunity and improve survival.
This randomized study compared the lymphocyte subpopulation and natural killer (NK) cell cytotoxicity after laparoscopically assisted resection with those after conventional open resection in patients with rectosigmoid carcinoma. The clinical parameters were recorded as secondary end points.
Methods
Patient selection
From September 1993, patients with rectosigmoid carcinoma seen in our center were randomized to receive either laparoscopically assisted or conventional open sigmoid colectomy and anterior resection according to a computer-generated random sequence kept by an independent operating theater coordinator. From June 1998 onward, patients without metastatic disease who consented to extra blood taking were recruited in this study.
Operative procedure
Premedication and anesthetic techniques were standardized. Induction was by 3 to 5 mg/kg thiopentone together with 2 µg/kg fentanyl given intravenously. Vecuronium was given for muscle relaxation, and anesthesia was maintained by ventilation with an O2 and N2O mixture and isoflurane. The operations were performed by surgeons experienced in both laparoscopic and colorectal surgery. Our laparoscopic techniques have been described previously [9]. In principle, we mobilized the relevant segment of the bowel, than transected the lymphovascular pedicle, the distal bowel, and the mesorectum intracorporeally. A port wound was extended to deliver the specimen with the protection of a plastic bag. The division of the remaining mesentery, the marginal artery, and the bowel was performed extracorporeally. The anvil of a circular stapler was inserted into the proximal bowel, the gut put back into the peritoneal cavity, pneumoperitoneum reestablished, and intracorporeal anastomosis performed with the stapler.
Postoperative care and data collection
Postoperatively, the patient’s diet was resumed as soon as bowel function returned clinically. Pethidine 1 mg/kg was given every 4 h on demand. The patients were discharged home when fully ambulatory.
The following parameters were measured prospectively: demographic data, operation time, blood loss and transfusion, postoperative analgesic requirement, pain score on a visual analog scale, time first flatus passing and opening bowel, time to resumption normal diet, duration of hospital stay, morbidity, and mortality. The specimens were fixed unpinned and examined for margins of clearance and Dukes’ staging. All the patients were followed up regularly at 3-month intervals for clinical examination and carcinoembryonic antigen testing.
Blood taking and assay
Blood was taken preoperatively, at 24 h, 72 h, and 8 days after operation, then anticoagulated in either ethylene diaminetetraacetic acid (EDTA) for immunophenotyping or preservative-free heparin for cytotoxicity testing.
Immunophenotyping of lmphocyte subsets
Complete blood counts and differential counts of white blood cells (WBC) were determined using an automated hematology analyzer (STKS; Coulter, Miami, FL, USA). For immunophenotypic analysis, the WBC count was adjusted to less than 1 × 107/ml with blood group AB plasma, if necessary, before staining with fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated antibodies (ImmunoTech; Coulter). These included one set each of CD14-PE/CD45-FITC, isotypic control-PE/FITC/PECy5, CD3-FITC/CD16+56-PE/CD19-PECy5, CD3-PECy5/CD4-FITC/CD8-PE, CD3-PECy5/CD25-PE/HLADR-FITC, CD3-PECy5/CD16-FITC/CD25-PE, and CD3-PECy5/CD25-PE/CD57-FITC. Next, 20 ul of monoclonal antibody were added to and mixed with 100 ul of EDTA-anticoagulated blood. This then was allowed to stand in the dark for 30 min at room temperature. The no-wash lysed whole-blood technique with the ImmunoPrep Reagent Kit and the Q-Prep Immunology Workstation was used according to the manufacturer’s protocol (ImmunoTech; Coulter).
Before the real run of patient samples, quality control analysis by Flow Check and Flow Set (Coulter) was performed to ensure proper laser alignment and intensity of the Coulter Epic XL cytometer. At least 7,000 events were acquired using the System II software (Coulter) of the cytometer. Sequential gating for the lymphocyte population of more than 90% purity and recovery was performed using forward scatter versus side scatter, followed by CD45-FITC versus CD14-PE and forward scatter versus side scatter again. Simultaneous interference from debris and nonspecific antibody bindings was eliminated. Background fluorescence was kept at less than 2% using isotypic controls. On the basis of the lymphocyte population gated in previously, the mean lymphocyte subsets of T cells (CD3+), activated T cells (CD3+ and HLA-DR+), non–major histocompatibility complex (MHC) restricted NK cells (CD3−, CD16+, and CD56+ or CD3−, CD25+, and CD57+), MHC-restricted NK-like cells (CD3+, CD16+, and CD56+ or CD3+, CD25+, and CD57+), T-helpers (CD3+ and CD4+ or CD3+ and CD8−), T-cytotoxic suppressors (CD3+ and CD8+ or CD3+ and CD4−) were measured. Data were rejected and tests were rerun if the summed percentages of lymphocyte subsets were outside the confidence interval of 100% ± 5%.
