Abstract
Background
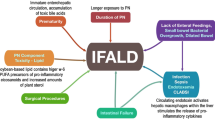
Intestinal failure-associated liver disease (IFALD) remains a serious problem in the treatment of infants with nutritional problems and short bowel syndrome.
Methods
A review of the recent literature from 2010 to 2016, concentrating on articles related to the pathophysiology of IFALD and to outcomes of novel nutritional and pharmacological therapies for neonatal cholestasis in the post-surgical neonate.
Results
The pathophysiology of IFALD relates to an increase sensitivity of the neonatal liver to cholestasis in the non-fed state; prolonged cholestasis almost inevitably results in liver damage which will progress from fibrosis to cirrhosis. Clinically discerned risk factors include premature birth, inflammation, sepsis, disruption of the enterohepatic circulation by creation of a proximal stoma, and the duration and type of parenteral nutritional support. Within the hepatocyte, the regulatory enzyme farsanoid receptor X (FXR) appears to play a pivotal role in the development of cholestasis. Recent studies have shown that its activity is suppressed by sepsis, and by plant phytosterols found in soy-based lipid preparations. This paradigm is reflected in the emerging consensus for the care of post-surgical neonates, which is based around a multi-disciplinary team approach. Using an algorithm-driven approach, an appropriate balance between caloric support and prevention of IFALD can be achieved.
Conclusions
Further prospective studies are required to further refine the optimal sequence of use of these therapies and the long-term effects on neurological development and hepatic function. However, with optimal care, the number of IF patients progressing to end-stage liver disease because of IFALD should be very low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The problem of nutrient malabsorption (intestinal failure or IF) following intestinal resection and the subsequent reliance on parenteral nutrition (PN) is common in neonates [1]. Worldwide, there is an increasing incidence of such babies, due to the increase in survival of premature infants, and the associated increased incidence of necrotizing enterocolitis (NEC). NEC is the most common cause of IF. The provision of nutritional support (PN) to support growth and allowing time for the remaining intestine to heal and adapt is the fundamental therapies for this patient group [2, 3]. This care is supportive; no therapies exist which can improve the function of the residual intestine or hasten the course of adaptation (the up-regulation of intestinal function). However, during this phase, the infant liver is uniquely susceptible to cholestasis. The reasons for this are not clear; although in general, outcomes are improving [1, 3, 4], cholestasis in infants supported with PN (parenteral nutrition associated cholestasis) and the subset of these patients who progress to liver disease after PN for intestinal failure (IFALD) is an unsolved problem. A recent meta-analysis of 3280 susceptible patients showed an incidence of 28.2 and 49.8% of PNAC and IFALD, respectively, with no change in incidence over the last decades [5]. This study highlighted the lack of direct evidence to support the use of many clinical therapies in the post-surgical neonate. While a number of therapies have been shown to be efficacious in the premature neonate requiring PN (where the major pathology is only prematurity), they have not been adequately evaluated in the post-surgical neonate. Thus, many of the recommendations for the prevention and treatment of IFALD are done on the basis of findings in the non-surgical premature population.
The pathophysiologic underpinnings of the improvement in outcomes achieved in centers which use a multi-disciplinary team to care for this patient population are the focus of this report [4]. Specifically, we will review the recent literature regarding the pathophysiology of PNAC and IFALD and the implications for the choice of lipid therapy in infants following intestinal resection. The findings will be presented by reviewing the pathophysiology of PNAC/IFALD, and then considering the prevention, treatment, rescue therapy, and the long-term outcomes of these therapies.
Pathophysiology In the fasting state, the normal stimulus for bile flow and bilirubin excretion is interrupted. Physiologically, in infants, this pathway is further stressed by the ongoing breakdown of fetal hemoglobin. Furthermore, if the infant undergoes surgical stress and is fasted, both sides of the equation are worsened; the breakdown of increases the production of bilirubin, while the stressed state reduces the conjugation and especially the excretion of bilirubin and bile acids [6]. This linkage between sepsis and cholestasis has been firmly established in animal models and is evident clinically in infants supported on PN [7, 8]. Studies examining the fundamental pathophysiology of PNAC have been primarily conducted in animals, but despite this limitation, a series of very relevant findings have recently been published. Key to these pathways is the Farsanoid receptor X receptor (FXR) (Fig. 1). It is likely that many of the negative effects of PN and other problems in the infant following intestinal surgery are related to this important regulator of liver activity.
