Abstract
Background
Growing evidence suggests that ozone (O3) protects the host against pathological conditions mediated by reactive oxygen species by increasing the activity of antioxidant enzymes. The purpose of the present study was to examine the effect of O3 on intestinal recovery and enterocyte turnover after intestinal ischemia–reperfusion (IR) injury in rats.
Methods
Male Sprague–Dawley rats were divided into four experimental groups: (1) sham rats underwent laparotomy; (2) sham-O3 rats underwent laparotomy and were treated with an ozone/oxygen mixture intraperitoneally and intraluminally (50 %/50 %); (3) IR rats underwent occlusion of both superior mesenteric artery and portal vein for 20 min followed by 48 h of reperfusion, and (4) IR-O3 rats underwent IR and were treated with an ozone/oxygen mixture similar to group 2. Intestinal structural changes, Park’s injury score, enterocyte proliferation and enterocyte apoptosis were determined 48 h following IR. Western blot was used to determine ERK and Bax protein levels. A non-parametric Kruskal–Wallis ANOVA test was used for statistical analysis with p < 0.05 considered statistically significant.
Results
Treatment of IR rats with O3 resulted in a significant increase in mucosal weight in jejunum (70 %) and ileum (32 %), mucosal DNA (twofold increase) and protein (35 %) in ileum, villus height and crypt depth in jejunum (61 and 16 %, correspondingly) and ileum (31 and 43 %, correspondingly) compared to IR animals. IR-O3 rats also had a significantly lower intestinal injury score as well as a lower apoptotic index in jejunum and ileum compared and IR animals. A significant increase in cell proliferation rates in IR-O3 animals was accompanied by increased levels of p-ERK protein.
Conclusions
Treatment with ozone prevents intestinal mucosal damage, stimulates cell proliferation and inhibits programmed cell death following intestinal IR in a rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal ischemia–reperfusion (IR) is a common, potentially fatal clinical entity that may be caused by mechanical obliteration of flow in blood vessels (occlusive ischemia) or by critical secondary reduction in splanchnic organ blood flow (non-occlusive ischemia) [1, 2]. Intestinal IR results in deprivation of vital nutrients, particularly oxygen, and activates a large variety of vascular and inflammatory mediators that trigger adhesion, migration, and activation of white blood cells, increase vascular leakage with fluid and protein efflux from blood vessels culminating in a severe local and systemic inflammatory response and multiple organ failure [3–7]. Although reinstitution of blood flow to ischemic tissues is critical for their salvage, the reperfusion of ischemic intestine may paradoxically enhance the vascular and tissue inflammatory response initiated by the ischemia and even aggravate some of the manifestations of the ischemic damage [1, 2]. The understanding of the central role of reactive oxygen species (ROS) in IR injury evoked concerns that hyperoxia could exacerbate the injury by adding extra oxygen to the system and thus increasing free radicals formation [8, 9].
Therapy of intestinal IR injury includes the pharmacological agents that can prevent ischemic injury as well as agents that can prevent production of reactive oxygen species and agents which can improve intestinal rehabilitation following the IR event. Medical ozone (O3) therapy uses a mixture of ozone and oxygen and appears to be a safe, economical, effective treatment for patients with advanced ischemic diseases, peritonitis, infected wounds, chronic skin ulcers, initial gangrene, burns, and others [10, 11]. The mechanisms of the positive effects of ozone are not yet fully understood and are mainly attributed to improvement in blood circulation and oxygen delivery to damaged tissues, enhanced general metabolism, up-regulation of the cellular antioxidant enzyme activity, activation of the immune system and neuro-endocrine system, and enhancement in the release of growth factors from platelets [12, 13]. Growing evidence suggests that O3 increases the activity of antioxidant enzymes, such as glutathione peroxidase and catalase, and supports the host against pathophysiological conditions mediated by reactive oxygen species (ROS) [14].
The purpose of the present study was to evaluate the effects of ozone therapy on enterocyte turnover (proliferation and death via apoptosis), and intestinal preservation and rehabilitation in a rat model of intestinal IR injury.