Cytotoxicity test
Effector cell isolation
Heparinized blood was diluted with an equal volume of magnesium- and calcium-free Hanks’ Balanced Salt Solution (HBSS; Gibco BRL; Life Technologies, Gaithersburg, MD, USA) and underlaid with an equal volume of Ficoll-Hypaque of specific gravity 1.077 (Pharmacia Biotech AB, Uppsala, Sweden). After centrifugation at 400 g for 30 min at room temperature, the plasma was discarded. Mononuclear cells (MNC) were harvested and washed twice in HBSS and once in RPMI 1640 (Gibco BRL) supplemented with 10% fetal calf serum (FCS; Gibco, BRL). The MNC were allowed to pass through a 40-µm cell strainer (Falcon, Becton Dickinson, NJ, USA) to remove any cell aggregates and debris, and the cell count was adjusted to 1 × 107/ml.
Target cell preparation
The cell line of human chronic myelogenous leukemia, K-562 (ATCC, CCL 243), was maintained in RPMI 1640 supplemented with 10% FCS at 5 × 105/ml. Target cells of 106/ml in culture were labeled with 2.5 µg/ml D275 (Molecular Probes, Eugene, OR, USA) and incubated overnight in a humidified 37°C incubator at 5% carbon dioxide (CO2). Labeled cells were washed twice with HBSS and once with RPMI. The cell count was adjusted to 1 × 106/ml before cytotoxicity testing on the condition that more than 90% K562 target cells yielded bright D275 fluorescence.
Cytotoxicity assay by flow cytometry [2]
Effector (E) and target (T) cells were mixed in 12 × 75-mm polypropylene tubes to give a total volume of 1 ml at each E:T ratios of 50:1, 25:1, 12:1, 6:1, and 3:1 in triplicate. Controls of effector and target cells in triplicate also were run in parallel. Cells were spun at 240 g for 1 min, then incubated in a humidified 37°C incubator at 5% CO2. A final concentration of 1 µg/µl propidium iodide (Molecular Probes) was added to the culture 15 min before the end of the 4-h incubation.
Whereas D275-stained live target cells yielded greenish cytoplasmic fluorescence, dead target cells showed a dual fluorescence of greenish cytoplasm and red nucleus. Dead effectors were stained red, and live effectors exhibited no fluorescence. Cultures were resuspended, and the cell cytotoxicity was determined for each E:T ratio in triplicate by analyzing at least 100,000 per culture using the Coulter Epic XL cytometer. Signals were acquired using the System II software with a discriminator set at 3.51 on the linear scale of forward angle light scatter to exclude interference attributable to cell debris. Fluorescence emitted from D275-labeled K562 target cells was acquired by setting signal volts at 509 and signal gain at 1, whereas fluorescence emitted by propidium iodide-stained dead cells was detected by setting signal volts at 540 and signal gain at 1. Signals from debris and red blood cells of low forward scatter and side scatter were excluded. Sequential gating of forward scatter versus side scatter followed by detection of green versus red fluorescence was performed, and the signals were displayed in log scale in FL1 and FL3, respectively. The percentage of dead target cells was defined as the number of cells showing dual fluorescence in relation to the number of D275-stained cells.
Data processing
The specific lysis at each E:T ratio was calculated by deducing the spontaneous lysis (the control without effector cells) from the total lysis. The data then were used to approximate an exponential equation that specifically summarized the cytotoxic activity of each sample [16]. The cytotoxic activity finally was presented as a 20% lytic unit.
Statistics
Data were analyzed by intention to treat. The chi-square test was used to compare categorical data, Student’s t-est to compare parametric data, and the Mann–Whitney U test to compare nonparametric data. A p value of 0.05 was considered significant.