NTCP Na+/taurocholate cotransporter: main transporter for Na+-dependent bile acid uptake from portal blood into hepatocyte. BSEP Bile salt export pump: ATP-dependent transport of monovalent bile acids. into bile; major determinant of bile salt dependent bile flow substrate: Monovalent and divalent bile acids. MRP 2 multidrug resistance-associated protein: ATP-dependent transport of organic anions into bile; major determinant of bile salt independent bile substrate: divalent but not monovalent bile acids, GSH, bilirubin mono/diglucuronide, LTC4, several other organic anions, as divalent amphipathic conjugates with glutathione. FXR Farnesoid X receptor activated by hydrophobic bile acids such as chenodeoxycholic acid (CDCA) > deoxycholic acid (DCA) = lithocholic acid (LCA) > cholic acid (CA). CYP7A1 cholesterol-7-α-hydroxylase rate-limiting step of bile acid production. substrate: cholesterol
The Farnesoid X receptor (FXR) is the major regulator of the excretion of conjugated metabolic byproducts (of which bilirubin is a major but not exclusive component), as well as bile acid homeostasis [8,9,10,11]. FXR regulates the expression of the major transporter proteins for bile acids and conjugated toxins at the bile duct canaliculus: the bile salt export pump (BSEP), the major bile acid efflux mechanism under physiologic conditions, and multidrug resistance-associated protein 2 (MRP2), which is the prime pathway for conjugated bilirubin and other metabolic breakdown products. However, FXR activity itself is regulated by bile acids, especially increased by hydrophobic bile acids, such as chenodeoxycholic acid (CDCA) > deoxycholic acid (DCA) = lithocholic acid (LCA) > cholic acid (CA). These are in large part ‘recycled’ from the enteric stream, and are partially dependent on active scavenging from the terminal ileum. It has been a consistent observation that cholestasis is worse in infants with a jejunostomy, which may be due to the elimination in the recycling of these bile salts and a secondary decrease in FXR activity [8,9,10]. Furthermore, FXR also regulates the expression of transporters which allow the uptake of these bile salts recirculating from the intestine into the hepatocyte (NATCP and OATCP). Finally, the FXR is the major regulator of ongoing synthesis of novel bile acids; FXR is a negative regulator of cholesterol 7α-hydroxylase, the rate-limiting step of the classic bile acid synthesis pathway. Thus, during normal physiological states, ongoing enteral nutrition and the feeding-related stimulus for bile secretion results in bile mixing with enteric contents. This then is a major component of the luminal phase of nutrient digestion. Conjugated bile salts are resorbed in the terminal ileum, and taken up by the hepatocyte via the NATCP and OATCP transporters. These conjugated bile salts then further activate the FXR receptor, further upregulating the expression of transporters bringing portal venous bile salts into the hepatocyte. In addition, increased in expression and activity are BESP and MDRP2, which caniliculi. Finally, activation of the FXR down-regulates the de novo synthesis of new bile salts [10].
Multiple lines of evidence have shown that infection and sepsis down-regulate FXR receptor activity [11]. This appears to be via direct actions of inflammatory cytokines to inhibit the activity of the FXR receptor [12, 13]. It also appears to involve signaling via the Toll-like receptor 4 pathway, which directly affects the hepatocyte, reducing FXR activity. PN has been shown to reduce gut barrier function and increase the likelihood of bacterial translocation, which is the major ligand of the TLR-4 receptor.
