Abstract
Background
The aim of this study is to investigate the impact of arginine on immune function and postoperative complications in colorectal cancer (CRC) patients.
Methods
We conducted a comprehensive search to identify eligible RCTs in various databases, such as PubMed, Cochrane Library, EMBASE, Web of Science, MEDLINE, China National Knowledge Infrastructure (CNKI), Wanfang, VIP Medicine Information System (VIP), and Chinese Biomedical Database (CBM). This study aimed to examine IgA, IgG, and IgM levels as well as CD4+ and CD8+ counts as well as the CD4+/CD8+ ratio. Anastomotic leaking, length of stay (LOS), and surgical site infection (SSI) were included as secondary outcomes. Stata (StataCorp, version 14.0) was utilized for data analysis. To ensure the results were reliable, we used meta-regression, sensitivity analysis, and publication bias analysis.
Results
A total of 24 publications (including 1883 patients) out of 681 that were retrieved fulfilled the inclusion criteria. The arginine group showed notable improvements in humoral immunity, with gains in IgA (SMD=0.45, 95% CI: 0.30-0.60), IgG (SMD=0.80, 95% CI: 0.64-0.96), and IgM (SMD=0.66, 95% CI: 0.39-0.93). With regards to cellular immunity, the arginine group exhibited a substantial increase in the CD4+ T cell count (SMD = 1.03, 95% CI: 0.67-1.38) compared to the control group. However, the CD4+/CD8+ ratio decreased significantly (SMD=1.37, 95% CI: 0.88-1.86) in the same arginine group, indicating a change in the balance between these two cell types. Additionally, the CD8+ T cell count showed a notable decrease (SMD=-0.70, 95% CI: -1.09 to -0.32) in the arginine group when compared to the control group. Anastomotic leakage was also considerably lower in the arginine group (SMD=-0.05, 95% CI: -0.08 to -0.02), the rate of SSIs was lower (RR = -0.02, 95% CI: -0.05-0), and the length of time patients spent in the hospital was shorter (SMD=-0.15, 95% CI: -0.38 to -0.08).
Conclusions
After radiation treatment for CRC, arginine improves immune function and decreases the risk of infection problems.
Trial registration
Registration with PROSPERO for this meta-analysis is number CRD42024520509.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, CRC accounts for 10% of all cancer cases and is the second most prevalent cancer-related killer [1]. Predictions show that colon cancer mortality will rise by 60% by 2035 and rectal cancer mortality by 71.5% [2]. Current estimates place CRC at number two on the list of cancer killers in the US by 2024, with males less than 50 years old being hit particularly hard by the disease [3].
Rigid resection is still the mainstay of CRC therapeutic regimens across the US, EU, UK, and China [4,5,6,7]. However, Severe complications such as anastomotic leakage (Incidence rate ranges from 5–19%) [8], bleeding (Incidence rate ranges from 1.3–1.5%) [9], and SSI (SSI, incidence rate ranges from 23–26%) [10, 11], continue to impact short-term outcomes by increasing postoperative mortality, prolonging hospital stay (LOS), escalating medical costs, and causing greater patient suffering [12]. Furthermore, postoperative complications after radical resection of CRC are known to affect long-term oncological outcomes [13].
Postoperative problems might arise from immunosuppression, which can be caused by factors such as tumor load, surgical stress, and malnutrition [14]. Patients having surgery for gastrointestinal cancer may benefit from perioperative immunnutrition since it lessens the likelihood of postoperative problems and decreases the length of time they spend in the hospital, according to recent studies [15, 16]. Immunonutrition formulations rely on arginine because of its important function in immune function modulation [17]. A large body of research dating back to the 1990s indicates that arginine supplementation during perioperative times improves immune function and reduces CRC patient complications [18,19,20]. However, a new randomized controlled trial with 176 colon cancer patients found that supplementing with arginine before surgery did not improve the results of the patient’s treatments [21].