Results
From June 1998 to August 1999, 40 patients with rectosigmoid carcinoma suitable for laparoscopically assisted resection but no metastatic disease who consented to extra blood taking were recruited for this study. The two groups of patients (laparoscopic and open groups) had comparable demographic data (Table 1).
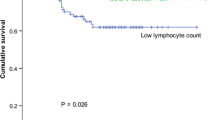
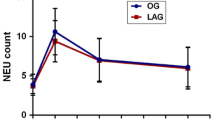
The lymphocyte subpopulation profiles and NK cell cytotoxicity are shown in Figs. 1,2,3,4,5,6,7,8,9,10. The preoperative cell counts of all the subpopulations and the NK cell function were comparable in the two groups. The typical changes after surgery also were observed [8]. An asterisk in the figures indicates a significant difference from the preoperative level. There was suppression in NK cell function; an increase in WBC count; a decrease in the total lymphocytes, total T cells (CD3+), helper T cells (CD3+ and CD4+), cytotoxic T cells (CD3+ and CD8+), NK cells (CD3−, CD16+, and CD56+), NK-like T cells (CD3+, CD16+, and CD56+); and T cell activation (CD3+ and HLA-Dr+).
A comparison of the laparoscopic and open groups postoperatively (a cross in the figure indicates a significant difference between the groups), showed no significant difference in NK cell cytotoxicity, WBC count, total lymphocytes, total T cells, helper T cells, total B cells, or NK cells at any time point. Suppression of NK-like T cells after laparoscopic surgery was significantly less on days 1 and 8. Summarizing the serial change with the area-under-curve method [11] also showed the difference to be significant (p = 0.015, by Mann–Whitney U test). The suppression of cytotoxic T cells also was significantly less in the laparoscopic group on day 8 (p = 0.04), but the difference between the two groups was shown to be marginally nonsignificant (p = 0.054, Mann–Whitney U test) when the serial change was summarized. The suppression of T cell activation was significantly less in the laparoscopic group on day 8, and the difference also was found to be significant (p = 0.045, Mann–Whitney U test) when the data were summarized.
The clinical outcome is shown in Table 2. The laparoscopic group had an earlier return of bowel function, was mobilized earlier, and required less analgesic. There was no operative mortality. Four patients in the laparoscopic group required conversion to open surgery. One patient in the open group had an anastomotic leak, which required reoperation and ileostomy. Another patient had an intraabdominal abscess, which was treated by aspiration under sonographic guidance. Up to the time of data analysis, there was no port-site or wound recurrence.
Discussion
The interaction between malignancy and host immunity has attracted attention for decades. The term “immunosurveillance” was put forward by Burnet and Thomas in the 1950s and 1960s, but supportive clinical evidence was lacking. Immunocompromised individuals have an increased incidence of virus-associated tumor, but not of other common cancers such as colon, lung, or breast tumor. In contrast, the incidence of rectal cancer and breast cancer was decreased in immunosuppressed cohorts [18, 19]. On the other hand, patients with malignancy have suppressed immunity, which may revert after removal of the tumor [6]. A persistent postoperative immunosuppressive state was associated with a higher probability of recurrence and a poor prognosis [12], although the causative relationship was uncertain. Many investigators have attempted to manipulate host immunity in an attempt to reduce recurrence and improve survival [3].
Surgical trauma itself is immunosuppressive [8] and it is believed generally that with cancer surgery, the additional immunosuppression resulting from the surgical trauma confers a growth advantage to micrometastases. This hypothesis seldom was tested before the 1990s because there was no alternative to conventional open resection. With the wide application of laparoscopic surgery, investigators have shown great interest in the impact of surgery on host immunity. Bruce et al. [1] showed that with cholecystectomy, NK cell numbers are depleted more by minilaparotomy than by the laparoscopic approach, whereas the NK cell function is not depressed. In a similar study [20], the same group also showed that, laparoscopy caused less reduction in the number of cells expressing T lymphocyte markers, activation markers, and NK cell markers. Da Costa et al. [4, 5] showed significantly more suppression of NK cell cytotoxicity, tumor growth, and metastases in mice that underwent laparotomy than in those that underwent laparoscopy.