Most recently, plant derived phytosterols, found in the lipid component of PN, have been shown to be potent inhibitors of FXR activity, both in vitro and in vivo [13,14,15]. This was shown most clearly in a recent study where added plant sterols were shown to directly inhibit FXR activity, so that fish-oil-derived lipids when given with exogenous plant phytosterols (stigmasterol) were as damaging to the liver as conventional soy-based lipid preparations [16] (Fig. 2).
Composition of major lipid emulsions available for neonates. Fat source (Soybean, Coconut (MCT), Fish, and Olive) is shown pictorally. Omega-3 and Omega-6 ratio relates to fatty acid composition of lipids, with metabolic and neuorologic implications. α-tocopherol content indicates antioxidant potential, Phytosterols are plant derived steroid compounds with potential toxicity to the neonatal liver
Clinical impact
The clinical correlate of the pathophysiologic pathways described above is the susceptibility of the post-surgical neonate to develop cholestasis, which then may progress to liver disease (IFALD) with conventional PN support. The therapies or treatment paradigms which optimize outcomes have developed empirically, as different clinical teams have used different nutritional support strategies (both parenteral and enteral nutrition) and ancillary therapies. Based on the outcomes of these strategies, an assessment of the relative recommendations for clinical care paradigms can be made; typically, there is greater evidence for the efficacy of a therapy in the prevention of PNAC. By extrapolation, these treatments can reasonably be applied to the post-surgical neonate, and IFALD but further direct study is warranted. Herein, we present the best available evidence of measures taken to prevent and reverse PNAC and IFALD.
Overview of nutritional considerations
The primary considerations in the care of the post-surgical neonate are the support of respiration and nutrition. As noted previously, the mainstay of nutritional support in the premature or post-surgical infant has been parenteral nutrition. It has now been well demonstrated that a multi-disciplinary team-based care pathway for infants with IF is the most significant factor in improving outcome [1, 3, 4].
Feeding protocols Part of such a care pathway is to continually advance the amount of enteral nutrition delivered, to stimulate adaptation [3, 17]. Increasing enteral nutrient delivery will have an effect on liver function; as noted in the discussion above, enteral recycling of bile salts is a powerful stimulus for the normalization of cholestasis. It appears that there is a requirement for >50% of nutrients to be delivered enterally to begin to reverse long standing or severe cholestasis. There is no clear evidence that any particular diet or feeding regimen is superior to another; however, all evidences suggest that breast milk (EBM) is the optimal enteral nutrition [2,3,4]. There are case reports which suggest that the use of milk fortifier and formula feeds is detrimental to the overall outcome in post-surgical infants, and likely increases the incidence of recurrent NEC [18]. There is also anecdotal evidence that if EBM is not available, then elemental formula is the optimal substitute [19]. It appears that the post-surgical neonate is more susceptible to the development of enteral sensitization to cow’s milk proteins, even if they are partially hydrolyzed [20]. In infants who can feed orally, there is a suggestion that promoting oral feeding optimizes the adaptive process [3, 21]. Physiologically, the admixture of saliva helps both the digestive process, and adds EGF to the nutrient stream. As well, oral feeding reinforces the suck and swallow reflex and helps to prevent oral aversion, which can be a significant problem in this population.
Use of choleretic agents
Studies on the use of cholecystokinin and tauroursodeoxycholic acid showed no significant effect of effect on the incidence of PNAC [22, 23]. However, many units continue to use them based in part on their relatively benign nature and favorable risk profile.
Use of prophylactic enteric antibiotics
From the discussion above, if sepsis increases cholestasis, then if is reasonable to consider antibiotics therapy as a means to reduce cholestasis. Multiple studies have suggested that careful prevention of sepsis and the use of enteral antibiotics for bacterial overgrowth reduce cholestasis [3, 24]. Two different RCTs focused on the possible prevention of PNAC with erythromycin in VLBW neonates. One used high-dose 12.5 mg/kg/dose every 6 h for 14 days with significantly lower incidence of PNAC, time to enteral feed, total TPN time, as well as reduced episodes of sepsis [25]. The other study used an intermediate erythromycin dose of 5 mg/kg/dose every 6 h for 14 days ad showed same findings as well as time required to achieve a body weight ≥2500 g (P < 0.05) were significantly shorter in treated infants [26]. Furthermore, the incidence of necrotizing enterocolitis (NEC) ≥stage IIa after 14 days of treatment was significantly lower in the erythromycin group. It is not clear that why these findings have not been more widely incorporated into clinical practice, but they certainly are worthy of consideration.