Materials and methods
Animals
Surgical procedures and animal care were conducted in compliance with the guidelines established by the “Guide for the Care and Use of Laboratory Animals”, Rappaport Faculty of Medicine, Technion (Haifa, Israel). Male Sprague–Dawley rats (250–300 g) were used in this study. Animals were housed in wire-bottomed cages and were acclimatized at 21 °C on 12:12-h light–dark cycle for a minimum of 3 days before the experiment. The rats were allowed access to water and chow ad libitum.
Experimental design
Thirty-two rats were divided randomly into four experimental groups of eight rats each: (1) Group A rats underwent midline laparotomy (sham); (2) Group B (sham-O3) rats underwent laparotomy and were treated with an ozone/oxygen mixture (0.7 mg/kg/day) intraperitoneally and intraluminally (50 %/50 %); (3) Group C (IR) rats underwent occlusion of both the superior mesenteric artery (SMA) and portal vein (PV) for 20 min followed by 72 h of reperfusion; and (4) Group D (IR-O3) rats underwent IR and were treated with an ozone/oxygen mixture similar to group 2.
Surgery
After overnight fasting, the animals were anesthetized with intraperitoneal ketamine (90 mg/kg) and xylasine (10 mg/kg). Using sterile techniques, the abdomen was opened using a midline incision. A sham operation was performed by identification and isolation of the SMA and PV without clamping. In IR animals, SMA and PV were occluded with a microbulldog clamp for 20 min. During the period of ischemia the abdominal wall incision was kept approximated to prevent fluid and heat loss. At the end of the ischemic period, the clamp was released, the rats were resuscitated with a 3-ml intraperitoneal injection of warm 0.9 % NaCl solution and the abdominal cavity was closed in two layers with a running suture of 3/0 Dexon. The rats were fasted for 24 h but were allowed free access to water.
Intestinal mucosal parameters
All animals were killed 72 h following the ischemia. The entire small intestine was carefully removed and placed on ice. Portions of intestine 10 cm distal to the ligament of Treitz and 10 cm proximal to the ileo-cecal region were removed, representing the proximal (jejunum) and distal (ileum) segments. Intestinal segments were split on the antimesenteric border, washed with cold saline, dried, and weighed. Each segment was rinsed thoroughly with physiological saline and opened longitudinally on its antimesenteric border to expose the intestinal mucosa. The mucosa was scraped from the underlying tissue with a glass slide. Total RNA, DNA and protein from the jejunum and ileum were extracted sequentially with a TRIzol reagent. DNA and protein were extracted by the method of Chromsczynski [15].
Histology
Tissue samples were removed from the jejunum and ileum and were immediately fixed in 4 % neutral-buffered formalin. The samples were then embedded in paraffin and sectioned. Deparaffinized 5 μm sections were stained with haematoxylin and eosin. The villus height and crypt depth for each specimen were measured using an objective mounted micrometer (100× magnification) and an optical microscope (10 × 100 magnification). Villus height and crypt depth data were from eight rats, and each measurement consisted of the mean of five villi and crypts.
The mucosal damage of the small bowel was graded using the Park score [16]: 0, normal mucosa; 1, subepithelial space at villus tip; 2, more extended subepithelial space; 3, epithelial lifting along villus sides; 4, denuded villi; 5, loss of villus tissue; 6, crypt layer infarction; 7, transmucosal infarction; 8, transmural infarction.
Enterocyte proliferation and apoptosis
Crypt cell proliferation was assessed using 5-bromodeoxyuridine (5-BrdU). Standard BrdU labeling reagent (Zymed Laboratories, Inc, San Francisco, CA) was injected intraperitoneally at a concentration of 1 ml/100 g body weight 2 h before killing. Tissue slices (5 μm) were stained with a biotinylated monoclonal anti-BrdU antibody system provided in a kit form (Zymed Laboratories, Inc, San Francisco, CA). An index of proliferation was determined as the ratio of crypt cells staining positively for BrdU per 10 crypts.