Consolidating these evidences has been difficult due to the diversity in Research Designs, methodology, demographies, and sample sizes, found in existing clinical trials. To put these questions to rest and determine arginine’s actual therapeutic value for CRC patients having radical surgery, researchers conducted a meta-analysis that drew from randomized, prospective clinical studies. Important new information regarding the role of arginine in immune function and postoperative complications in CRC patients was uncovered in this meta-analysis.
Materials and methods
Protocol registration
The protocol was registered with PROSPERO in March 2024 (registration number: CRD42024520509).
Eligibility criteria
In accordance with the PRISMA and Cochrane Handbook for Systematic Reviews of Interventions, this meta-analysis was carried out. Research was considered for inclusion if it met the following criteria: (1) was a published randomized controlled trial, (2) included patients with a colon or rectal cancer diagnosis who underwent radical surgery, (3) included an intervention group that received arginine-enhanced immunonutrition during the perioperative period and a control group that received routine nutritional support, (4) reported on at least one of the outcomes under investigation. Studies were excluded if they: (1) were duplicate studies, (2) were irrelevant, (3) were laboratory studies, (4) were animal studies, (5) lacked accessible data or the original text, (6) were published in languages other than English or Chinese.
Search methodology
Two researchers worked separately to search all available databases (up to March 10, 2024) for relevant articles: PubMed, Cochrane Library, Web of science, MEDLINE, EMBASE, Wanfang, CNKI, CBM, VIP, etc. The following terms were used in the search: (colon OR rectal OR colorectal) AND (cancer OR tumor OR carcinoma OR neoplasm) AND (arginine OR argininosuccinic acid OR immunonutrition OR immunomodulatory) AND (random OR randomized OR RCTs OR clinical trial). A combination of MeSH and free-text information was also considered. The detailed search strategies were provided in Supplement 1.
Study selection
A thorough examination of the complete texts of selected publications followed the screening of possibly relevant literature by reviewing titles and abstracts. The inclusion or deletion of a certain article was decided upon according to the established criteria. Two evaluators, Zan Ouyang and Ping Chen, independently assessed each article’s research design, study subjects, implementation plan, and results according to the established criteria and collected pertinent information. In cases of disagreement, a third party was consulted.
Data extraction and analysis
Zan Ouyang and Li Zhou, working separately, retrieved the following data: (i) the name and publication year of the first author, (ii) the enrollment total of patients and their corresponding age ranges/distributions were recorded; (iii) the experimental and controlled treatment plans; (iv) primary and secondary observational indicators, including IgA, IgG, IgM, CD4+, CD8+, and the CD4+/CD8+ ratio, and (v) Complications after surgery, namely anastomotic leakage, LOS, and SSI. We talked things out and, if that didn’t work, we brought in an outsider.
Quality assessment
To determine how well the included RCTs performed, researchers used the Cochrane Collaboration’s bias assessment method. This evaluation paid special attention to the following critical areas: the production of random sequences, the concealment of allocations, the blinding of participants and staff, the blinding of outcome assessment, the management of incomplete outcome data, selective reporting, and other possible biases.
Statistical analysis
The statistical analysis was conducted using Stata (StataCorp, version 14.0), with a 95% confidence interval (CI) employed for estimation. Continuous data from laboratory tests and LOS were shown as mean ± standard deviation, whereas dichotomous variables like SSI and anastomotic leakage were calculated as relative risks (RR), and forest plots were presented as risk difference (RD). A high level of homogeneity was indicated by P<0.1 and I2<50%, respectively, when evaluating the heterogeneity among the included studies, whereas a high level of heterogeneity was denoted by the contrary. A random-effects model with heterogeneity analysis was utilized in heterogeneous studies, while a fixed-effects model was utilized in homogeneous studies. To check for publication bias, we utilized a funnel plot, and to see how high-risk papers affected the meta-analysis as a whole, we ran a sensitivity analysis.