Parenteral nutrition: prevention or prophylaxis of cholestasis
The general considerations for the use of different PN strategies are highly dependent on the clinical circumstances. In the otherwise well infant, who is likely to require 2–3 weeks of PN, it is reasonable to use soy-based lipid emulsion (SB-LE). The world wide experience with this lipid formulation is vast, the likelihood of cholestasis is low, and the cost is the lowest amongst available lipid formulations. [27,28,29] Furthermore, the soy-based lipid preparation is higher in ∞-6 fatty acids which are required for neurological development [30], (see Fig. 2). However, close monitoring for cholestasis is required. If there is a trend to an increase in bilirubin levels above the normal range (>18–25 μmol/L), then an aggressive approach to reduce cholestasis is appropriate. These strategies are reviewed in the next section.
Combination lipids SMOF-LE
For infants at high risk of PNAC or IFALD, such as those who are post NEC and post intestinal resection, there is no strong evidence that the prophylactic use of alternative lipid formulations or lipid reduction strategies will prevent cholestasis [28, 31]. In addition, there are ongoing concerns about the long-term neurological consequences of a reduced lipid strategy [30, 32]. To avoid the hepatotoxicity seen with sole use of soy-based LE, yet allow for essential fat to be delivered, combination lipid emulsions have been formulated. The commercially available combination of 30% soybean oil, 30% MCT, 25% olive oil, and 15% fish oil (SMOF-LE) seems to combine the benefits of individual fatty acids while minimizing side effects (Fig. 2). It is notable that the ration of omega 3: to omega 6 fat in this formulation is identical to that in human breast milk. Rayyan et al. compared the use of SMOF-LE versus soybean-LE in preterm infants [33]; in this study, the amount was increased gradually to a goal of 3 g/kg/day for 14 days. SMOF-LE group demonstrated lower bilirubin level and lower N-6/N-3 fatty acid ratio and a lower linolenic acid compared to the comparison soybean-based LE treated group.
Accordingly, for infants at risk for PNAC, in view of the accumulating evidence that the balanced lipid formulation (SMOF-LE) is safe, and meets the developing infant’s nutritional requirements for fat, many experienced centers are switching to using such formulations as the primary lipid source. (Wales P, personal communication) [33, 34].
PN: treatment of established cholestasis
In an infant support with PN, if cholestasis occurs, the response is dependent on the degree of cholestasis, and the underlying causes of intestinal failure. In general, three overlapping strategies have evolved: reducing lipid intake, the use of alternate lipid therapy, such as SMOF-LE, or the use of fish-oil-based lipid emulsions. (FO-LE). Fish-oil-based protocols have typically also involved a reduction in lipid intake to 1 gm/kg/day.
Limiting daily parenteral lipid intake to <1 gm/kg/day
The evidence from retrospective, cohort studies and small scale randomized controlled trials clearly shows that limiting SB-LE intake to <1 g/kg/day as opposed to conventional 2.5–3 g/kg/day reduces bilirubin level in infants with established cholestasis [32]. This occurs along with a persistence in transaminase elevation suggesting that there is ongoing liver damage. Another point of interest is whether lipid restriction impacts growth. In a recent case series, nine infants who are receiving TPN for >12 months with soy-based lipid emulsion content <1 g/day displayed improved weight and height z scores at the end of the study [32]. Essential fatty acid deficiency was observed in two of the patients due to temporary stop of lipid emulsion to improve cholestasis. This improved after restarting the lipids in both patients who were mostly TPN-dependent with >70% of calories intake. Further controlled trials are required to verify these findings and to study the effect of lipid restriction on neural development.