Additional 5-μm thick sections were prepared to establish the degree of enterocyte apoptosis. Immunohistochemistry for Caspase-3 (Caspase-3 cleaved concentrated polyclonal antibody; dilution 1:100; Biocare Medical, Walnut Greek, CA) was performed for identification of apoptotic cells using a combination of the streptavidin-biotin-peroxidase method and microwave antigen retrieval on formalin-fixed, paraffin-embedded tissues according to the manufacturer’s protocols. The apoptotic index (AI) was defined as the number of apoptotic cells per ten villi. A qualified pathologist blinded to the source of intestinal tissue performed all measurements.
Western blotting
Tissue was homogenized in RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 % NP-40, 2 mM EDTA, supplemented with a cocktail of protease (Roche Diagnostic) and phosphatase cocktail inhibitors (Sigma). Protein concentrations were determined by Bradford reagent according to the manufacturer’s instructions. Samples containing equal amounts of total protein (30 μg) were dissolved by SDS-PAGE under reducing conditions. After electrophoresis, proteins were transferred to PVDF membrane and probed with various primary antibody to anti-Caspase-3 antibody (1:2,500 dilution, sc-7382), anti-phospho-ERK antibodies (1:2,500 dilution, sc-7383), and two other antibodies anti-ERK2 (1:2,000 dilution, Cell Signaling #9108) and anti-β-Tubulin (1:5,000 dilution, Sigma T6557) and used for the protein normalization. Horseradish peroxidase-conjugated secondary antibody was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA) and an enhanced chemiluminescent substrate from Biological Industries (Kibbutz Beth HaEmek, Israel). The optical density of the specific protein bands was quantified by using a densitometer (Vilber Lourmat, Lion, France).
Statistical analysis
The data are expressed as the mean ± SEM. A one-way ANOVA for comparison, followed by Tukey’s test for pair-wise comparison was used for statistical analysis. Prism software was used (GraphPad Software, Inc., San Diego, CA); and statistical significance was defined as p < 0.05.
Results
Intestinal mucosal parameters
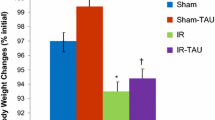
Treatment with ozone of sham animals (Group B) resulted in a significant increase in jejunal mucosal weight (10.6 ± 0.3 vs. 8.8 ± 0.6 mg/cm length/100 g body weight, p < 0.05) compared to sham animals (Group A) as well as in a trend toward increase in ileal mucosal weight; however, this trend was not statistically significant. The IR injury (Group C) had a significant decrease in mucosal weight in jejunum (6.7 ± 0.8 vs. 8.8 ± 0.6 and 10.6 ± 0.3 mg/cm length/100 g body weight, p < 0.05, correspondingly) and ileum (6.2 ± 0.1 vs. 8.2 ± 0.7 and 9.6 ± 0.4 mg/cm length/100 g body weight, p < 0.05, correspondingly) compared to control (Group A) and sham-O3 animals (Group B) (Fig. 1). Following ozone administration, IR rats (Group D) demonstrated a significant increase in jejunal bowel weight (22.5 ± 1.5 vs. 18 ± 1.5 mg/cm length/100 g body weight, p < 0.05) as well as in jejunal (11.4 ± 1.5 vs. 6.7 ± 0.8 mg/cm length/100 g body weight, p = 0.007) and ileal (8.2 ± 0.6 vs. 6.2 ± 0.3 mg/cm length/100 g body weight, p = 0.004) mucosal weight compared to IR-untreated animals (Group C).
Intestinal mucosal DNA and protein levels
As demonstrated in Fig. 2, IR caused a significant decrease in mucosal DNA content in jejunum (fivefold decrease, p < 0.001) and ileum (twofold decrease, p = 0.007) and mucosal protein content in the jejunum (twofold decrease, p < 0.001) and ileum (65 ± 12 vs. 98 ± 10 μg/cm length/100 g body weight, p = 0.03) compared to sham animals (Group A). In the ozone-treated IR group (Group D), rats demonstrated a significant increase in jejunal (threefold increase, p < 0.05) and ileal (twofold increase, p < 0.01) mucosal DNA as well as ileal mucosal protein (35 % increase, p < 0.05) content compared to the untreated IR group (Group C).