Results
Study selection outcome
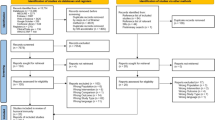
There were 681 relevant articles found, with 560 being duplicates. Review papers, conference abstracts, animal studies, and case reports accounted for 77 of the 77 items that were discarded after abstract and title screening. This left 44 publications for further consideration. Our full-text examination revealed that 12 publications lacked suitable control groups, 3 were inaccessible, and 5 had data that could not be extracted. As a result, all of these articles were removed. After careful review, a total of 24 publications were ultimately deemed suitable for inclusion in the meta-analysis. Figure 1 depicts the retrieval procedure.
Study characteristics
You may find a summary of the 24 studies’ features in Table 1. From 1999 to 2024, a grand total of 1,883 individuals were involved in the investigations; 905 were assigned to the experimental group and 978 were assigned to the control group. Ten studies utilized enteral nutrition (EN), while 14 employed parenteral nutrition (PN). Of these, 20 detailed the specific use of arginine, while 4 did not specify the arginine dosage. 13 studies focused on patients with CRC, and 10 specifically targeted colon cancer. Regarding immune parameters, 13 trials (18,20,24,25,26,27,29,31,34,36,38,40,41) reported CD4+ T cell content, 11 reported CD8+ T cell content (18,25,26,27,29,30,34,36,38,40,41), and 14 reported the CD4+/CD8+ ratio in peripheral blood (18,25,26,27,29,30,31,32,34,36,38,39,40,41). For humoral immunity, 13 trials reported IgA levels (18,23,25,26,27,29,30,31,32,33,36,38,41), 14 (18,23,25,26,27,29,30,31,32,33,36,38,40,41) reported IgM levels, and another 14 (18,23,25,26,27,29,30,31,32,33,36,38,40,41) reported IgG levels in peripheral blood. Additionally, anastomotic leakage was addressed in 11 trials (19,21,22,23,24,28,30,32,33,35,37), SSI in 12 trials (18,19,21,22,23,24,28,30,32,33,35,37), and length of hospital stay in 10 trials (19,21,22,23,24,28,30,32,35,37).
Study quality assessment
Two researchers worked separately to do the quality assessments in Review Manager 5.3. To examine the included RCTs’ methodological quality, they used the bias risk assessment tool offered by the Cochrane Collaboration. The results are presented in Fig. 2A and B. Of the 24 RCTs, two did not specify whether random grouping was used, resulting in a high-risk rating. Another 12 articles did not clearly describe the method for generating the random sequence, which led to an unclear risk assessment. However, the remaining 10 RCTs thoroughly described their methods for generating random sequences. None of the included RCTs used blinding procedures, and the allocation concealment was unclear. There was no evidence of biased reporting or missing data in any of the research.
The Cochrane Collaboration Network GRADE was employed to assess the quality of evidence for this analysis. The results of the evaluation of these ten indicators showed that the certainty levels for CD8 + and PA were very low, levels for IgA, IgM, IgG, CD3 + and CD4+/CD8 + were low, and CD4+, TP, ALB were moderate. There are potential reasons for the downgrade: (1) The included studies had significant variations in randomization, allocation concealment, and blinding; (2) The sample size included in the original studies was limited; (3) Significant heterogeneity between studies. The downgrade is a certain degree indicating the publication bias of included studies, the positive results were published publicly or potential negative results were not reported which results in publication bias and downgrading of the level of evidence. The results of GRADE were provided in Supplement 2.
Results of meta-analysis
Effect of arginine on humoral immunity of patients with CRC
After conducting the heterogeneity test, it was decided whether the combined analysis of humoral immune function indicators, specifically IgA, IgG, and IgM, should be performed using a fixed-effects model or a random-effects model. In Fig. 3, you can see the outcomes. Thirteen studies reporting IgA levels involving seven hundred fifty participants were included in the analysis using a fixed-effects model (I2 = 29.9%, P = 0.145). Figure 3A shows that there was a significant difference in IgA levels between the arginine group and the control group (Z = 6.04, p < 0.001, SMD = 0.45, 95% CI: 0.30–0.60). Out of 14 randomized controlled trials, 810 individuals reported their IgG levels. Figure 3B shows that the arginine group had significantly greater IgG levels compared to the control group, using a fixed-effects model (I²=14.9%, P = 0.291). The statistical analysis yielded a Z = 10.82, p < 0.001, SMD = 0.80, 95% CI: 0.64–0.96.14 randomized controlled trials with 810 participants reported IgM levels. Figure 3C shows that the arginine group had significantly greater IgM levels compared to the control group, as revealed by a random-effects model (I²=69.3%, p < 0.001), SMD = 0.66, 95% CI: 0.39–0.93, and Z = 8.95, p < 0.001.These findings suggest that arginine improves humoral immune function after radical surgery for CRC patients.