Use of alternative lipids
In patients with mild to moderate cholestasis, it is reasonable to consider the use of alternative lipid strategies. A recent trial comparing SB-LE to SMOF-LE showed improved normalization of bilirubin with SMOF treatment and a conventional lipid dosing protocol (2.5–3 gm/kg/day) [33]. The alternative strategy is the use of a fish-oil-based lipid emulsion (FO-LE), which as noted above, is typically done with a reduction in lipid dosing. There are now a number of smaller studies which support the use of FO-LE in infants with PNAC or IFALD [35, 36].
In a review of the literature and meta-analysis, Park et al. reviewed the effect of FO-LE on PNAC and found that using FO-LE was more likely to reverse PNAC compared to SB-LE (OR 6.14; 95% CI 2.27, 16.6; P < 0.01 [37]. It is notable that FO-LE did not prevent the development of PNAC (OR 0.56; 95% CI 0.28, 1.10; P = 0.09). There are no studies which compare the efficacy of SMOF vs FO-LE in cholestasis. In light of the satisfactory results reported with SMOF-LE in mild to moderate cholestasis, it would seem reasonable to use this therapy (with typical lipid dosing e.g. 2.5–3 gm/kg/day), so long as the bilirubin is <50 μmol/L. However, with severe cholestasis (>50 μmol/L), because of the greater experience with the use of FO-LE, it is appropriate to recommend FO-LE (with lipid minimization) [38, 39].
Long-term effects
The critical factor in judging the efficacy of any therapy for infants is the long-term outcome. There have been a number of reports of the neurological outcomes of infants who require major surgical interventions and long-term PN support [40, 41]. The findings are of concern; there is a significant long-term neurological morbidity in this population, particular those treated for NEC. It is not clear from the present studies what, if any, of the neurological impairment seen in this population may be due to nutritional issues. However, it is clear that any studies of the long-term effects of novel lipid strategies must include neurological outcomes as an outcome measure.
From the perspective of the long-term liver function, it is important to understand that the long-term cholestasis (>50 μmol/L bilirubin, for >2 months) is almost certainly associated with some degree of liver fibrosis [42]. The use of FO-LE and a lipid reduction strategy appears to greatly improve the cholestasis and preserve liver function [43], but the effects on fibrosis or more significant cirrhosis are not known In addition, it is not clear what the long-term effects on liver function, and future liver growth is, and these must also be also part of the long-term follow-up of these patients.
Conclusion
All the current studies show that no one type of lipid emulsion is perfect to use in preterm infants. There is clear evidence that using soy-based lipid emulsion can lead to cholestasis and potentially irreversible cirrhosis that goes beyond the duration of use of parenteral nutrition.
The best available evidence suggests greater advantage gained using SMOF-LE. The current evidence suggests that fish-oil-based lipid emulsion as sole treatment or in combination (SMOF) is effective in reversing cholestasis and preserving liver function. However, these novel lipid therapies cannot reverse histologic changes; it is likely that the long-term hepatic injury persists after the cessation of TPN and normalization of liver function. The long-term effects of novel lipid strategies that preterm neonates on neurological development are an important area for longer term studies.