Microscopic bowel appearance
Treatment of sham animals with ozone did not change significantly intestinal injury score compared to sham non-treated animals (Fig. 3). IR injury (Group C) led to a significant increase in the mean intestinal injury grade (Park’s criteria) in jejunum (2.5 ± 0.5 vs. 1.2 ± 0.6, p < 0.05) and ileum (3.2 ± 0.5 vs. 1 ± 0.3, p = 0.005) compared to sham animals. Treatment of IR rats with ozone/oxygen mixture (Group D) resulted in a significant decrease in intestinal injury score in ileum (twofold decrease, p < 0.05) compared to IR non-treated rats as well as in a trend toward a decrease in injury grade in jejunum; however, this trend was not statistically significant.
Administration of ozone/oxygen mixture in sham rats (Group B) resulted in a trend toward increase in jejunal villus height and crypt depth compared to sham animals (Group A); however, this trend did not achieve statistical significance (Fig. 4). IR rats (Group C) demonstrated a significant decrease in villus height in jejunum (427 ± 37 vs. 592 ± 47 μm, p = 0.008) and ileum (334 ± 41 vs. 443 ± 27 μm, p < 0.05) as well as crypt depth in ileum (138 ± 7 vs. 192 ± 13 μm, p = 0.001) compared to sham animals (Group A). Treatment with ozone/oxygen mixture (Group D) resulted in a significant increase in villus height in jejunum (620 ± 38 vs. 427 ± 37 μm, p = 0.008) and ileum (438 ± 39 vs. 334 ± 41 μm, p < 0.05) as well as crypt depth in jejunum (223 ± 10 vs. 192 ± 12 μm, p = 0.001) and ileum (198 ± 13 vs. 138 ± 7 μm, p < 0.05) compared to IR-nontreated animals (Group C).
Cellular proliferation and apoptosis
IR injury (Group C) resulted in a significant inhibition of cell proliferation in jejunum (128 ± 5 vs. 160 ± 6 BrdU-positive cells/10 crypts, p < 0.05) and ileum (125 ± 5 vs. 160 ± 5 BrdU-positive cells/10 crypts, p < 0.05) compared to sham animals (Fig. 5). Treatment with ozone (Group D) led to a significant increase in cell proliferation rates in jejunum (137 ± 2 vs. 128 ± 5 BrdU-positive cells/10 crypts, p < 0.05) and ileum (136 ± 2 vs. 125 ± 5 BrdU-positive cells/10 crypts, p < 0.05) compared to IR-animals (Group C).
The frequency of apoptotic cells was increased in the animals after the IR insult in jejunum (5.7 ± 0.3 vs. 2.2 ± 0.2 apoptotic cells/10 villi, p < 0.001) and ileum (6.7 ± 1.5 vs. 1.6 ± 0.5, p = 0.002) compared to sham animals (Fig. 6). The apoptotic index decreased following administration of ozone (Group D) in jejunum (26 % decrease, p < 0.005) and ileum (twofold decrease, p < 0.05) compared to IR-untreated animals (Group C).
Western blotting
Treatment with ozone of control animals (Group B) resulted in a trend toward increase in p-ERK protein levels compared to sham animals (Fig. 7). Decreased cell proliferation rates in IR animals (Group C) were accompanied by decreased levels of p-ERK protein levels compared to control rats. An increased cell apoptosis was also accompanied by an increased caspase-3 protein levels. Treatment with ozone (Group D) resulted in a significant increase in both p-ERK protein levels, which correlated with increased cell proliferation rates in this group compared to IR-untreated animals (Group C). IR-O3 animals demonstrated a significant decrease in caspase-3 protein levels that correlated with decreased cell apoptosis in this group.