In our previous study [7], we were not able to show any difference in immunosuppression between open and laparoscopic resection of colorectal cancer because of the small sample size. On reviewing the result, we found it appropriate to repeat the study with the following modifications: increased the sample size, extended recruitment criteria to increase the general applicability of the result, analysis of the data by intention to treat (i.e., difficult cases with conversion, complication, or transfusion should not be excluded). Although other factors such as narcotic consumption and carbon dioxide pneumoperitoneum are known to affect immunologic function, they are inherent properties of the specific surgical approach and should not be used as exclusion criteria.
In this study we showed that after laparoscopic resection of rectosigmoid cancer, patients required less analgesic and recovered earlier. The postoperative profiles of the lymphocyte subpopulation and NK cell cytotoxicity showed a trend similar to that in the open group and to the trend previously reported for conventional open surgery [8]. In addition, we showed that the magnitude of suppression of some components (i.e., NK-like T cell, T cell activation, and marginally, the cytotoxic T cell) was less after laparoscopic surgery. Because the cytotoxic T cell [13] and, more recently, the NK-like T cell [17] have been shown to play an important role in tumor immunosurveillance, the reduced suppression of these components may have a beneficial effect on tumor recurrence and patient survival.
We were unable to show any difference between the two study groups in NK cell depletion or cytotoxicity, which has been shown in cholecystectomy [1, 20] and in the animal model [4, 5]. Possible explanations suggest that resection of colorectal tumor is more complicated and causes more tissue trauma than cholecystectomy, resulting in no demonstrable immunologic benefit; the effect of tumor on NK cell depletion or cytotoxicity in real life may be different from that of benign diseases or an implanted tumor; the current group of patients by chance had more advanced diseases, as evidenced by the higher incidence of transfusion, procedure conversion, and complication than in our previous study, thus masking the immunologic benefit.
In conclusion, there is evidence that laparoscopic resection for rectosigmoid tumor preserves host immune function better than conventional open surgery because of less tissue trauma. Whether this will lead to a better clinical outcome can be determined only by the long-term results of prospective randomized trials.
References
DM Bruce CB Walker SD Heys NR Binnie DB Gough O Eremin (1997) ArticleTitleEffects of minimal access surgery on natural cytotoxicity. Br J Surg 84 29 Occurrence Handle10.1046/j.1365-2168.1997.02444.x Occurrence Handle9043442
L Chang GA Gusewitch DB Chritton JC Folz LK Lebeck SL Nehlsen-Cannarella (1993) ArticleTitleRapid-flow cytometric assay for the assessment of natural killer cell activity. J Immunol Methods 166 45–54 Occurrence Handle10.1016/0022-1759(93)90327-4 Occurrence Handle1:CAS:528:DyaK2cXmtlen Occurrence Handle8228287
U Chattopadhyay (1999) ArticleTitleTumour immunotherapy: developments and strategies. Immunol Today 20 480–482 Occurrence Handle10.1016/S0167-5699(99)01526-1 Occurrence Handle1:CAS:528:DyaK1MXntFGrs7g%3D Occurrence Handle10576778
ML Da Costa HP Redmond N Finnegan M Flynn DJ Bouchier-Hayes (1998) ArticleTitleLaparotomy and laparoscopy differentially accelerate experimental flank tumour growth. Br J Surg 85 1439–1442 Occurrence Handle10.1046/j.1365-2168.1998.00853.x Occurrence Handle1:STN:280:DyaK1cvltlaqtg%3D%3D Occurrence Handle9782033
ML Da Costa P Redmond DJ Bouchier-Hayes (1998) ArticleTitleThe effect of laparotomy and laparoscopy on the establishment of spontaneous tumor metastasis. Surgery 124 516–525 Occurrence Handle10.1067/msy.1998.89410 Occurrence Handle1:STN:280:DyaK1cvgvVWisw%3D%3D Occurrence Handle9736904