References
Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R et al (2012) Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 161(4):723–728
Kocoshis SA (2010) Medical management of pediatric intestinal failure. Semin Pediatr Surg 19(1):20–26
Sigalet D, Boctor D, Brindle M, Lam V, Robertson M (2011) Elements of successful intestinal rehabilitation. J Pediatr Surg 46(1):150–156
Stanger JD, Oliveira C, Blackmore C, Avitzur Y (2013) Wales PA The impact of multi-disciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: a systematic review and meta-analysis. J Pediatr Surg 48:983–992
Lauriti G, Zani A, Aufieri R, Cananzi M, Chiesa PL, Eaton S, Pierro A (2014) Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr 38(1):70–85. doi:10.1177/0148607113496280
Karpen SJ (2002) Update on the etiologies and management of neonatal cholestasis. Clin Perinat. 29:159–180
Geier A, Mb Wagner, Dietrich CG, Trauner M (2007) Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim et Biophys Acta 1773:283–308
Beath SV, Davies P, Papadopolou A et al (1996) Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg 31:604–606
Lacaille F, Gupte G, Colomb V, D’Antiga L, Hartman C, Hojsak I, Kolacek S, Puntis J, Shamir R; ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation (2015) Intestinal failure-associated liver disease: a position paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J Pediatr Gastroenterol Nutr 60(2):272–283. doi:10.1097/MPG.0000000000000586
Wagner M, Zollner G (2009) Trauner M New molecular insights into the mechanisms of cholestasis J. Hepatology 51:565–580
Burrin DG, Ng K, Stoll B, Pipaón MSG (2014) Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr 5:82–91. doi:10.3945/an.113.004796
Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA et al (2010) Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr 140:2193–2200
Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C (2003) Repression of farnesoid X receptor during the acute phase response. J Biol Chem 278:8988–8995
Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, Karpen SJ (2007) Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res 62:301–306
Forchielli ML, Bersani G, Tala S et al (2010) The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids 45:63. doi:10.1007/s11745-009-3371
Karim CEK, Anderson AL, Devereaux MW et al (2013) Phytosterols promote liver injury and kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med 5(206):206137
Goulet O, Olieman J, Ksiazyk J, Spolidoro J, Tibboe D, Köhler H, Vural Yagci R, Falconer J, Grimble G, Beattie RM (2013) Neonatal short bowel syndrome as a model of intestinal failure: physiological background for enteral feeding. Clinical Nutrition 32:162–171
Cristofalo EA, Schanler RJ, Blanco CL et al (2013) Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 163:1592–1595
Stamm DA, Hait E, Litman HJ, Mitchell PD, Duggan C (2016) High prevalence of eosinophilic gastrointestinal disease in children with intestinal failure. J Pediatr Gastroenterol Nutr 63(3):336–339
Mazon A, Solera E, Alentado N et al (2008) Frequent IgE sensitization to latex, cow’s milk, and egg in children with short bowel syndrome. Pediatr Allergy Immunol 19:180–183
Javid PJ, Collier S, Richardson D, Iglesias J, Gurac K, Lob C, Kim HB, Duggan C, Tom Jaksic T (2005) The role of enteral nutrition in the reversal of parenteral nutrition-associated liver dysfunction in infants. J Pediatr Surg 40:1015–1018
Teitelbaum DH, Tracy TF Jr, Aouthmany MM, Llanos A, Brown MB, Yu S et al (2005) Use of cholecystokinin-octapeptide for the prevention of parenteral nutrition-associated cholestasis. Pediatrics 115:1332–1340
Heubi JE, Wiechmann DA, Creutzinger V, Setchell KD, Squires R Jr, Couser R et al (2002) Tauroursodeoxycholic acid (TUDCA) in the prevention of total parenteral nutrition-associated liver disease. J Pediatr 141:237–242
Meehan JJ, Georgeson KE (1997) Prevention of liver failure in parenteral nutrition-dependent children with short bowel syndrome. J Pediatr Surg 32:473–475
Ng PC, Lee CH, Wong SPS et al (2007) High-dose oral erythromycin decreased the incidence of parenteral nutrition-associated cholestasis in preterm infants. Gastroenterology 132:1726–1739
Ng YY, Su PH, Chen JY et al (2012) Efficacy of intermediate-dose oral erythromycin on very low birth weight infants with feeding intolerance. Pediatr Neonatol. 53:34–40