Effect of arginine on cellular immunity in patients with CRC
We investigated for heterogeneity to choose the right model before pooling the indications of cellular immune function, which include CD4+ T cells, CD8+ T cells, and the CD4+/CD8+ ratio. Figure 4 displays the results. Thirteen studies that reported on CD4+ T cell counts and included 589 people were analyzed using a random-effects model (I2 = 74.4%, P < 0.001). Figure 4A shows that compared to the control group, the arginine group had significantly increased CD4+ T cell numbers (Z = 11.22, p < 0.001, SMD = 1.03, 95% CI: 0.67–1.38). Eleven randomized controlled trials (RCTs) examined CD8+ T cell counts in 536 individuals. The study revealed that the arginine group had significantly reduced CD8+ T cell counts compared to the control group, using a random-effects model (I2 = 77.2%, P < 0.001) (Z = 7.87, p < 0.001, SMD=-0.70, 95% CI: -1.09 to -0.32; Fig. 4B). The CD4+/CD8+ ratio was reported in 14 RCTs that included 796 individuals. The results of the random-effects model, which demonstrated a significantly higher CD4+/CD8+ ratio in the arginine group compared to the control group (Z = 13.07, p < 0.001, SMD = 1.37, 95% CI: 0.88–1.86; Fig. 4C), were presented. According to these results, arginine controls cellular immune function in CRC patients after severe surgery.
Effect of arginine on post-operative complications and LOS in patients with CRC
To make sure the results were reliable, we checked for heterogeneity among the studies before doing the summary analysis. Figure 5 displays the outcomes. Twelve research examining SSI rates with 1,239 participants utilized a fixed-effects model (I2 = 0%, P = 0.825). Comparing the arginine and control groups, we find that the former had reduced SSI rates (Z = 3.31, P = 0.001, RR = -0.02, 95% CI: -0.05-0.00; Fig. 5A). Anastomotic leakage rates were reported in eleven RCTs in a total of 1,180 participants. Anastomotic leakage was shown to be less common in the arginine group compared to the control group, according to a fixed-effects model (I2 = 8.0%, P = 0.367; Fig. 5B). The P-value was 0.039, and the SMD was − 0.05. On LOS, ten randomized controlled trials documented 1,160 individuals. Based on the results from a random-effects model (I2 = 71.0%, P < 0.001), the arginine group exhibited a shorter length of stay (LOS) compared to the control group, as depicted in Fig. 5C. The statistical analysis indicated a significant difference, with a Z-score of 2.58, a p-value of 0.010, a standardized mean difference (SMD) of -0.15, and a 95% confidence interval (CI) ranging from − 0.38 to 0.08.
Robustness assessment for the sensitivity of pooled analysis
Sensitivity analysis
The pooled results for IgM, CD4+, CD8+, CD4+/CD8+, and LOS showed high heterogeneity (≥ 50%) across the included studies. Thus, a leave-one-out sensitivity analysis was performed to evaluate their robustness. Figure 6 displays the results. Excluding any one trial had no discernible effect on the heterogeneity, according to the sensitivity analysis for CD4+ outcomes (Fig. 6A), suggesting that the combined CD4+ results were robust. The sensitivity analyses of CD8+, CD4+/CD8+, IgM, and LOS all came to similar conclusions, demonstrating that these combined findings are robust (Fig. 6B, C and D, and 6E, respectively).