Discussion
Many experiments have shown beneficial effects of ozone on ischemia–reperfusion injury in different organs and tissues. Intraperitoneal application of ozone creates a positive impact on histologic and biochemical markers on ovarian IR injury in a rat [17]. Ozone oxidative preconditioning ameliorates the hepatic injury associated with IR and has a stimulatory effect on liver cell regeneration following 70 % hepatectomy in rats [18]. The protective effect of ozone (intraperitoneal, 4 mg/kg) on testicular ischemia–reperfusion injury in a rat is associated with elevated nitrous oxide production [19]. Ozone therapy decreases the tissue oxidative stress parameters (lipid peroxidation, protein oxidation, and nitrite plus nitrate) and increases the antioxidant enzyme activities (superoxide dismutase and glutathione peroxidase) during renal ischemia–reperfusion in a rat model [20]. Although little is known about the protective effects of ozone in intestinal IR, the positive effects of ozone therapy have been described in many gastrointestinal disorders. Ozone therapy decreases inflammation, edema, and oxidative stress during experimental colitis [21]. The use of ozone notably reduced the incidence of postoperative inflammatory complications, incompetence of intestinal anastomoses, and animal mortality [22]. Ozone preconditioning decreases tissue damage and increases antioxidant enzyme activity in an experimental model of methotrexate-induced intestinal injury [23]. The mechanisms of this positive effect of ozone therapy in the gastrointestinal tract are poorly understood. In a recent pilot study, we evaluated the effect of ozone therapy on intestinal mucosal homeostasis in healthy rat and compared oral versus intraperitoneal route of O3 administration. We have shown that ozone stimulates intestinal mucosal homeostasis in the normal gastrointestinal tract and this effect was greater when ozone was used intraperitoneally (compared to oral route administration) (non-published data).
Since ozone has a positive effect during IR events in different organs, we hypothesized in the present study that this agent could enhance intestinal regeneration following intestinal IR injury. Ozone might stimulate enterocyte turnover by a direct stimulation of proliferation, cell migration, or by inhibition of enterocyte apoptosis. Alternatively, it might exert its stimulating effect on mucosal restitution by stimulating the release of various cytokines or peptide growth factors or through changes in ROS production. Our experimental protocol was based on pilot studies which demonstrated that occlusion of the SMA for 30 min causes small changes, while occlusion of both the SMA and the PV for 20 min leads to visually severe but reversible intestinal ischemia.
Similar to our previous experience [24, 25], the IR event resulted in an intestinal mucosal injury manifested by an increased Park’s intestinal injury score, and led to mucosal hypoplasia. The observed decreased bowel and mucosal weight, decreased mucosal DNA and protein, and decreased villus height and crypt depth support this statement. Decrease in mucosal DNA and protein also suggests diminished cell metabolism, which is consistent with decreased epithelial cell proliferation. Taken together, the decreased enterocyte proliferation and increased cell death indicate decreased gut epithelial cell turnover. Although this study did not address altered function, based on conventional thinking of intestinal function, the lower villus height in the jejunum and ileum following IR suggests a decrease in intestinal absorptive surface area and impaired nutrient absorption. The less significant damage that was observed in the proximal jejunum may be explained by collateral blood flow from the celiac axis.
Treatment with an ozone/oxygen mixture exerted obvious beneficial effects on mucosal preservation and recovery. Intestine from ozone-treated rats exhibited reversal of the loss of and injury to villus epithelium, and a decrease in the Park injury score. The intestinal mucosa was indistinguishable from normal bowel, with evidence of minimal epithelial disruption. In addition, the mucosal hypoplasia caused by IR was ameliorated in part by ozone administration. This claim is based on the increased mucosal weight, increased mucosal DNA and protein, and increased villus height and crypt depth after treatment with ozone. Furthermore, proliferation of crypt cells increased significantly following ozone administration and was closely correlated with increased crypt depth. Increased villus height and crypt depth are the result of increased cell production and accelerated migration along the villus, and are a marker of increased absorptive surface area. Although the inhibitory effect of ozone on cell proliferation in malignant cell lines has been previously described [26], several experiments have demonstrated an epithelial hyperplasia following ozone administration [27]. Our data fit with these studies. The increase in enterocyte proliferation in our experiment was associated with up-regulation of p-ERK protein expression. The transmission of extracellular proliferation and differentiation signals into their intracellular targets is mediated by a signaling cascade culminating in mitogen-activated protein kinase (MAPK). MAPKs are serine/threonine-specific protein kinases that regulate various cellular activities, such as gene expression, mitosis, cell proliferation, differentiation and apoptosis [28]. One of the MAPK signaling pathways triggered by cytokines or growth factors is the extracellular signal-related kinase (ERK) pathway.