Kapoor V, Glover R, Malviya MN (2015) Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst Rev. doi:10.1002/14651858.CD009172.pub2
Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, Miles JM, Valentine CJ, Kochevar M, Novel Nutrient Task Force, Intravenous Fat Emulsions Workgroup, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors (2012) A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 27(2):150–192
Nandivada P, Fell GL, Gura KM, Puder M (2016) Lipid emulsions in the treatment and prevention of parenteral nutrition-associated liver disease in infants and children. Am J Clin Nutr 103(2):629S–634S. doi:10.3945/ajcn.114.103986
Innes S (2009) Omega-3 fatty acids and neural development to 2 years of age: do we know enough for dietary recommendations? J Pediatr Gastroenterol Nutr 48(Supplement):S16–S24
Finn KL, Chung M, Rothpletz-Puglia P (2015) Byham-Gray L impact of providing a combination lipid emulsion compared with a standard soybean oil lipid emulsion in children receiving parenteral nutrition: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 39(6):656–667
Lam G, Strogach IG, Baron N, Thompson JF (2016) Normal growth and essential fatty acid status in children with intestinal failure on lipid limitation. J Pediatr Gastroenterol Nutr 62(2):335–340
Diamond IR, Grant RC, Pencharz PB, de Silva N, Feldman BM, Fitzgerald P, Sigalet D, Dicken B, Turner J, Marchand V, Ling SC, Moore AM, Avitzur Y, Wales PW (2016) Preventing the progression of intestinal failure-associated liver disease in infants using a composite lipid emulsion: a pilot randomized controlled trial of SMOFlipid. JPEN J Parenter Enteral Nutr. doi:10.1177/0148607115626921
Rayyan M, Devlieger H, Jochum F, Allegaert K (2012) Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized double-blind study in preterm infants. JPEN J Parenter Enteral Nutr 36(1 Suppl):81S–94S PMID: 22237883
Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RAM, Lopes S, Duggan C (2008) Puder M safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 121:e679–e686
Lam HS, Tam YH, Poon TC, Cheung HM, Yu X, Chan BP, Lee KH, Lee BS, Ng PC (2014) A double-blind randomised controlled trial of fish oil-based versus soy-based lipid preparations in the treatment of infants with parenteral nutrition-associated cholestasis. Neonatology 105:290–296. doi:10.1159/000358267
Park HW, Lee NM, Kim JH, Kim KS, Kim SN (2015) Parenteral fish oil-containing lipid emulsions may reverse parenteral nutrition-associated cholestasis in neonates: a systematic review and meta-analysis. J Nutr 145(2):277–283. doi:10.3945/jn.114.204974
Koletzko B, Goulet O (2010) Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care 13(3):321–326. doi:10.1097/MCO.0b013e3283385407
Park HW, Lee NM, Kim JH, Kim KS, Kim SN (2014) Parenteral fish oil—containing lipid emulsions may reverse parenteral nutrition–associated cholestasis in neonates: a systematic review and meta-analysis. J Nutr Dis. doi:10.3945/jn.114.204974
Blakely ML, Tyson JE, Lally KP et al (2006) Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 117(4):e680–e687
Schulzke SM, Deshpande CG, Patole SK (2007) Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis a systematic review of observational studies. Arch Pediatr Adolesc Med 161:583–590
Fitzgibbons SC, Jones BA, Hulla MA, Zurakowskic D, Duro D, Duggan C, Boctor D, Sigalet DL, Jaksic T (2010) Relationship between biopsy-proven parenteral nutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Ped Surg 45:95–99
Nandivada P, Chang MI, Potemkin AK, Carlson SJ, Cowan E, Oʼloughlin AA, Mitchell PD, Gura KM, Puder M (2015) The natural history of cirrhosis from parenteral nutrition-associated liver disease after resolution of cholestasis with parenteral fish oil therapy. Ann Surg 261(1):172–179. doi:10.1097/SLA.0000000000000445
Acknowledgements
These studies were supported by the Professorship in Pediatric Surgical Research of the Alberta Children’s Hospital Research Foundation and by Sidra Medical and Research Center, Doha Qatar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Al-Shahwani, N.H., Sigalet, D.L. Pathophysiology, prevention, treatment, and outcomes of intestinal failure-associated liver disease. Pediatr Surg Int 33, 405–411 (2017). https://doi.org/10.1007/s00383-016-4042-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-4042-7