Meta-regression analysis
The main outcomes, which include IgM, CD4+, CD8+, and CD4+/CD8+, showed substantial variation across investigations. As a result, we applied meta-regression analysis to find out where the differences were coming from and how much of an effect confounding variables had on the reliability of our pooled results. We examined the route of arginine administration, tumor kind, sample size, and duration of treatment as possible influential confounding factors after consulting and discussing the matter. In Table 2, the outcomes of the meta-regression were presented. Univariate and multivariate analysis revealed the administration route (PN or EN) of arginine had a significant influence on the results of CD4+, which indicated the heterogeneity may originate from this covariate. The remaining variable had no significant influence on the pooled effects of CD8+, CD4+/CD8+ and IgM in either the single or multi-factor regression analyses.
Test of publication bias
The inclusion of RCTs was evaluated for publication bias using Egger’s test, and the results can be shown in Fig. 7. The following findings have been deduced from Egger’s test: A p-value of 0.75 and a coefficient of 0.77 are shown for CD4+ in Fig. 7A. No publication bias has been identified because the P-value is greater than the stated significance level of 0.05. The same conclusion applies to the other six Egger’s tests: For CD8+ (Fig. 7B), P = 0.86, Coefficient = 0.51. For IGA (Fig. 7D), P = 0.73, Coefficient=-0.43. For IGG (Fig. 7E), P = 0.66, Coefficient = 0.49. For IGM (Fig. 7F), P = 0.90, Coefficient = 0.24. For SSI (Fig. 7G), P = 0.59, Coefficient = 0.22. For LOS (Fig. 7H), P = 0.91, Coefficient = 0.24. In all cases, the P-values exceed 0.05, indicating no significant publication bias. However, Egger’s test result for CD4+/CD8+ (Fig. 7C) showed a P-value of 0.018, with a coefficient of 5.57 and a 95% CI ranging from 1.15 to 9.98, indicating significant publication bias. Following this, we re-examined the original studies and hypothesized that factors such as small sample sizes, intention-to-treat (ITT) analysis, and the lack of blinding in many studies might have influenced these biases. These factors could potentially impact the conclusions regarding CD4+/CD8+.
Discussion
The amino acid arginine is considered conditionally essential and plays a pivotal role in various metabolic processes within the human body. These processes include: (1) Participation in the urea cycle and facilitation of nitrogen-containing waste transportation [42, 43], (2) involvement as an intermediate in protein synthesis across diverse types within the body [42, 43], (3) serving as a precursor for polyamine and hydroxyproline synthesis, both crucial for repair mechanisms [44], (4) acting as a precursor in nitric oxide (NO) synthesis, a major vasodilator involved in intracellular signal transduction, stimulation of NK cell activation, and tumor growth inhibition [45, 46], (5) enhancing lymphocyte count in peripheral blood and exerting a positive regulatory effect on cellular immunity, primarily governed by T lymphocytes under specific conditions [47]. The exact cause of the abnormally low arginine levels seen in trauma, surgical procedures, and tumor patients is still unclear [48], but one possible explanation is the presence of type I arginase, an enzyme that breaks down arginine into ornithine, expressed by immature bone marrow primitive cells in the lymphatic and circulatory systems [49]. In colon cancer patients who have undergone radical surgery, the risk of infection is greatly increased due to the enormous depletion of arginine, which greatly hinders cellular immune activity [50, 51].