The present study also compared the rate of apoptosis and the presence of apoptosis-related proteins that regulate cell death. After IR the number of apoptotic cells increased in both bowel segments. The increase in enterocyte apoptosis was associated with up-regulation of bax (pro-apoptotic gene) expression and down-regulation of bcl-2 (anti-apoptotic gene) expression [24, 25]. In the gut, Bcl-2 is mostly expressed at the base of the colonic crypt, but less so in the small intestinal mucosa, whereas Bax is predominant in the crypts of the small intestine. It is generally accepted that Bax/Bcl-2 ratio appears to determine the cell’s fate, whereby excess Bcl-2 confers survival and excess Bax promotes cell death. In the present study, increased enterocyte apoptosis following IR was associated with increase in caspase-3 protein levels. Treatment with ozone resulted in a significant decrease in enterocyte apoptosis and in caspase-3 protein levels. Acceleration of the proliferation of intestinal cells together with decreased apoptosis may indicate an adaptive mechanism to increase enterocyte mass. Literature data concerning the effects of ozone on cell death via apoptosis are controversial. Several experiments have shown that ozone induces cell apoptosis [29]. The mechanism of ozone-induced cell injury is poorly understood. One hypothesis is that ozone induces lipid peroxidation and that these peroxidated lipids produce oxidative stress and DNA damage. In contrast, a recent study has shown that acute ozone exposure compromised cell membrane integrity severely, while repetitive, short-duration exposures to ozone up-regulated cellular plasticity via induction of anti-apoptotic pathways in a treatment regimen-specific manner [30].
In conclusion, the present study demonstrates that ozone administration attenuates the intestinal mucosal injury caused by IR and enhances intestinal recovery. This benefit correlates with elevated cell proliferation and decreased cell death via apoptosis. Further clinical trials may be necessary to determine whether ozone/oxygen mixture may be beneficial as an agent to stimulate intestinal recovery in patients after intestinal ischemia.
References
Granger DN, Hollwarth ME, Park DA (1986) Ischemia reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl 548:47–56
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190:255–266
Mangino MJ, Mangino JE, Murphy MK, Anderson CB (1996) Arachidonic acid metabolism in intestinal hypothermic preservation injury. Cryobiology 33:404–412
Kurose I, Argenbright LW, Wolf R, Lianxi L, Granger DN (1997) Ischemia/reperfusion-induced microvascular dysfunction: role of oxidants and lipid mediators. Am J Physiol 272:H2976–H2982
Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M (2001) The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res 99:134–141
Schoenberg MH, Poch B, Younes M, Schwarz A, Baczako K, Lundberg C, Haglund U, Beger HG (1991) Involvement of neutrophils in postischaemic damage to the small intestine. Gut 32:905–1112
Sun Z, Wang X, Deng X, Boriesson A, Wallen R, Hallberg E, Andersson R (2000) Phagocytic and intestinal endothelial and epithelial barrier function during the early stage of small intestinal ischemia and reperfusion injury. Shock 13:209–216
Benke PJ (1988) Jessica in the well: ischemia and reperfusion injury. JAMA 259:1326
Davis JC (1988) Jessica in the well: ischemia and reperfusion injury. JAMA 259:3558
Re L, Mawsouf MN, Menéndez S, León OS, Sánchez GM, Hernández F (2008) Ozone therapy: clinical and basic evidence of its therapeutic potential. Arch Med Res 9:17–26
Bocci V (2004) Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators Infl amm 13:3–11
Bocci VA (2006) Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res 37:425–435