A Espi J Arenas E Garcia-Granero E Marti S Lledo (1996) ArticleTitleRelationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum 39 429–434 Occurrence Handle1:STN:280:ByiD3s3nsFA%3D Occurrence Handle8878504
PM Hewitt SM Ip SP Kwok SS Somers K Li KL Leung WY Lau AK Li (1998) ArticleTitleLaparoscopic assisted vs open surgery for colorectal cancer: comparative study of immune effects. Dis Colon Rectum 41 901–909 Occurrence Handle1:STN:280:DyaK1czktFGiug%3D%3D Occurrence Handle9678378
TW Lennard BK Shenton A Borzotta et al. (1985) ArticleTitleThe influence of surgical operations on components of the human immune system. Br J Surg 72 771–776 Occurrence Handle1:STN:280:BimD3c7itF0%3D Occurrence Handle2412626
KL Leung SP Kwok WY Lau et al. (1997) ArticleTitleLaparoscopic assisted resection of rectosigmoid carcinoma : immediate and medium-term results. Arch Surg 132 761–764 Occurrence Handle1:STN:280:ByiA2svktFc%3D Occurrence Handle9230862
KL Leung PB Lai RL Ho et al. (2000) ArticleTitleSystemic cytokine response after laparoscopic assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg 231 506–511 Occurrence Handle10.1097/00000658-200004000-00008 Occurrence Handle1:STN:280:DC%2BD3c3htlaitg%3D%3D Occurrence Handle10749610
JNS Matthews DG Altman MJ Campbell P Royston (1990) ArticleTitleAnalysis of serial measurements in medical research. BMJ 300 230–235 Occurrence Handle1:STN:280:By%2BC1c7mvFQ%3D
DC McMillan GD Fyffe HA Wotherspoon TG Cooke CS McArdle (1997) ArticleTitleProspective study of circulating T-lymphocyte subpopulations and disease progression in colorectal cancer. Dis Colon Rectum 40 1068–1071
CJ Melief WM Kast (1991) ArticleTitleCytotoxic T lymphocyte therapy of cancer and tumor escape mechanism. Semin Cancer Biol 2 347–354 Occurrence Handle1:STN:280:By2C38jgtlQ%3D Occurrence Handle1773050
V Paolucci B Schaeff M Schneider C Gutt (1999) ArticleTitleTumor seeding following laparoscopy: international survey. World J Surg 23 989–995 Occurrence Handle10.1007/s002689900613 Occurrence Handle1:STN:280:DyaK1MvksVWksw%3D%3D Occurrence Handle10512937
RE Pollock E Lotzova (1987) ArticleTitleSurgical-stress-related suppression of natural killer cell activity: a possible role in tumor metastasis. Nat Immun Cell Growth Regul 6 269–278 Occurrence Handle1:STN:280:BieB3c7ps1A%3D Occurrence Handle3329294
HF Pross MG Baines P Rubin P Shragge MS Patterson (1981) ArticleTitleSpontaneous human lymphocyte-mediated cytotoxicity against tumor target cells: IX. The quantitation of natural killer cell activity. J Clin Immunol 1 51–63 Occurrence Handle1:STN:280:Bi2C2c3ps1w%3D Occurrence Handle7334070
MJ Smyth KY Thia SE Street et al. (2000) ArticleTitleDifferential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 191 661–668 Occurrence Handle10.1084/jem.191.4.661 Occurrence Handle1:CAS:528:DC%2BD3cXhsVKqsL0%3D Occurrence Handle10684858
T Stewart R Henderson H Grayson G Opelz (1997) ArticleTitleReduced incidence of rectal cancer, compared to gastric and colonic cancer, in a population of 73,076 men and women chronically immunosuppressed. Clin Cancer Res 3 51–55 Occurrence Handle1:STN:280:DyaK1M%2FjsFamsA%3D%3D Occurrence Handle9815537
T Stewart SC Tsai H Grayson R Henderson G Opelz (1995) ArticleTitleIncidence of de novo breast cancer in women chronically immunosuppressed after organ transplantation. Lancet 346 796–798 Occurrence Handle1:STN:280:ByqH3crgslU%3D Occurrence Handle7674744
CB Walker DM Brace SD Heys DB Gough NR Binnie O Eremin (1999) ArticleTitleMinimal modulation of lymphocyte and natural killer cell subsets following minimal access surgery. Am J Surg 177 48–54 Occurrence Handle10.1016/S0002-9610(98)00290-6 Occurrence Handle1:STN:280:DyaK1M7lsV2isA%3D%3D Occurrence Handle10037308
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, K., Tsang, K., Ng, M. et al. Lymphocyte subsets and natural killer cell cytotoxicity after laparoscopically assisted resection of rectosigmoid carcinoma . Surg Endosc 17, 1305–1310 (2003). https://doi.org/10.1007/s00464-002-9172-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-002-9172-4