In patients undergoing radical resection for CRC, this meta-analysis confirms the impact of perioperative arginine supplementation on immune function and postoperative outcomes from three angles. At the outset, arginine significantly enhances humoral immune function following CRC radical resection. To add to that, it improves the cellular immune function of the patients. Thirdly, it keeps patients out of the hospital for shorter periods of time and reduces the risk of anastomotic leaks and infections at the surgical site. With respect to humoral immunity, three important markers showed notable improvements in the arginine group: IgA (SMD = 0.45, 95% CI: 0.30–0.60), IgG (SMD = 0.80, 95% CI: 0.64–0.96), and IgM (SMD = 0.66, 95% CI: 0.39–0.93). In terms of cellular immune function, pooled analysis revealed that the arginine group had significantly higher CD4+T cell counts (SMD = 1.03, 95% CI: 0.67–1.38) and CD4+/CD8+ ratios (SMD = 1.37, 95% CI: 0.88–1.86) compared to the control group, while the CD8+T cell count decreased significantly (SMD=-0.70, 95% CI: -1.09 to -0.32). These results show that the arginine group had significantly improved cellular immune activity. In addition, when looking at the data as a whole, it was found that the arginine group had a lower rate of SSIs (RR=-0.02, 95% CI: -0.05-0), a shorter LOS (SMD=-0.15, 95% CI: -0.38-0.08), and a significantly lower incidence of anastomotic leakage (SMD=-0.05, 95% CI: -0.08 to -0.02) compared to the control group.
The lack of arginine, as a central factor causing immunosuppression in CRC patients after radical surgery, warrants greater attention from clinicians and researchers. This study found that arginine improved immune function and decreased postoperative infection problems in CRC patients following radical surgery. Nevertheless, it is important to highlight the potential limitations of this integrated analysis. All the studies included in the analysis were not designed with single or double blinding, which increases the risk of detection bias. Additionally, the results of the GRADE assessment indicated the presence of publication bias among the studies could downgrade the level of evidence. Undetected bias was also found in studies with small sample sizes and missing ITT analysis, which may have contributed to potential bias. Furthermore, the metaregression analysis, using both univariate and multivariate methods, identified the method of administering arginine as a potential factor contributing to significant heterogeneity, casting doubt on the validity of the results in this combined analysis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and supplementary material.
References
World Health Organization. Cancer fact sheet. Geneva: WHO Media Centre; 2024. https://www.who.int/zh/news-room/fact-sheets/detail/colorectal-cancer. Cited 2024 March 21.
Araghi M, Soerjomataram I, Jenkins M, et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer. 2019;144(12):2992–3000.
Siegel RL, Giaquinto AN, Jemal A, Cancer statistics. CA Cancer J Clin. 2024;74(1):12–49.
Argilés G, Tabernero J, Labianca R, et al. Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Localised colon cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–305.
Davies J, Chew C, Bromham N, et al. National Institute for Health and Care Excellence (NICE). NICE 2020 guideline for the management of colorectal cancer. Lancet Oncol. 2022;23(6):e247.
General Office of National Health Commission of the People’s Republic of China, Oncology Branch of the Chinese Medical Association. The Standard for Diagnosis and Treatment of Chinese Colorectal Cancer(2023 version)[J]. Med J Peking Union Med Coll Hosp. 2023;14(4):706–33.
NCCN. (2024). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer. Version 2.2024. [PDF document]. Retrieved from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102(5):462–79.
Lavikainen LI, Guyatt GH, Sallinen VJ, et al. Systematic reviews and Meta-analyses of the Procedure-specific risks of thrombosis and bleeding in General Abdominal, Colorectal, Upper Gastrointestinal, and Hepatopancreatobiliary surgery. Ann Surg. 2024;279(2):213–25.
Gachabayov M, Senagore AJ, Abbas SK, et al. Perioperative hyperglycemia: an unmet need within a surgical site infection bundle. Tech Coloproctol. 2018;22(3):201–7.
Milinis K, Shalhoub J, Coupland AP, et al. The effectiveness of graduated compression stockings for prevention of venous thromboembolism in orthopedic and abdominal surgery patients requiring extended pharmacologic thromboprophylaxis. J Vasc Surg Venous Lymphat Disord. 2018;6(6):766–e7772.
Pak H, Maghsoudi LH, Soltanian A, et al. Surgical complications in colorectal cancer patients. Ann Med Surg (Lond). 2020;55:13–8.
Lawler J, Choynowski M, Bailey K, et al. Meta-analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. BJS Open. 2020;4(5):737–47.