Bocci V, Zanardi I, Travagli V (2011) Oxygen/ozone as a medical gas mixture. A critical evaluation of the various methods clarifies positive and negative aspects. Med Gas Res 28:1–6
Bocci V (1996) Does ozone therapy normalize the cellular redox balance? Implications for therapy of human immunodeficiency virus infection and several other diseases. Med Hypotheses 46:150–154
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15:532–536
Park PO, Haglund U, Bulkley GB (1990) The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 107:574–580
Aslan MK, Boybeyi Ö, Şenyücel MF, Ayva Ş, Kısa Ü, Aksoy N, Soyer T, Cesur Ö, Çakmak M (2012) Protective effect of intraperitoneal ozone application in experimental ovarian ischemia/reperfusion injury. J Pediatr Surg 47:1730–1734
Gultekin FA, Cakmak GK, Turkcu UO, Yurdakan G, Demir FE, Comert M (2013) Effects of ozone oxidative preconditioning on liver regeneration after partial hepatectomy in rats. J Invest Surg 26:242–252
Ekici S, Doğan Ekici AI, Öztürk G, Benli Aksungar F, Sinanoğlu O, Turan G, Lüleci N (2012) Comparison of melatonin and ozone in the prevention of reperfusion injury following unilateral testicular torsion in rats. Urology 80:899–906
Oztosun M, Akgul EO, Cakir E, Cayci T, Uysal B, Ogur R, Ozcan A, Ozgurtas T, Guven A, Korkmaz A (2012) The effects of medical ozone therapy on renal ischemia/reperfusion injury. Ren Fail 34:921–925
Altinel O, Demirbas S, Cakir E, Yaman H, Ozerhan IH, Duran E, Cayci T, Akgul EO, Ersoz N, Uysal B, Kurt B, Yasar M, Oter S, Peker Y (2011) Comparison of hyperbaric oxygen and medical ozone therapies in a rat model of experimental distal colitis. Scand J Clin Lab Invest 71:185–192
Lelyanov AD, Sergienko VI, Ivliev NV, Emel’yanov VV, Guseva ED (2004) Effects of sodium hypochlorite and ozone on healing of intestinal anastomosis in simulated strangulation colorectal obstruction. Bull Exp Biol Med 137:103–105
Kesik V, Uysal B, Kurt B, Kismet E, Koseoglu V (2009) Ozone ameliorates methotrexate-induced intestinal injury in rats. Cancer Biol Ther 8:1623–1628
Sukhotnik I, Slijper N, Chemodanov E, Bashenko Y, Shaoul R, Coran AG, Mogilner JG (2011) Parenteral omega-3 fatty acids (Omegaven) modulate intestinal recovery following intestinal ischemia-reperfusion in a rat. J Pediatr Surg 46:1353–1360
Sukhotnik I, Slijper N, Chemodanov E, Bashenko Y, Shaoul R, Coran AG, Mogilner J (2010) Effect of simvastatin on intestinal recovery following gut ischemia-reperfusion injury in a rat. Ped Surg International 26:105–110
Cannizzaro A, Verga Falzacappa CV, Martinelli M, Misiti S, Brunetti E, Bucci B (2007) Source O(2/3) exposure inhibits cell progression affecting cyclin B1/cdk1 activity in SK-N-SH while induces apoptosis in SK-N-DZ neuroblastoma cells. J Cell Physiol 213:115–125
Cho HY, Hotchkiss JA, Harkema JR (1999) Inflammatory and epithelial responses during the development of ozone-induced mucous cell metaplasia in the nasal epithelium of rats. Toxicol Sci 51:135–145
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Kosmider B, Loader JE, Murphy RC, Mason RJ (2010) Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells. Free Radic Biol Med 48:1513–1524
Brink CB, Pretorius A, van Niekerk BP, Oliver DW, Venter DP (2008) Studies on cellular resilience and adaptation following acute and repetitive exposure to ozone in cultured human epithelial (HeLa) cells. Redox Rep 13:87–100
Acknowledgments
This work was supported by Rabinovitz Research Fund (2013) and Israel Society of Clinical Pediatrics (HIPAK) Research Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Haj and I. Sukhotnik equally contributed to preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Haj, B., Sukhotnik, I., Shaoul, R. et al. Effect of ozone on intestinal recovery following intestinal ischemia–reperfusion injury in a rat. Pediatr Surg Int 30, 181–188 (2014). https://doi.org/10.1007/s00383-013-3448-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-013-3448-8