Zhang Y, Rajput A, Jin N, et al. Mechanisms of Immunosuppression in Colorectal Cancer. Cancers (Basel). 2020;12(12):3850.
Khan A, Wong J, Riedel B, et al. The impact of peri-operative Enteral Immunonutrition on post-operative complications in gastrointestinal Cancer surgery: a Meta-analysis. Ann Surg Oncol. 2023;30(6):3619–31.
Sánchez-Guillén L, Arroyo A. Immunonutrition in patients with colon cancer. Immunotherapy. 2020;12(1):5–8.
Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–54.
Song JX, Qing SH, Huang XC, et al. Effect of parenteral nutrition with L-arginine supplementation on postoperative immune function in patients with colorectal cancer. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(6):545–7.
Braga M, Gianotti L, Vignali A, et al. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805–14.
Szefel J, Ślebioda T, Walczak J, et al. The effect of l-arginine supplementation and surgical trauma on the frequency of myeloid-derived suppressor cells and T lymphocytes in tumour and blood of colorectal cancer patients. Adv Med Sci. 2022;67(1):66–78.
Lee SY, Lee J, Park HM, et al. Impact of Preoperative Immunonutrition on the outcomes of Colon cancer surgery: results from a Randomized Controlled Trial. Ann Surg. 2023;277(3):381–6.
Achilli P, Mazzola M, Bertoglio CL, et al. Preoperative immunonutrition in frail patients with colorectal cancer: an intervention to improve postoperative outcomes. Int J Colorectal Dis. 2020;35(1):19–27.
Chen R, Cai JL, et al. Effect of immune-enhanced enteral diet on postoperative immunological function in patients with colorectal cancer. Chin J Gastrointest Surg. 2005;8(4):328–30.
Finco C, Magnanini P, Sarzo G, et al. Prospective randomized study on perioperative enteral immunonutrition in laparoscopic colorectal surgery. Surg Endosc. 2007;21(7):1175–9.
Gao ZK, Chen QL, et al. Effects of arginine-enriched TPN and standard TPN on the immune function of patients with carcinoma. Parenter Enter Nutr. 2000;7(2):66–9.
Gong H, Lin H, et al. The effects of early enteral immunologic nutrition on the nutritional status,immune function and inflammatory response of patients with colorectal cancer. Shaanxi Med J. 2019;48(5):575–577580.
He J. Li Q,Ge HY.Effects of arginine-enriched parenteral nutrition on the immune function of patients with colorectal carcinoma. Shanghai Med J. 2006;29(6):359–62.
Horie H, Okada M, Kojima M, et al. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36(12):1063–8.
Hu XH, Zhang Q, Wang TZ, et al. The effect of L-arginine on the immune function in patients with colorectal cancer.Chinese. J Clin Oncol Rehabilitation. 2001;8(5):4–5.
Liang WX, Zhang T, Hong Y, et al. The effect of preoperative enteral immunonutrition on immune function in patients with colorectal cancer. Parenter Enter Nutr. 2006;13(6):346–9.
Li LH. The impact of arginine on immune function in perioperative patients with colorectal cancer. Shandong Med J. 2002;42(17):48.
Liu G, Lao JM,Guo Z, et al. The impact of early postoperative enteral immune nutrition on nutritional status, immune function, and inflammatory response in patients with colorectal cancer. J Med Theory Pract. 2024;37(2):231–3.
Liu SJ, Yang XH, Liu JW, et al. Enteral immune nutrition for preoperative preparation in patients with colorectal carcinoma. J Jinan University (Natural Sci Med Ed). 2004;25(6):752–4.
Liu XC. Zhang L.The influence of total parenteral nutrition added with arginine on cellular immune function of colonic cancer after operation. J Colorectal Anal Surg. 2007;13(4):216–20.
Moya P, Soriano-Irigaray L, Ramirez JM, et al. Perioperative Standard Oral Nutrition Supplements Versus Immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol: a Multicenter Randomized Clinical Trial (SONVI Study). Med (Baltim). 2016;95(21):e3704.
Qin HL. Effects of L-arginine on the immunof{Velkoski, 2021 #10}unction after operation and chemotherapy in patients with colorectal cancer. Parenter Enter Nutr. 2000;7(1):13–6.
Velkoski J, Grimaldi F, DI Meo L, et al. Immunonutrition in elective colorectal surgery and early inflammatory response. Minerva Surg. 2021;76(5):407–14.
Ya HQ, Wang WX, Huang SR, et al. Effects of Total Parenteral Nutrition Supplemented with Glutamine and Arginine on Postoperative Immune Function in Patients with Colorectal Cancer. Cancer Res Prev Treatment. 2007;34(12):965–7.
Yang DG, Yang FH, Sui YL, et al. The effect of preoperative total parenteral nutrition enriched with arginine on immune function for patients with colorectal cancer. Parenter Enter Nutr. 1999;6(2):66–8.
Zhang CA. Li QL.Effect of arginine and glutamine enhanced parenteral nutrition on post-operative stress status and immune function in colon cancer patients. J Fourth Military Med Univ. 2008;29(2):178–80.
Zhuang ST, Li QZ, Cai YJ, et al. The impact of early postoperative enteral immune nutrition on immune function and inflammatory response in patients with colorectal cancer. Chin J Clin (Electronic Edition). 2013;7(16):117–9.
Gupta MN, Uversky VN. Biological importance of arginine: a comprehensive review of the roles in structure, disorder, and functionality of peptides and proteins. Int J Biol Macromol. 2024;257(Pt 1):128646.
Du T, Han J. Arginine metabolism and its potential in treatment of Colorectal Cancer. Front Cell Dev Biol. 2021;9:658861.
Arribas-López E, Zand N, Ojo O, et al. The effect of amino acids on Wound Healing: a systematic review and Meta-analysis on arginine and glutamine. Nutrients. 2021;13(8):2498.
Gambardella J, Khondkar W, Morelli MB, et al. Arginine and endothelial function. Biomedicines. 2020;8(8):277.
Keshet R, Erez A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis Model Mech. 2018;11(8):dmm033332.
Martí I, Líndez AA, Reith W. Arginine-dependent immune responses. Cell Mol Life Sci. 2021;78(13):5303–24.
Starikova EA, Rubinstein AA, Mammedova JT, et al. Regulated arginine metabolism in Immunopathogenesis of a wide range of diseases: is there a way to pass between Scylla and Charybdis? Curr Issues Mol Biol. 2023;45(4):3525–51.
Kim SH, Roszik J, Grimm EA, et al. Impact of l-Arginine metabolism on Immune Response and Anticancer Immunotherapy. Front Oncol. 2018;8:67.
Sindhu R, Supreeth M, Prasad SK, et al. Shuttle between arginine and lysine: influence on cancer immunonutrition. Amino Acids. 2023;55(11):1461–73.
Karimian J, Hadi A, Salehi-Sahlabadi A, et al. The Effect of Arginine Intake on Colorectal Cancer: a systematic review of literatures. Clin Nutr Res. 2019;8(3):209–18.
Acknowledgements
Our greatest acknowledgment is to the authors who made detailed data available for this meta-analysis and all our colleagues in this study for their hard work.
Conflict of interest
We demonstrated that this paper is new and has no conflicts of interest to disclose.
Funding
None.
Author information
Authors and Affiliations
Contributions
Zan Ouyang and Ping Chen performed the search and drafted the manuscript. Zan Ouyang, Min Zhang and Sijia Wu performed the data extraction and analyzed the data. Zongying Qin and Li Zhou designed the study and amended the original draft. All authors equally involved and equally contributed into the study conduction.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ouyang, Z., Chen, P., Zhang, M. et al. Arginine on immune function and post-operative obstructions in colorectal cancer patients: a meta-analysis. BMC Cancer 24, 1089 (2024). https://doi.org/10.1186/s12885-024-12858-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12858-7